Characteristic Analysis of Trigonelline Contained in Raphanus sativus Cv. Sakurajima Daikon and Results from the First Trial Examining Its Vasodilator Properties in Humans
Abstract
1. Introduction
2. Materials and Methods
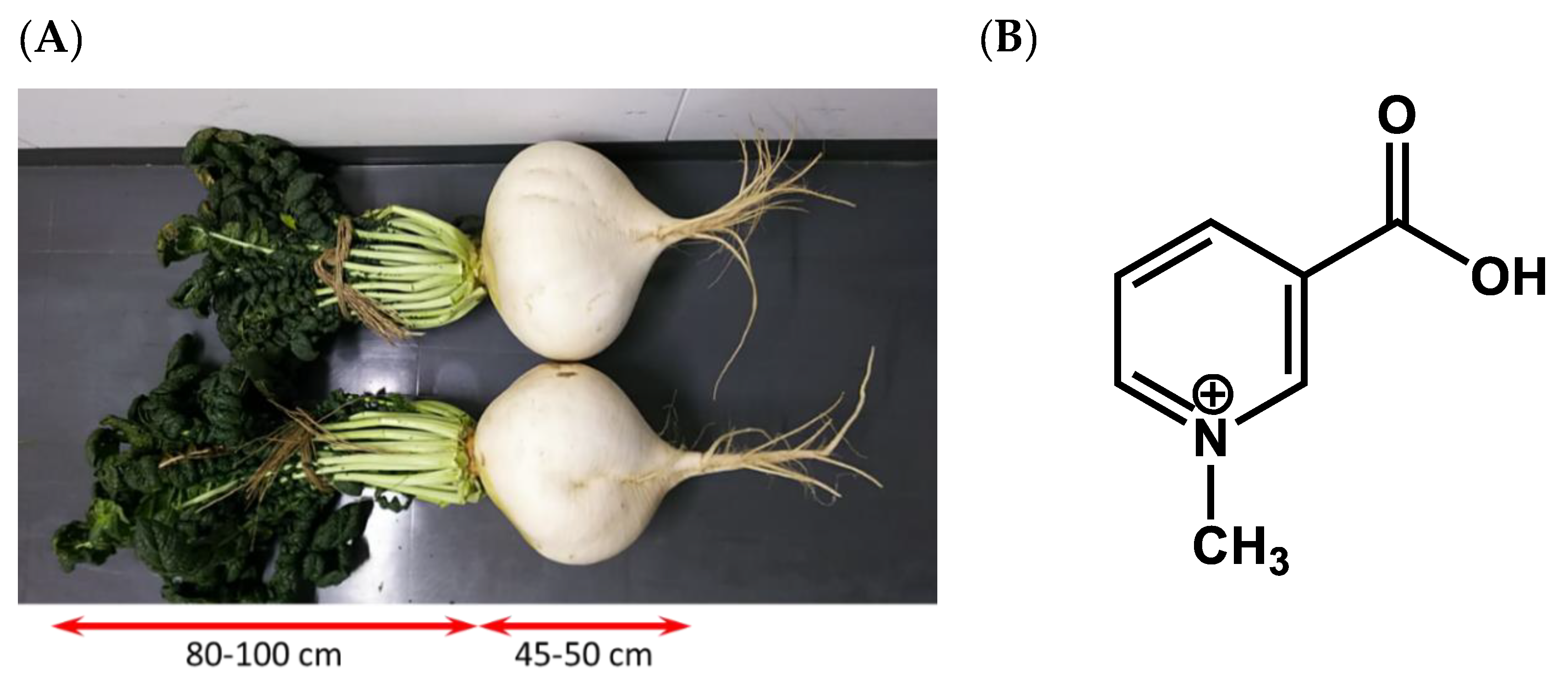
2.1. Materials
2.2. Quantification of Trigonelline
2.3. Cooking and Processing
2.4. First Trial of Sakurajima Radish Examining the Vasodilator Property in Humans
2.5. Statistical Analyses
3. Results
3.1. Comparison of Trigonelline Content in Sakurajima Radish
3.2. Differences in Trigonelline Content between Sakurajima Radish Varieties
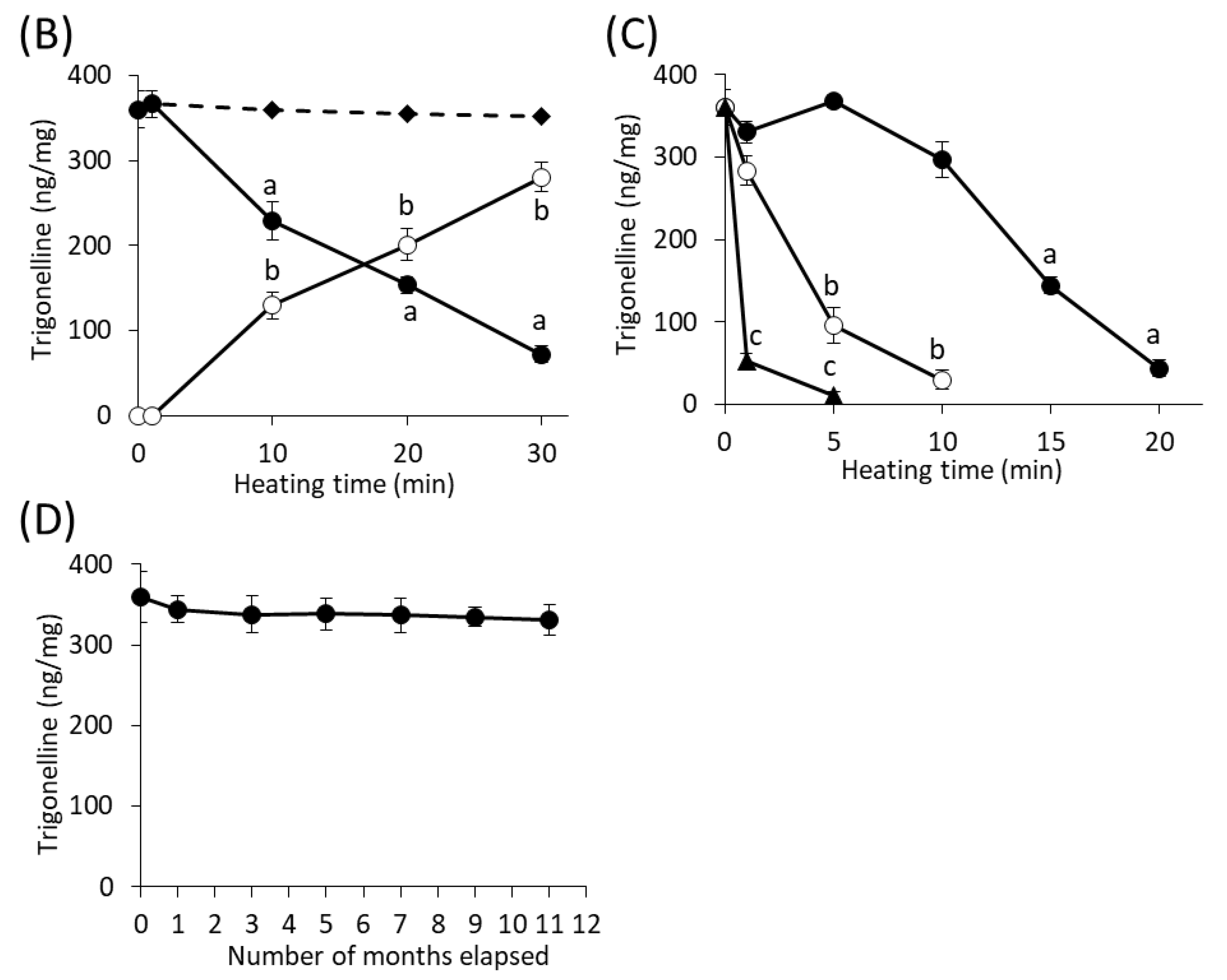
3.3. Effects of Cooking and Processing Sakurajima Radishes on Trigonelline Contents
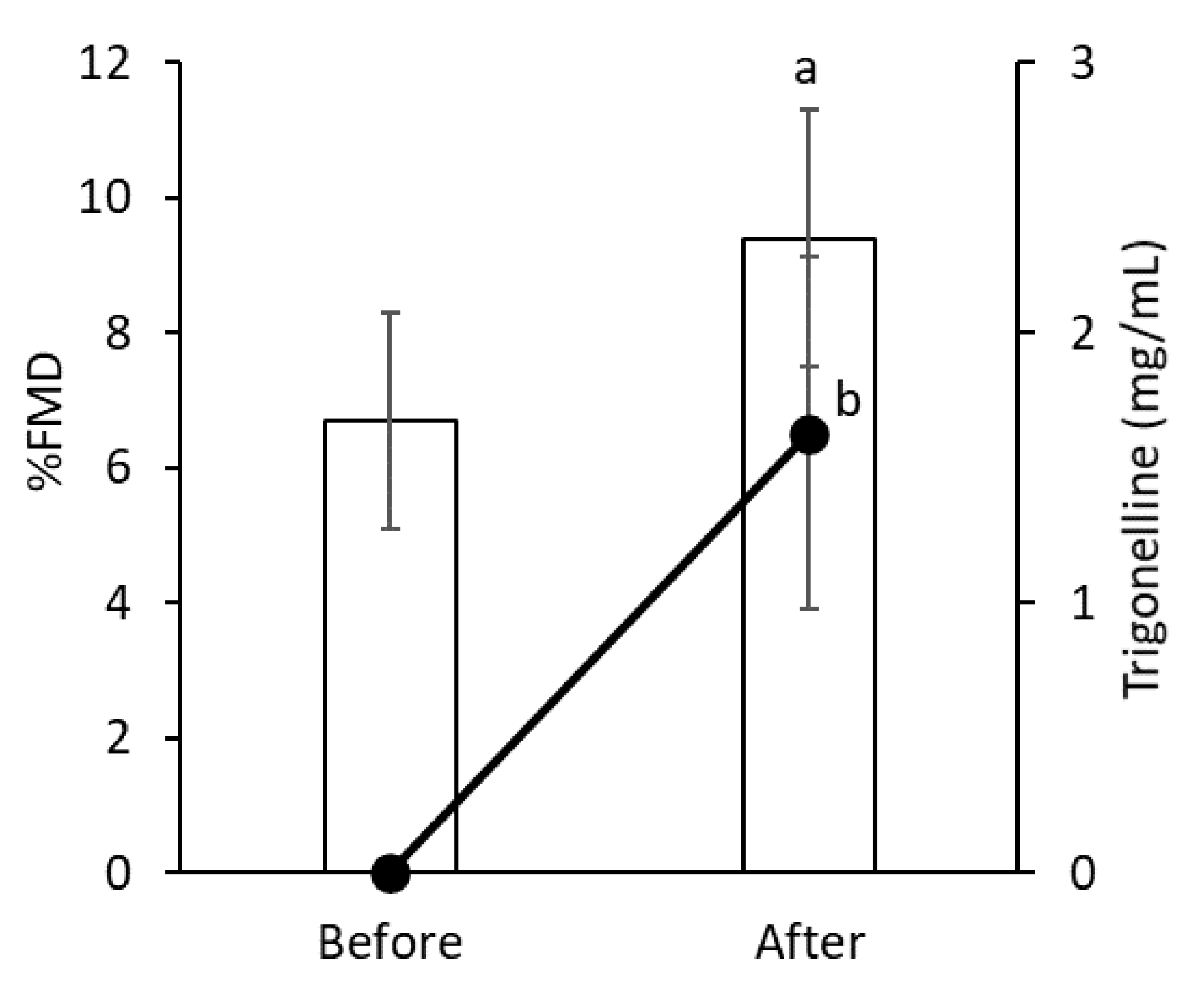
3.4. First Trial of Sakurajima Radish Examining the Vasodilator Property in Humans
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kuroda, R.; Kazumura, K.; Ushikata, M.; Minami, Y.; Kajiya, K. Elucidating the improvement in vascular endothelial function from Sakurajima daikon and its mechanism of action: A comparative study with Raphanus sativus. J. Agri. Food Chem. 2018, 66, 8714–8721. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, M.; Yamanouchi, H.; Sahara, H.; Iwanaga, T.; Kuroda, R.; Yamamoto, A.; Minami, Y.; Sekijima, M.; Yamada, K.; Kajiya, K. Catechin and caffeine contents in green tea at different harvest periods and their metabolism in miniature swine. Food Sci. Nutr. 2019, 7, 2769–2778. [Google Scholar] [CrossRef]
- Kajiya, K.; Yamanouchi, H.; Tanaka, Y.; Hayashi, H.; Minami, Y. Capsicum cultivated under adverse conditions produces high concentrations of antioxidants and capsaicinoids. J. Agri. Sci. 2020, 12, 1–14. [Google Scholar] [CrossRef][Green Version]
