Improvement of Learning and Memory in Senescence-Accelerated Mice by S-Allylcysteine in Mature Garlic Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Preparation of Matured Garlic Extract
2.2. Cell Culture
2.3. Immunofluorescence and Image Quantification
2.4. Behavioral Experiments
2.4.1. Working Memory
2.4.2. Memory Acquisition Test and Retention Test
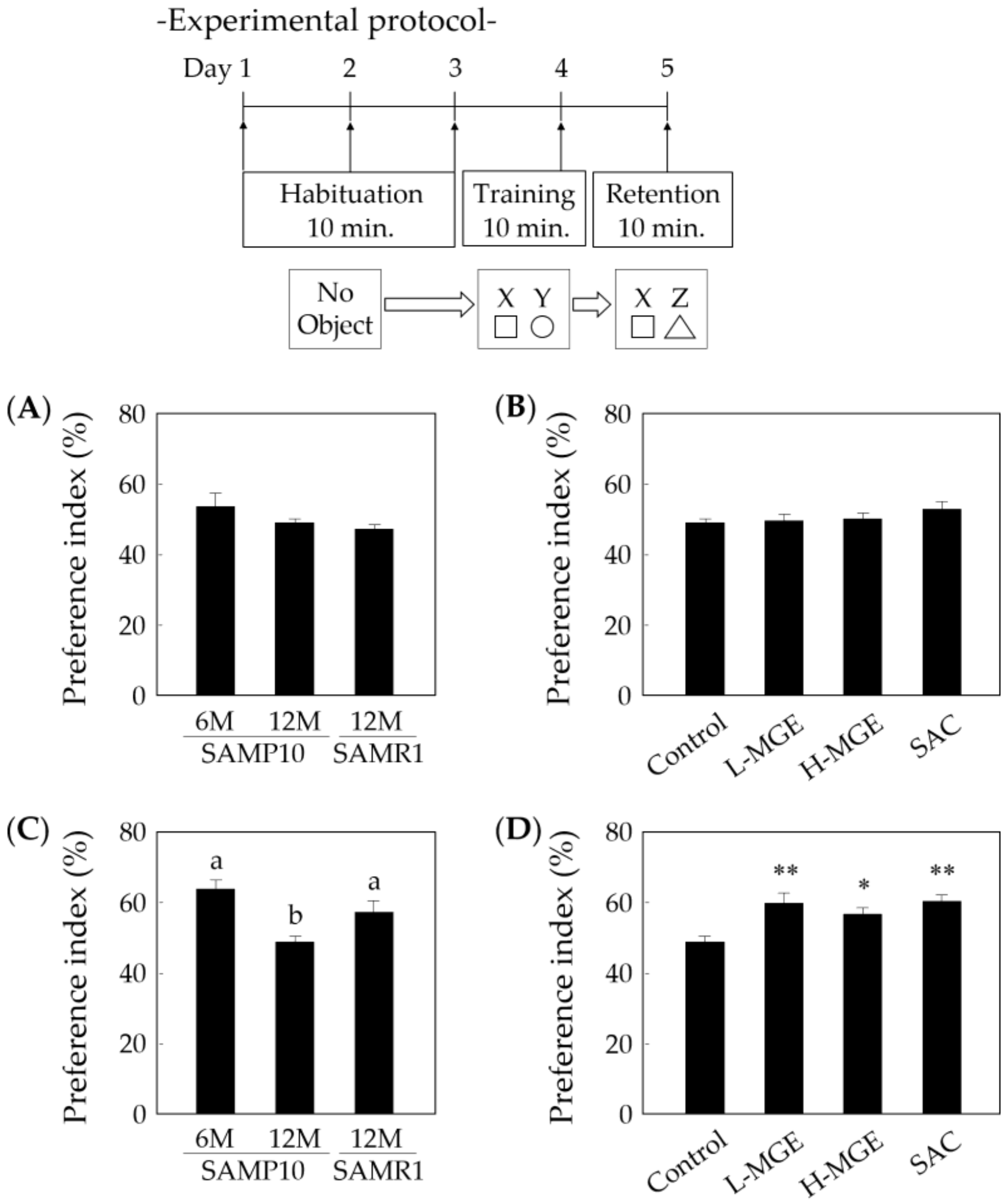
2.4.3. Novel Object Recognition Test
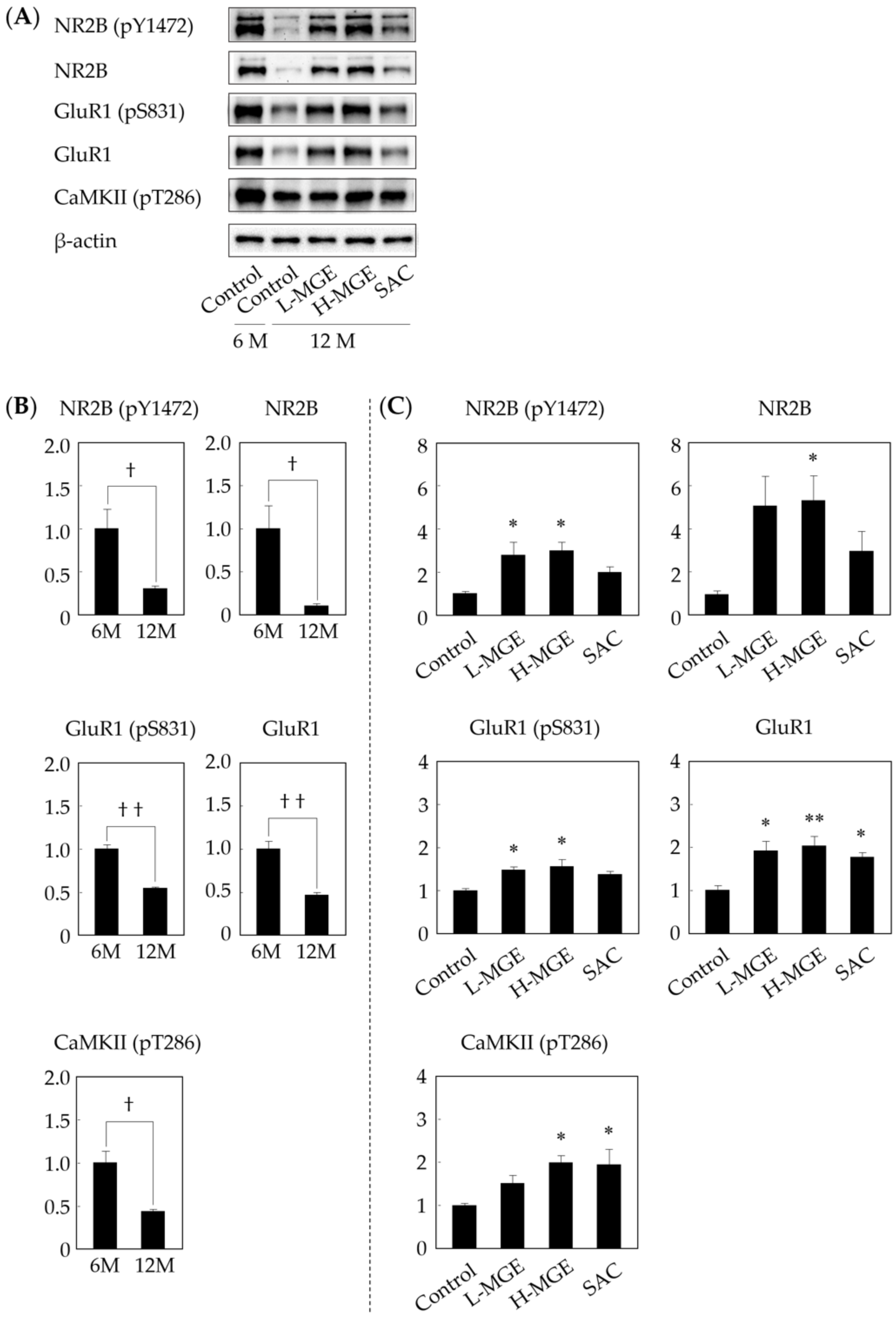
2.4.4. Western Immunoblotting
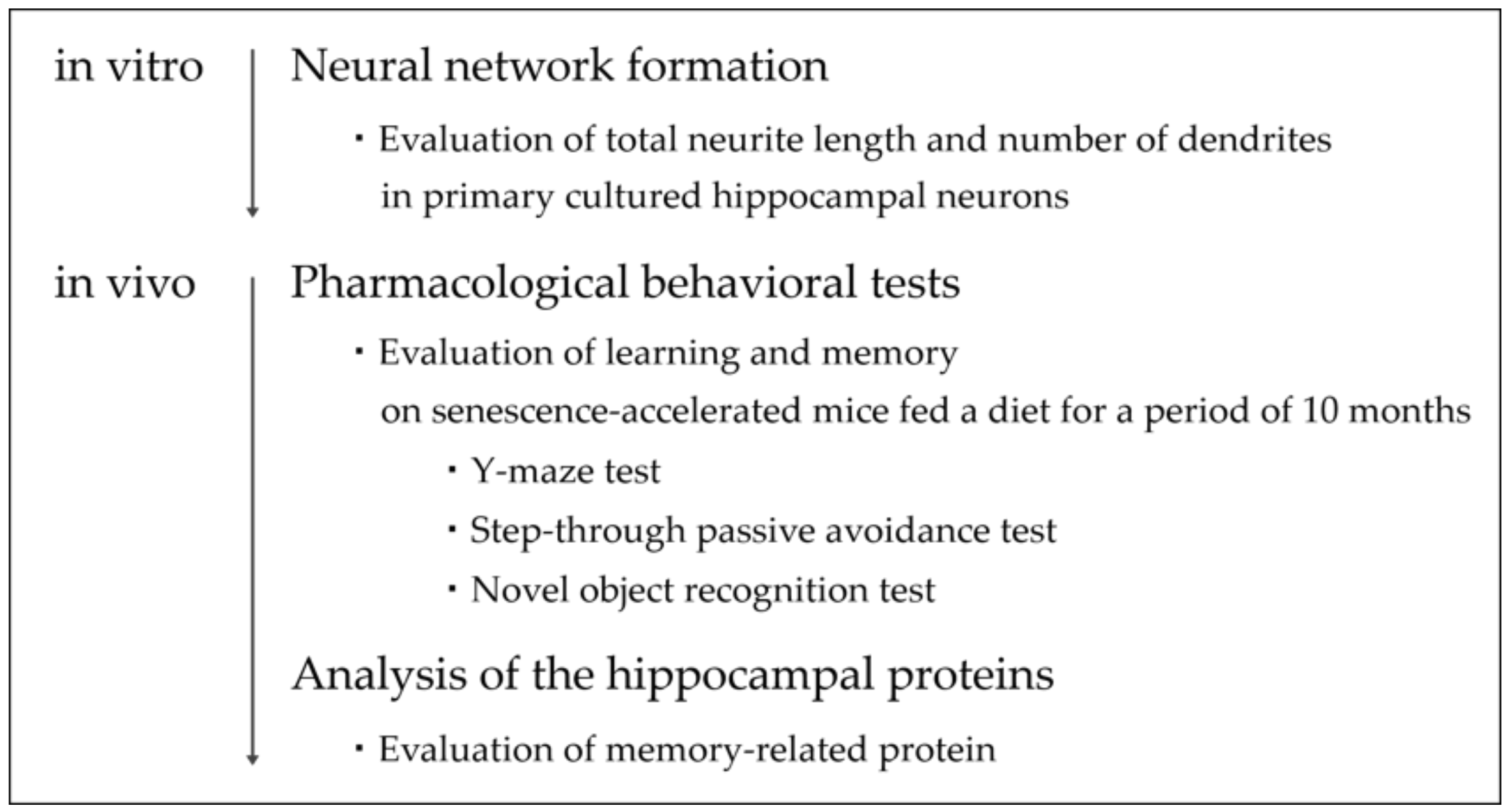
2.5. Flowchart
2.6. Statistical Analyses
3. Results
3.1. Effects of MGE on the Total Neurite Length and Number of Dendrites in Primary Cultured Hippocampal Neurons
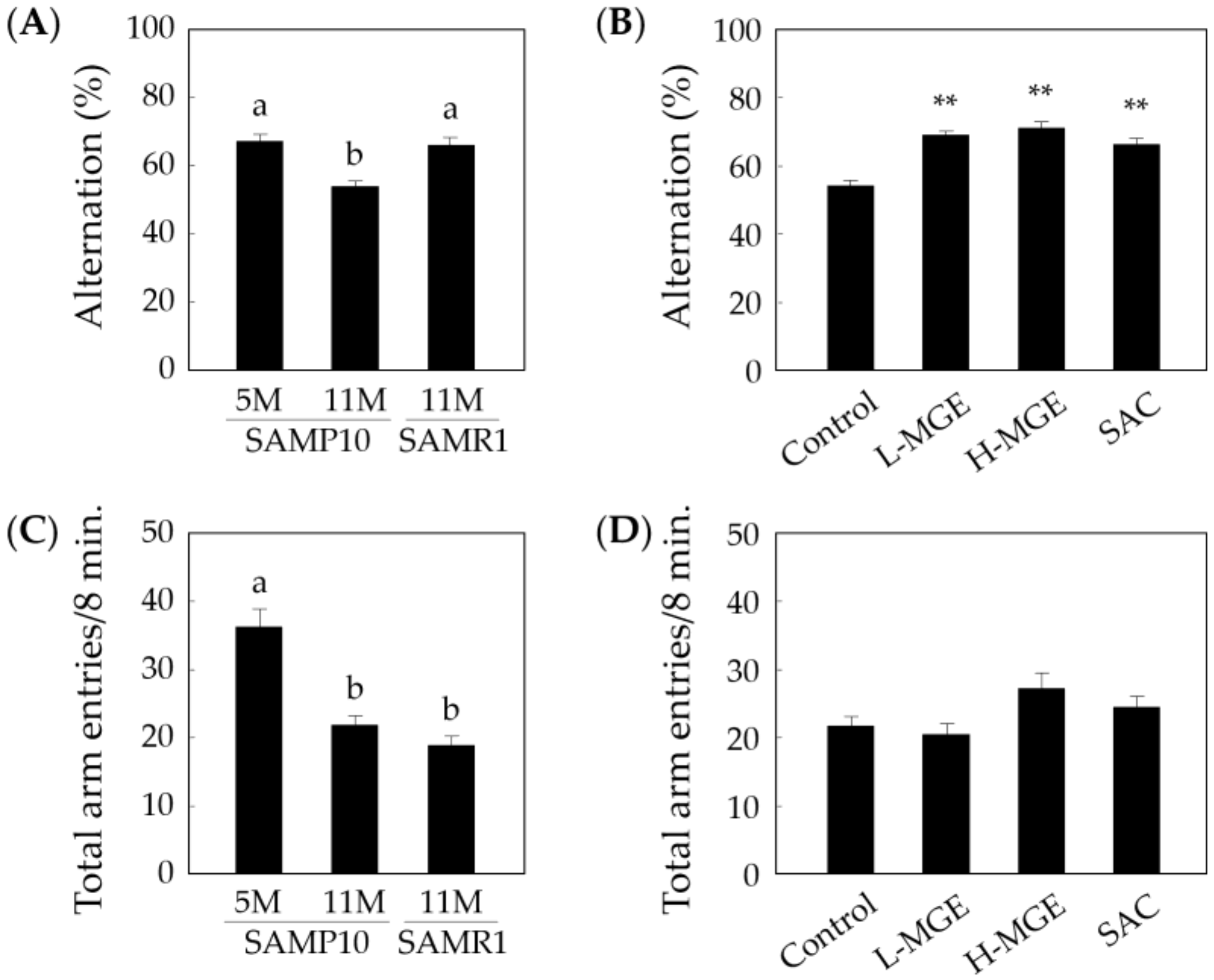
3.2. Y-Maze Test
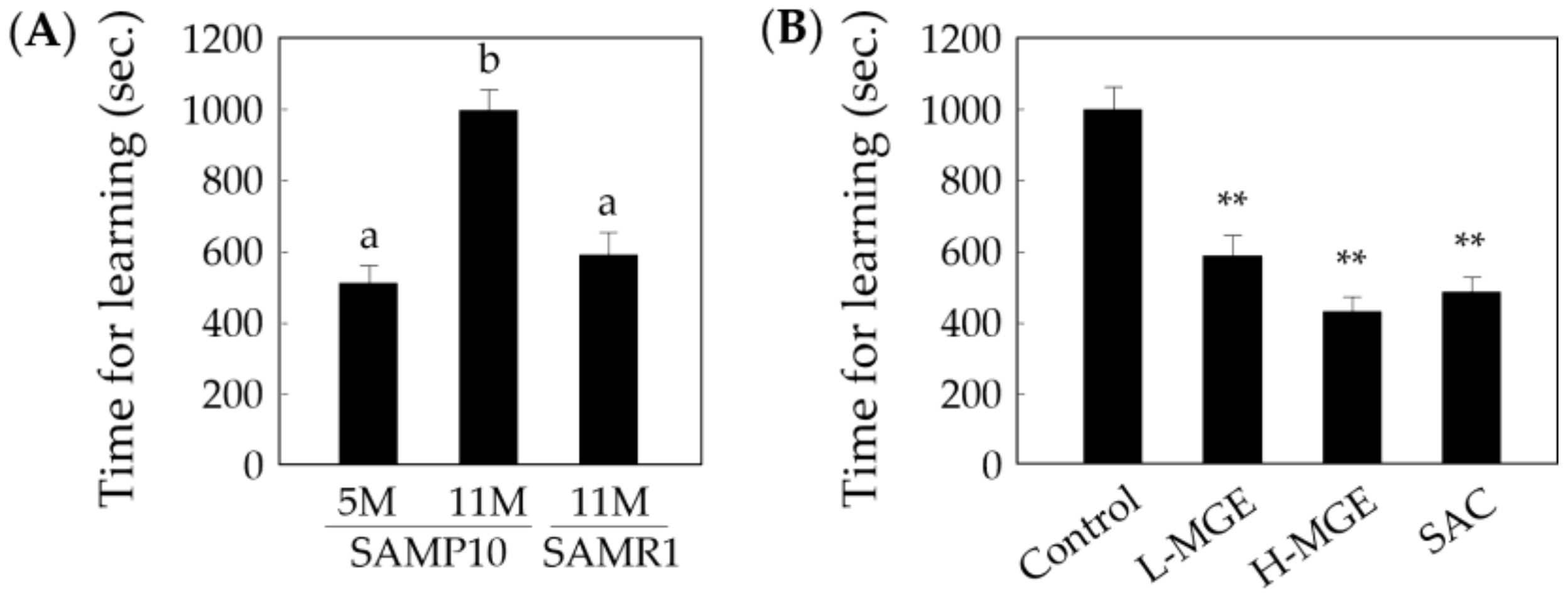
3.3. Step-Through Passive Avoidance Test
3.4. Novel Object Recognition Test
3.5. Western Immunoblotting
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hahn, G. History, folk medicine, and legendary uses of garlic. In Garlic: The Science and Therapeutic Applications of Allium sativum L. and Related Species, 2nd ed.; Koch, H.P., Lawson, L.D., Eds.; William & Wilkins: Baltimore, MA, USA, 1996; pp. 1–24. [Google Scholar]
- Block, E. The chemistry of garlic and onions. Sci. Am. 1985, 252, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.K.; Dewar, H.A.; Newell, D.J.; Das, B. Controlled trial of the effect of cycloalliin on the fibrinolytic activity of venous blood. Atherosclerosis 1977, 27, 347–351. [Google Scholar] [CrossRef]
- Yoshinari, O.; Shiojima, Y.; Igarashi, K. Anti-obesity effects of onion extract in Zucker diabetic fatty rats. Nutrients 2012, 4, 1518–1526. [Google Scholar] [CrossRef]
- Watabe, K.; Tanaka, S.; Yamaguchi, H. Studies on the mechanism of antihypertensive action of a constituents of garlic, γ-glutamyl-S-allyl-cysteine (GSAC). Oyo Yakuri (Pharmacomet.) 2006, 71, 79–87. (In Japanese) [Google Scholar]
- Nakasone, Y.; Nakamura, Y.; Yamamoto, T.; Yamaguchi, H. Effect of a traditional Japanese garlic preparation on blood pressure in prehypertensive and mildly hypertensive adults. Exp. Ther. Med. 2013, 5, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Nagae, S.; Ushijima, M.; Hatano, S.; Imai, J.; Kasuga, S.; Matsuura, H.; Itakura, Y.; Higashi, Y. Pharmacokinetics of the garlic compound S-allylcysteine. Planta Med. 1994, 60, 214–217. [Google Scholar] [CrossRef]
- Yan, C.K.; Zeng, F.D. Pharmacokinetics and tissue distribution of s-allylcysteine in rats. Asian J. Drug Metab. Pharm. 2005, 5, 61–69. [Google Scholar]
- Moriguchi, T.; Matsuura, H.; Kodera, Y.; Itakura, Y.; Katsuki, H.; Saito, H.; Nishiyama, N. Neurotrophic activity of organosulfur compounds having a thioallyl group on cultured rat hippocampal neurons. Neurochem. Res. 1997, 22, 1449–1452. [Google Scholar] [CrossRef]
- Moriguchi, T.; Nishiyama, N.; Saito, H.; Katsuki, H. Trophic effects of aged garlic extract (AGE) and its fractions on primary cultured hippocampal neurons from fetal rat brain. Phytother. Res. 1996, 10, 468–472. [Google Scholar] [CrossRef]
- Calamandrei, G.; Alleva, E. Neuronal growth factors, neurotrophins and memory deficiency. Behav. Brain Res. 1995, 66, 129–132. [Google Scholar] [CrossRef]
- Kosuge, Y.; Koen, Y.; Ishige, K.; Minami, K.; Urasawa, H.; Saito, H.; Ito, Y. S-allyl-L-cysteine selectively protects cultured rat hippocampal neurons from amyloid beta-protein- and tunicamycin-induced neuronal death. Neuroscience 2003, 122, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Kosuge, Y.; Saito, H.; Uchiyama, T.; Wada, T.; Shimba, S.; Ishige, K.; Miyairi, S.; Makishima, M.; Ito, Y. Neuroprotective effect of S-allyl-l-cysteine derivatives against endoplasmic reticulum stress-induced cytotoxicity is independent of calpain inhibition. J. Pharmacol. Sci. 2016, 130, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Kosuge, Y.; Endo-Umeda, K.; Miyagishi, H.; Ishige, K.; Makishima, M.; Ito, Y. Protective effect of S-allyl-L-cysteine against endoplasmic reticulum stress-induced neuronal death is mediated by inhibition of calpain. Amino Acids. 2014, 46, 385–393. [Google Scholar] [CrossRef]
- Gupta, V.B.; Rao, K.S. Anti-amyloidogenic activity of S-allyl-L-cysteine and its activity to destabilize Alzheimer’s beta-amyloid fibrils in vitro. Neurosci. Lett. 2007, 429, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.B. Effect of aged garlic extract on APP processing and tau phosphorylation in Alzheimer’s transgenic model Tg2576. J. Ethnopharmacol. 2006, 108, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T.; Hosokawa, M.; Takeshita, S.; Irino, M.; Higuchi, K.; Matsushita, T.; Tomita, Y.; Yasuhira, K.; Hamamoto, H.; Shimizu, K.; et al. A new murine model of accelerated senescence. Mech. Ageing Dev. 1981, 17, 183–194. [Google Scholar] [CrossRef]