- Vanhoutte, P.M.; Shimokawa, H.; Feletou, M.; Tang, E.H.C. Endothelial dysfunction and vascular disease—A 30th anniversary update. J. Agri. Food Chem. 2018, 66, 8714–8721. [Google Scholar] [CrossRef] [PubMed]
- Bekendam, R.H.; Iyu, D.; Passam, F.; Stopa, J.D.; De Ceunynck, K.; Muse, O.; Bendapudi, P.K.; Garnier, C.L.; Gopal, S.; Crescence, L.; et al. Protein disulfide isomerase regulation by nitric oxide maintains vascular quiescence and controls thrombus formation. J. Thromb. Haemost. 2018, 16, 2322–2335. [Google Scholar] [CrossRef] [PubMed]
- Gliozzi, M.; Scicchitano, M.; Bosco, F.; Musolino, V.; Carresi, C.; Scarano, F.; Maiuolo, J.; Nucera, S.; Maretta, A.; Paone, S.; et al. Modulation of Nitric Oxide Synthases by Oxidized LDLs: Role in Vascular Inflammation and Atherosclerosis Development. Int. J. Mol. Sci. 2019, 20, 3294. [Google Scholar] [CrossRef] [PubMed]
- Guinness World Records. Heaviest Radish. Available online: http://www.guinnessworldrecords.com/world-records/heaviest-radish (accessed on 23 June 2020).
- American Chemical Society. Compounds in ‘monster’ radish could help tame cardiovascular disease. J. Agric. Food Chem. 2018. Available online: https://www.acs.org/content/acs/en/pressroom/presspacs/2018/acs-presspac-august-8-2018/compounds-in-monster-radish-could-help-tame-cardiovascular-disease.html (accessed on 23 June 2020).
- Toshinari, O.; Sato, H.; Igarashi, K. Anti-diabetic effects of pumpkin its components, trigonelline and nicotinic acid, on Goto-Kakizaki rats. Biosci. Biotechnol. Biochem. 2009, 73, 1033–1041. [Google Scholar] [CrossRef]
- Zhou, J.; Chan, L.; Zhou, S. Trigonelline: A plant alkaloid with therapeutic potential for diabetes and central nervous system disease. Curr. Med. Chem. 2012, 19, 3523–3531. [Google Scholar] [CrossRef]