- Takeda, T.; Hosokawa, M.; Higuchi, K. Senescence-accelerated mouse (SAM): A novel murine model of accelerated senescence. J. Am. Geriatr. Soc. 1991, 39, 911–919. [Google Scholar] [CrossRef]
- Shimada, A.; Ohta, A.; Akiguchi, I.; Takeda, T. Inbred SAM-P/10 as a mouse model of spontaneous, inherited brain atrophy. J. Neuropathol. Exp. Neurol. 1992, 51, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Shimada, A.; Hosokawa, M.; Ohta, A.; Akiguchi, I.; Takeda, T. Localization of atrophy-prone areas in the aging mouse brain: Comparison between the brain atrophy model SAM-P/10 and the normal control SAM-R/1. Neuroscience 1994, 59, 859–869. [Google Scholar] [CrossRef]
- Shimada, A.; Tsuzuki, M.; Keino, H.; Satoh, M.; Chiba, Y.; Saitoh, Y.; Hosokawa, M. Apical vulnerability to dendritic retraction in prefrontal neurones of ageing SAMP10 mouse: A model of cerebral degeneration. Neuropathol. Appl. Neurobiol. 2006, 32, 1–14. [Google Scholar] [CrossRef]
- Shimada, A.; Keino, H.; Satoh, M.; Kishikawa, M.; Hosokawa, M. Age-related loss of synapses in the frontal cortex of SAMP10 mouse: A model of cerebral degeneration. Synapse 2003, 48, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Shimada, A.; Ohta, A.; Akiguchi, I.; Takeda, T. Age-related deterioration in conditional avoidance task in the SAM-P/10 mouse, an animal model of spontaneous brain atrophy. Brain Res. 1993, 608, 266–272. [Google Scholar] [CrossRef]
- Miyamoto, M. Characteristics of age-related behavioral changes in senescence-accelerated mouse SAMP8 and SAMP10. Exp. Gerontol. 1997, 32, 139–148. [Google Scholar] [CrossRef]
- Okuma, Y.; Murayama, T.; Tha, K.K.; Yamada, C.; Hosokawa, M.; Ishikawa, A.; Watanabe, R.; Maekawa, M.; Nomura, Y. Learning deficiency and alterations in acetylcholine receptors and protein kinase C in the brain of senescence-accelerated mouse (SAM)-P10. Mech. Ageing Dev. 2000, 114, 191–199. [Google Scholar] [CrossRef]