- Kano, Y.; Fukuoka, N. Role of endogenous cytokinin in the development of hollowing in the root of Japanese radish (Raphanus sativus L.). Sci. Hortic. 1996, 65, 105–115. [Google Scholar] [CrossRef]
- Kano, Y.; Fukuoka, N. Effects of soil temperature on hollowness in Japanese radish (Raphanus sativus L. cv. ‘Gensuke’). Sci. Hortic. 1995, 61, 157–166. [Google Scholar] [CrossRef]
- Thijssen, D.H.J.; Bruno, R.M.; van Mil, A.C.C.M.; Holder, S.M.; Faita, F.; Greyling, A.; Zock, P.L.; Taddei, S.; Deanfield, J.E.; Luscher, T.; et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur. Heart J. 2019, 40, 2534–2547. [Google Scholar] [CrossRef]
- Corretti, M.C.; Anderson, T.J.; Benjamin, E.J.; Celermajer, D.; Charbonneau, F.; Creager, M.A.; Deanfield, J.; Drexler, H.; Gerhard-Herman, M.; Herrington, D.; et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J. Am. Coll. Cardiol. 2002, 39, 257–265. [Google Scholar] [CrossRef]
- Neunteufl, T.; Katzenschlager, R.; Hassan, A.; Klaar, U.; Schwarzacher, S.; Glogar, D.; Bauer, P.; Weidinger, F. Systemic endothelial dysfunction is related to the extent and severity of coronary artery disease. Atherosclerosis 1997, 129, 111–118. [Google Scholar] [CrossRef]
- Stennert, A.; Maier, H.G. Trigonelline in coffee. II. Content of green, roasted and instant coffee. Zeitschrift fur Lebensmittel-Untersuchung und Forschung 1994, 199, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Matsuzaki, M.; Kanazawa, S.; Tokiwano, T.; Yoshizawa, Y.; Kato, M. Conversion of nicotinic acid to trigonelline is catalyzed by N-methyltransferase belonged to motif B-methyltransferase family in Coffea Arabica. Biochem. Biophys. Res. Commun. 2014, 452, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Q.; Matsui, A.; Ashihara, H. Biosynthesis of trigonelline from nicotinate mononucleotide in mungbean seedlings. Phytochemistry 2008, 69, 390–395. [Google Scholar] [CrossRef]