- Onodera, T.; Watanabe, R.; Tha, K.K.; Hayashi, Y.; Murayama, T.; Okuma, Y.; Ono, C.; Oketani, Y.; Hosokawa, M.; Nomura, Y. Depressive behavior and alterations in receptors for dopamine and 5-hydroxytryptamine in the brain of the senescence accelerated mouse (SAM)-P10. Jpn. J. Pharmacol. 2000, 83, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, N.; Moriguchi, T.; Saito, H. Beneficial effects of aged garlic extract on learning and memory impairment in the senescence-accelerated mouse. Exp. Gerontol. 1997, 32, 149–160. [Google Scholar] [CrossRef]
- Moriguchi, T.; Saito, H.; Nishiyama, N. Anti-ageing effect of aged garlic extract in the inbred brain atrophy mouse model. Clin. Exp. Pharmacol. Physiol. 1997, 24, 235–242. [Google Scholar] [CrossRef]
- Unno, K.; Takabayashi, F.; Kishido, T.; Oku, N. Suppressive effect of green tea catechins on morphologic and functional regression of the brain in aged mice with accelerated senescence (SAMP10). Exp. Gerontol. 2004, 39, 1027–1034. [Google Scholar] [CrossRef]
- Unno, K.; Yamamoto, H.; Ohtaki, T.; Ishikawa, Y.; Noda, S.; Maeda, K.; Fujitani, K.; Miyazaki, H.; Takabayashi, F.; Sasaki, T.; et al. Active component in green tea catechins and effective intake period for prevention of age-related brain dysfunction. J. Anti Aging Med. 2011, 8, 75–81. [Google Scholar] [CrossRef][Green Version]
- Unno, K.; Ishikawa, Y.; Takabayashi, F.; Sasaki, T.; Takamori, N.; Iguchi, K.; Hoshino, M. Daily ingestion of green tea catechins from adulthood suppressed brain dysfunction in aged mice. Biofactors 2008, 34, 263–271. [Google Scholar] [CrossRef]
- Ray, B.; Chauhan, N.B.; Lahiri, D.K. Oxidative insults to neurons and synapse are prevented by aged garlic extract and S-allyl-L-cysteine treatment in the neuronal culture and APP-Tg mouse model. J. Neurochem. 2011, 117, 388–402. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, R.O.; Fox, J.G. Federal support for training in laboratory animal medicine should be reinstated. Lab. Anim. Sci. 1999, 49, 348. [Google Scholar] [PubMed]
- Ibi, D.; Nagai, T.; Nakajima, A.; Mizoguchi, H.; Kawase, T.; Tsuboi, D.; Kano, S.; Sato, Y.; Hayakawa, M.; Lange, U.C.; et al. Astroglial IFITM3 mediates neuronal impairments following neonatal immune challenge in mice. Glia 2013, 61, 679–693. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, M.; Inoue, K. Nociceptin/orphanin FQ and nocistatin on learning and memory impairment induced by scopolamine in mice. Br. J. Pharmacol. 1999, 127, 655–660. [Google Scholar] [CrossRef]
- Itoh, J.; Ukai, M.; Kameyama, T. Dynorphin A-(1–13) markedly improves scopolamine-induced impairment of spontaneous alternation performance in mice. Eur. J. Pharmacol. 1993, 236, 341–345. [Google Scholar] [CrossRef]
- Nagai, T.; Yamada, K.; Kim, H.C.; Kim, Y.S.; Noda, Y.; Imura, A.; Nabeshima, Y.; Nabeshima, T. Cognition impairment in the genetic model of aging klotho gene mutant mice: A role of oxidative stress. FASEB J. 2003, 17, 50–52. [Google Scholar] [CrossRef]
- Kamei, H.; Nagai, T.; Nakano, H.; Togan, Y.; Takayanagi, M.; Takahashi, K.; Kobayashi, K.; Yoshida, S.; Maeda, K.; Takuma, K.; et al. Repeated methamphetamine treatment impairs recognition memory through a failure of novelty-induced ERK1/2 activation in the prefrontal cortex of mice. Biol. Psychiatry 2006, 59, 75–84. [Google Scholar] [CrossRef]
- Shi, S.H.; Hayashi, Y.; Petralia, R.S.; Zaman, S.H.; Wenthold, R.J.; Svoboda, K.; Malinow, R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science 1999, 284, 1811–1816. [Google Scholar] [CrossRef]
- Liao, D.; Scannevin, R.H.; Huganir, R. Activation of silent synapses by rapid activity-dependent synaptic recruitment of AMPA receptors. J. Neurosci. 2001, 21, 6008–6017. [Google Scholar] [CrossRef]
- Hansen, M.R.; Devaiah, A.K.; Bok, J.; Zha, X.; Green, S.H. Ca2+/calmodulin-dependent protein kinases II and IV function similarly in neurotrophic signaling but differ in their effects on neurite growth in spiral ganglion neurons. J. Neurosci. Res. 2003, 72, 169–184. [Google Scholar] [CrossRef]
- Taha, S.; Hanover, J.L.; Silva, A.J.; Stryker, M.P. Autophosphorylation of alphaCaMKII is required for ocular dominance plasticity. Neuron 2002, 36, 483–491. [Google Scholar] [CrossRef]
- Lisman, J.; Schulman, H.; Cline, H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat. Rev. Neurosci. 2002, 3, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Giese, K.P. Calcium/calmodulin-dependent kinase II and Alzheimer’s disease. Mol. Brain 2015, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Lovinger, D.M. Communication networks in the brain: Neurons, receptors, neurotransmitters, and alcohol. Alcohol Res. Health 2008, 31, 196–214. [Google Scholar] [PubMed]
- Mamiya, T.; Ukai, M. [Gly(14)]-Humanin improved the learning and memory impairment induced by scopolamine in vivo. Br. J. Pharmacol. 2001, 134, 1597–1599. [Google Scholar] [CrossRef] [PubMed]