- Zheng, X.Q.; Hayashibe, E.; Ashihara, H. Changes in trigonelline (N-methylnicotinic acid) content and nicotinic acid metabolism during germination of mungbean (Phaseolus aureus) seeds. J. Exp. Bot. 2005, 56, 1615–1623. [Google Scholar] [CrossRef]
- Nordestgaard, B.G. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: New insights from epidemiology, genetics, and biology. Circ. Res. 2016, 118, 547–563. [Google Scholar] [CrossRef]
- Anwar, S.; Bhandari, U.; Panda, B.P.; Dubey, K.; Khan, W.; Ahmad, S. Trigonelline inhibits intestinal microbial metabolism of choline and its associated cardiovascular risk. J. Pharm. Biomed. Anal. 2018, 159, 100–112. [Google Scholar] [CrossRef]
- Sharma, L.; Lone, N.A.; Knott, R.M.; Hassan, A.; Abdullah, T. Trigonelline prevents high cholesterol and high fat diet induced hepatic lipid accumulation and lipo-toxicity in C57BL/6J mice, via restoration of hepatic autophagy. Food Chem. Toxicol. 2018, 121, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Du, X.; Zhang, Z.; Zhou, J. Trigonelline inhibits caspase 3 to protect β cells apoptosis in streptozotocin-induced type 1 diabetic mice. Eur. J. Pharmacol. 2018, 836, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Q.; Wang, C.; Lou, Z.; Li, Q. Trigonelline reduced diabetic nephropathy and insulin resistance in type 2 diabetic rats through peroxisome proliferator-activated receptor-γ. Exp. Ther. Med. 2019, 18, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, A.E.; Olthof, M.R.; Meeuse, J.C.; Seebus, E.; Heine, R.J.; van Dam, R.M. Acute effects of decaffeinated coffee and the major coffee components chlorogenic acid and trigonelline on glucose tolerance. Diabetes Care 2009, 32, 1023–1025. [Google Scholar] [CrossRef] [PubMed]