- Fortin, N.J.; Agster, K.L.; Eichenbaum, H.B. Critical role of the hippocampus in memory for sequences of events. Nat. Neurosci. 2002, 5, 458–462. [Google Scholar] [CrossRef]
- Hughes, R.N. The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci. Biobehav. Rev. 2004, 28, 497–505. [Google Scholar] [CrossRef]
- Ressler, K.; Davis, M. Genetics of childhood disorders: L. Learning and memory, part 3: Fear conditioning. J. Am. Acad. Child. Adolesc. Psychiatry 2003, 42, 612–615. [Google Scholar] [CrossRef]
- Murray, E.A.; Richmond, B.J. Role of perirhinal cortex in object perception, memory, and associations. Curr. Opin. Neurobiol. 2001, 11, 188–193. [Google Scholar] [CrossRef]
- Tanaka, K.Z.; Pevzner, A.; Hamidi, A.B.; Nakazawa, Y.; Graham, J. Cortical representations are reinstated by the hippocampus during memory retrieval. Neuron 2014, 84, 347–354. [Google Scholar] [CrossRef]
- Richter-Levin, G.; Akirav, I. Amygdala-hippocampus dynamic interaction in relation to memory. Mol. Neurobiol. 2000, 22, 11–20. [Google Scholar] [CrossRef]
- Davis, H.P.; Squire, L.R. Protein synthesis and memory: A review. Psychol. Bull. 1984, 96, 518–559. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tiedge, H. Translational control at the synapse. Neuroscientist 2004, 10, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Gerrow, K.; Triller, A. Synaptic stability and plasticity in a floating world. Curr. Opin. Neurobiol. 2010, 20, 631–639. [Google Scholar] [CrossRef]
- Nicoll, R.A.; Malenka, R.C. Contrasting properties of two forms of long-term potentiation in the hippocampus. Nature 1995, 377, 115–118. [Google Scholar] [CrossRef]
- Nayak, A.; Zastrow, D.J.; Lickteig, R.; Zahniser, N.R.; Browning, M.D. Maintenance of late-phase LTP is accompanied by PKA-dependent increase in AMPA receptor synthesis. Nature 1998, 394, 680–683. [Google Scholar] [CrossRef]
- Hollmann, M.; Heinemann, S. Cloned glutamate receptors. Annu. Rev. Neurosci. 1994, 17, 31–108. [Google Scholar] [CrossRef]
- Derkach, V.; Barria, A.; Soderling, T.R. Ca2+/calmodulin-kinase II enhances channel conductance of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proc. Natl. Acad. Sci. USA 1999, 96, 3269–3274. [Google Scholar] [CrossRef]
- Nicoll, R.A. Expression mechanisms underlying long-term potentiation: A postsynaptic view. Philos. Trans. R. Soc. B Biol. Sci. 2003, 358, 721–726. [Google Scholar] [CrossRef]
- Gocel, J.; Larson, J. Evidence for loss of synaptic AMPA receptors in anterior piriform cortex of aged mice. Front. Aging Neurosci. 2013, 5, 39. [Google Scholar] [CrossRef]
- Bahr, B.A.; Godshall, A.C.; Hall, R.A.; Lynch, G. Mouse telencephalon exhibits an age-related decrease in glutamate (AMPA) receptors but no change in nerve terminal markers. Brain Res. 1992, 589, 320–326. [Google Scholar] [CrossRef]
- Nicoletti, V.G.; Condorelli, D.F.; Dell’Albani, P.; Ragusa, N.; Giuffrida Stella, A.M. AMPA-selective glutamate receptor subunits in the rat hippocampus during aging. J. Neurosci. Res. 1995, 40, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, K.R. Aging of glutamate receptors: Correlations between binding and spatial memory performance in mice. Mech. Ageing Dev. 1998, 104, 227–248. [Google Scholar] [CrossRef]
- Wenk, G.L.; Barnes, C.A. Regional changes in the hippocampal density of AMPA and NMDA receptors across the lifespan of the rat. Brain Res. 2000, 885, 1–5. [Google Scholar] [CrossRef]
- Cantanelli, P.; Sperduti, S.; Ciavardelli, D.; Stuppia, L.; Gatta, V.; Sensi, S.L. Age-dependent modifications of AMPA receptor subunit expression levels and related cognitive effects in 3xTg-AD mice. Front. Aging Neurosci. 2014, 6, 200. [Google Scholar] [CrossRef]
- Guntupalli, S.; Widagdo, J.; Anggono, V. Amyloid-β-induced dysregulation of AMPA receptor trafficking. Neural Plast. 2016, 2016, 3204519. [Google Scholar] [CrossRef]
- Jurado, S. AMPA receptor trafficking in natural and pathological aging. Front. Mol. Neurosci. 2018, 10, 446. [Google Scholar] [CrossRef]
- Amada, N.; Aihara, K.; Ravid, R.; Horie, M. Reduction of NR1 and phosphorylated Ca2+/calmodulin-dependent protein kinase II levels in Alzheimer’s disease. Neuroreport 2005, 16, 1809–1813. [Google Scholar] [CrossRef]
- Bliss, T.V.; Collingridge, G.L. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 1993, 361, 31–39. [Google Scholar] [CrossRef]
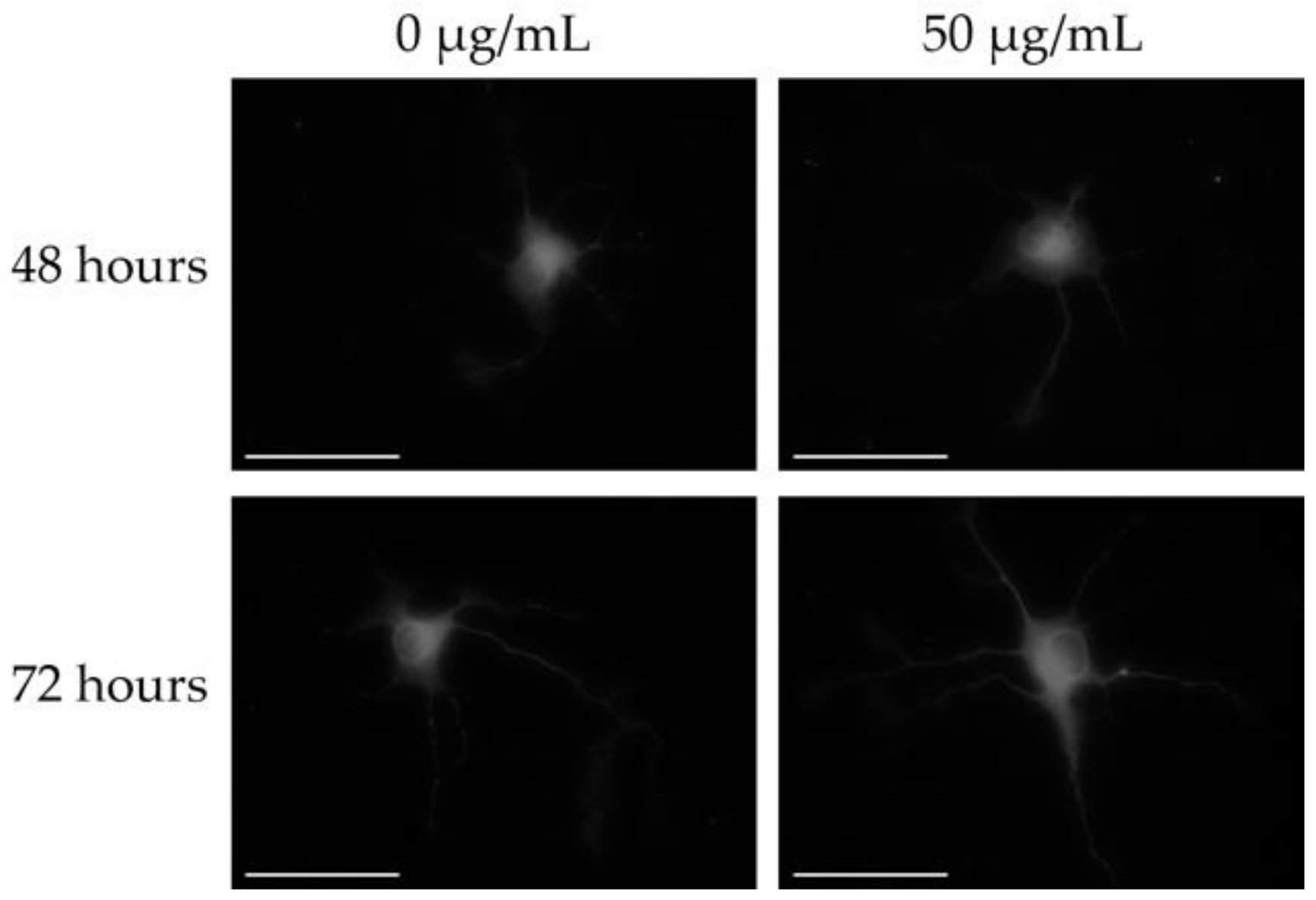
| Concentration | 48 h | 72 h | |
|---|---|---|---|
| Total Neurite Length, μm | Total Neurite Length, μm | ||
| MGE (μg/mL) | 0 | 278.9 ± 7.24 | 362.9 ± 8.60 |