- Nathan, J.; Panjwani, S.; Mohan, V.; Joshi, V.; Thakurdesai, P.A. Efficacy and safety of standardized extract of Trigonella foenum-graecum L. seeds as an adjuvant to L-Dopa in the management of patients with Parkinson’s disease. Phytother. Res. 2014, 28, 172–178. [Google Scholar] [CrossRef]
- Bakuradze, T.; Lang, R.; Hofmann, T.; Eisenbrand, G.; Schipp, D.; Galan, J.; Richling, E. Consumption of a dark roast coffee decreases the level of spontaneous DNA strand breaks: A randomized controlled trial. Eur. J. Nutr. 2015, 54, 149–156. [Google Scholar] [CrossRef]

| Plant | Type | Relative Value (%) |
|---|---|---|
| Radish | Sakurajima | 100 |
| Aokubi | 1.75 | |
| Coffee | Fruit | 81.74 |
| Roast | 17.15 | |
| Extraction | 0.01 | |
| Buttercup | Seeds | 1.50 |
| Squash | Fruit | 1.00 |
| Before | After | p Value | ||
|---|---|---|---|---|
| Systolic BP | mmHg | 120 ± 13.6 | 115.8 ± 10.8 | 0.72 |
| Diastolic BP | mmHg | 70.8 ± 11.0 | 68.2 ± 10.7 | 0.66 |
| Pulse | /min | 73.6 ± 13.7 | 70.9 ± 11.7 | 0.07 |
| Body weight | kg | 58.1 ± 9.4 | 58.0 ± 9.4 | 1.0 |
| Before | After | p Value | ||
|---|---|---|---|---|
| WBC | /μL | 5785.7 ± 1287.8 | 6228.6 ± 1658.1 | 0.12 |
| Hb | g/dL | 14.6 ± 1.6 | 14.5 ± 1.5 | 0.58 |
| Plt | ×104/μL | 27.2 ± 5.9 | 26.1 ± 5.8 | 0.10 |
| LDL-C | mg/dL | 108.5 ± 21.1 | 113.6 ± 29.0 | 0.33 |
| HDL-C | mg/dL | 79.5 ± 18.3 | 77.7 ± 14.9 | 0.47 |
| TG | mg/dL | 110.1 ± 123.4 | 60.8 ± 33.0 | 0.04 |
| FPG | mg/dL | 87.5 ± 4.4 | 90.4 ± 7.1 | 0.08 |
| UA | mg/dL | 4.4 ± 1.5 | 4.9 ± 1.6 | 0.23 |
| BUN | mg/dL | 13.4 ± 3.0 | 12.6 ± 2.8 | 0.24 |
| Cr | mg/dL | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.71 |