| 5 | 306.7 ± 9.04 * | 419.3 ± 9.53 ** | |
| 50 | 323.5 ± 8.77 ** | 430.7 ± 9.40 ** | |
| 500 | 392.8 ± 8.18 ** | 435.2 ± 9.30 ** | |
| SAC (ng/mL) | 0 | 259.1 ± 6.85 | 342.9 ± 8.15 |
| 10 | 300.3 ± 8.04 ** | 445.8 ± 12.9 ** | |
| 100 | 294.8 ± 7.08 ** | 433.5 ± 11.3 ** | |
| 1000 | 303.8 ± 7.56 ** | 398.1 ± 7.56 ** |
| Concentration | 48 h | 72 h | |
|---|---|---|---|
| Number of Dendrites | Number of Dendrites | ||
| MGE (μg/mL) | 0 | 5.29 ± 0.137 | 5.29 ± 0.186 |
| 5 | 5.86 ± 0.176 * | 7.16 ± 0.217 ** | |
| 50 | 6.06 ± 0.166 ** | 6.88 ± 0.196 ** | |
| 500 | 6.81 ± 0.207 ** | 7.92 ± 0.226 ** | |
| SAC (ng/mL) | 0 | 5.34 ± 0.190 | 5.32 ± 0.148 |
| 10 | 6.19 ± 0.166 ** | 7.35 ± 0.242 ** | |
| 100 | 6.20 ± 0.163 ** | 6.76 ± 0.203 ** | |
| 1000 | 5.88 ± 0.133 | 6.46 ± 0.180 ** |
| Mice | Age | Number of Animals Success (Left) Failure (Right) | Memory Retention (%) | |
|---|---|---|---|---|
| SAMP10 | 6 M (young) | 8 | 0 | 100 |
| SAMP10 | 12 M (old) | 7 | 8 | 46.7 |
| SAMR1 | 12 M (old) | 7 | 4 | 63.6 |
| Mice | Diet | Number of Animals Success (Left) Failure (Right) | Memory Retention (%) | |
|---|---|---|---|---|
| SAMP10 | Control | 7 | 8 | 46.7 |
| SAMP10 | L-MGE | 9 | 4 | 69.2 |
| SAMP10 | H-MGE | 10 | 3 | 76.9 |
| SAMP10 | SAC | 11 | 4 | 73.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashimoto, M.; Nakai, T.; Masutani, T.; Unno, K.; Akao, Y. Improvement of Learning and Memory in Senescence-Accelerated Mice by S-Allylcysteine in Mature Garlic Extract. Nutrients 2020, 12, 1834. https://doi.org/10.3390/nu12061834
Hashimoto M, Nakai T, Masutani T, Unno K, Akao Y. Improvement of Learning and Memory in Senescence-Accelerated Mice by S-Allylcysteine in Mature Garlic Extract. Nutrients. 2020; 12(6):1834. https://doi.org/10.3390/nu12061834
Chicago/Turabian StyleHashimoto, Masakazu, Tsuyoshi Nakai, Teruaki Masutani, Keiko Unno, and Yukihiro Akao. 2020. "Improvement of Learning and Memory in Senescence-Accelerated Mice by S-Allylcysteine in Mature Garlic Extract" Nutrients 12, no. 6: 1834. https://doi.org/10.3390/nu12061834
APA StyleHashimoto, M., Nakai, T., Masutani, T., Unno, K., & Akao, Y. (2020). Improvement of Learning and Memory in Senescence-Accelerated Mice by S-Allylcysteine in Mature Garlic Extract. Nutrients, 12(6), 1834. https://doi.org/10.3390/nu12061834