| Na | mEq/L | 142.0 ± 1.4 | 140.6 ± 1.3 | 1.00 |
| K | mEq/L | 4.2 ± 0.3 | 4.2 ± 0.2 | 1.00 |
| Cl | mEq/L | 101.6 ± 1.4 | 103.1 ± 1.0 | 1.00 |
| AST | IU/L | 20.5 ± 4.7 | 20.3 ± 4.2 | 0.89 |
| ALT | IU/L | 16.1 ± 8.4 | 16.5 ± 8.1 | 0.65 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasaki, M.; Nonoshita, Y.; Kajiya, T.; Atsuchi, N.; Kido, M.; Chu, D.-C.; Juneja, L.R.; Minami, Y.; Kajiya, K. Characteristic Analysis of Trigonelline Contained in Raphanus sativus Cv. Sakurajima Daikon and Results from the First Trial Examining Its Vasodilator Properties in Humans. Nutrients 2020, 12, 1872. https://doi.org/10.3390/nu12061872
Sasaki M, Nonoshita Y, Kajiya T, Atsuchi N, Kido M, Chu D-C, Juneja LR, Minami Y, Kajiya K. Characteristic Analysis of Trigonelline Contained in Raphanus sativus Cv. Sakurajima Daikon and Results from the First Trial Examining Its Vasodilator Properties in Humans. Nutrients. 2020; 12(6):1872. https://doi.org/10.3390/nu12061872
Chicago/Turabian StyleSasaki, Maho, Yuri Nonoshita, Takashi Kajiya, Nobuhiko Atsuchi, Megumi Kido, Djong-Chi Chu, Lekh Raj Juneja, Yuji Minami, and Katsuko Kajiya. 2020. "Characteristic Analysis of Trigonelline Contained in Raphanus sativus Cv. Sakurajima Daikon and Results from the First Trial Examining Its Vasodilator Properties in Humans" Nutrients 12, no. 6: 1872. https://doi.org/10.3390/nu12061872
APA StyleSasaki, M., Nonoshita, Y., Kajiya, T., Atsuchi, N., Kido, M., Chu, D.-C., Juneja, L. R., Minami, Y., & Kajiya, K. (2020). Characteristic Analysis of Trigonelline Contained in Raphanus sativus Cv. Sakurajima Daikon and Results from the First Trial Examining Its Vasodilator Properties in Humans. Nutrients, 12(6), 1872. https://doi.org/10.3390/nu12061872