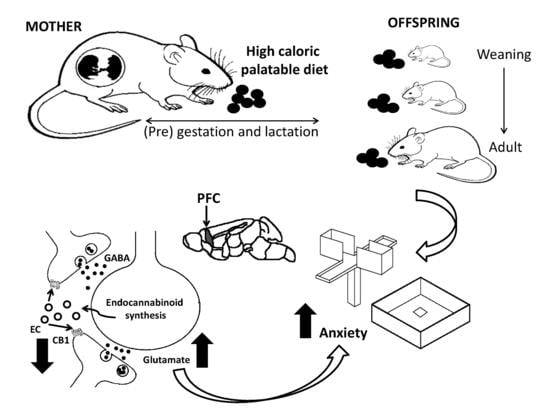
Sex-Specific Anxiety and Prefrontal Cortex Glutamatergic Dysregulation Are Long-Term Consequences of Pre-and Postnatal Exposure to Hypercaloric Diet in a Rat Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals
2.3. Diets
2.4. Experimental Design
2.5. Sample Collection
2.6. RNA Isolation and Real-Time Quantitative PCR Analysis
2.7. Western Blot Analysis
2.8. Behavioral Studies
2.9. Statistical Analysis
3. Results
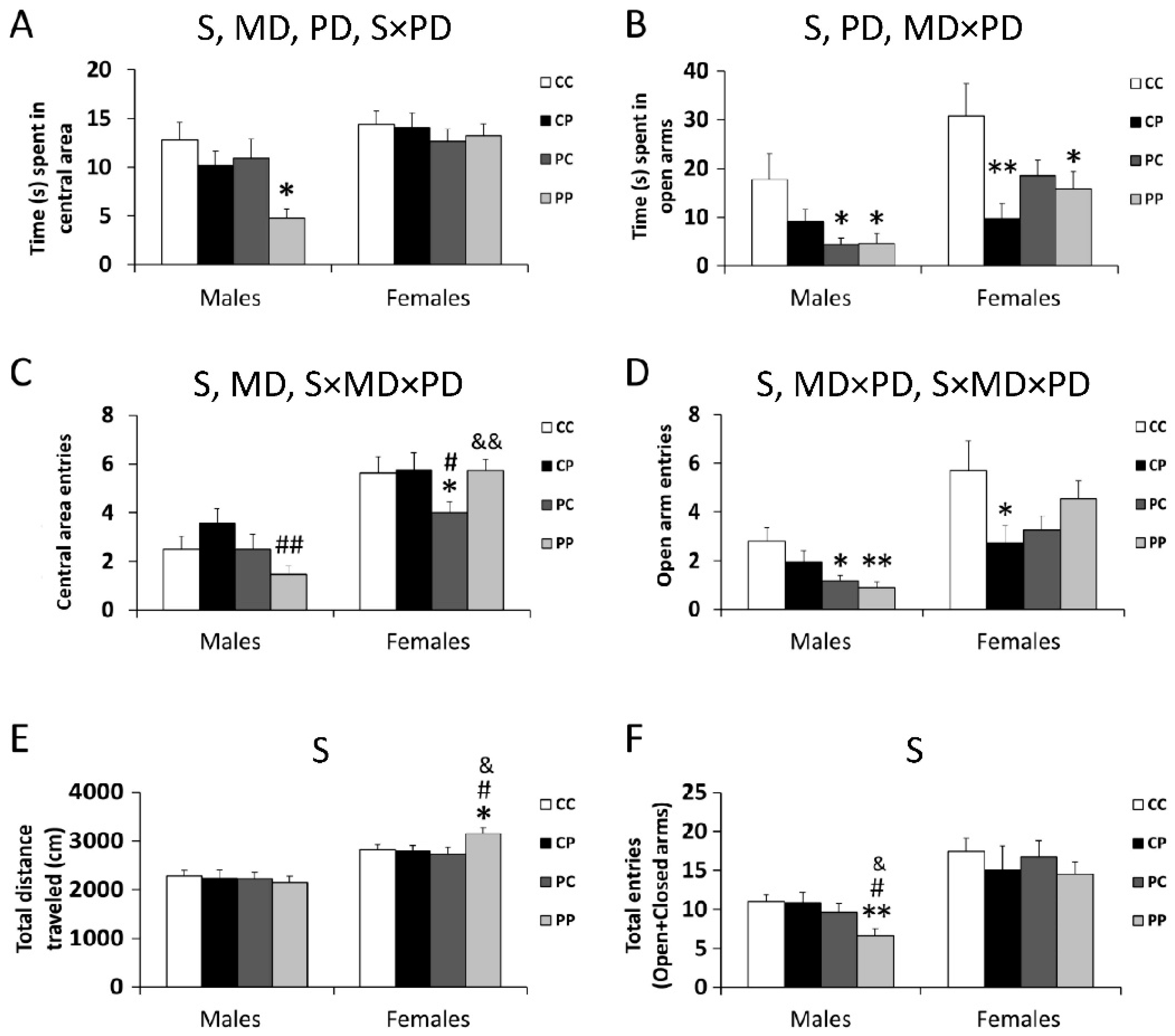
3.1. Effect of Maternal and/or Postnatal Hypercaloric Diet on Offspring Behavior
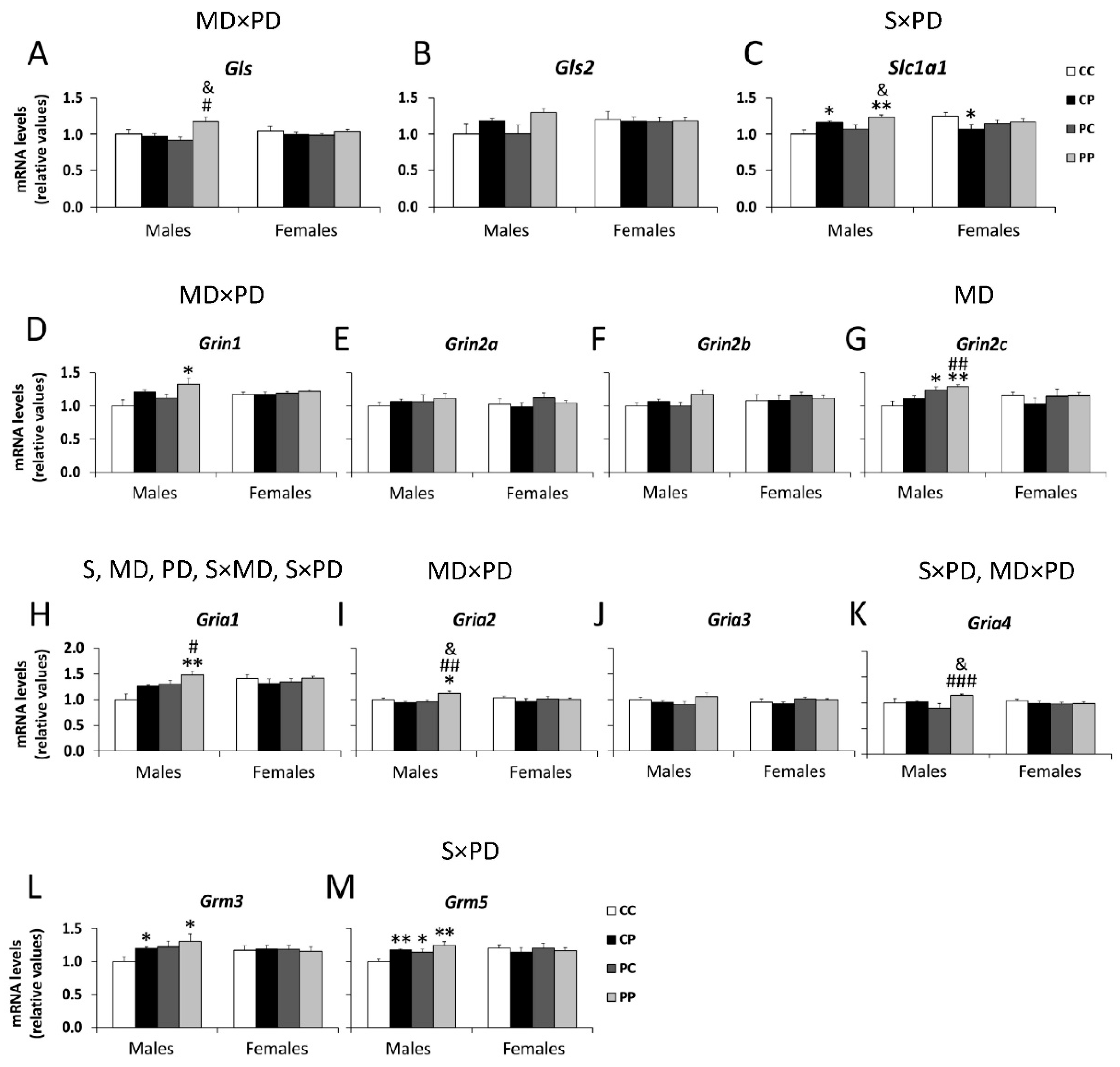
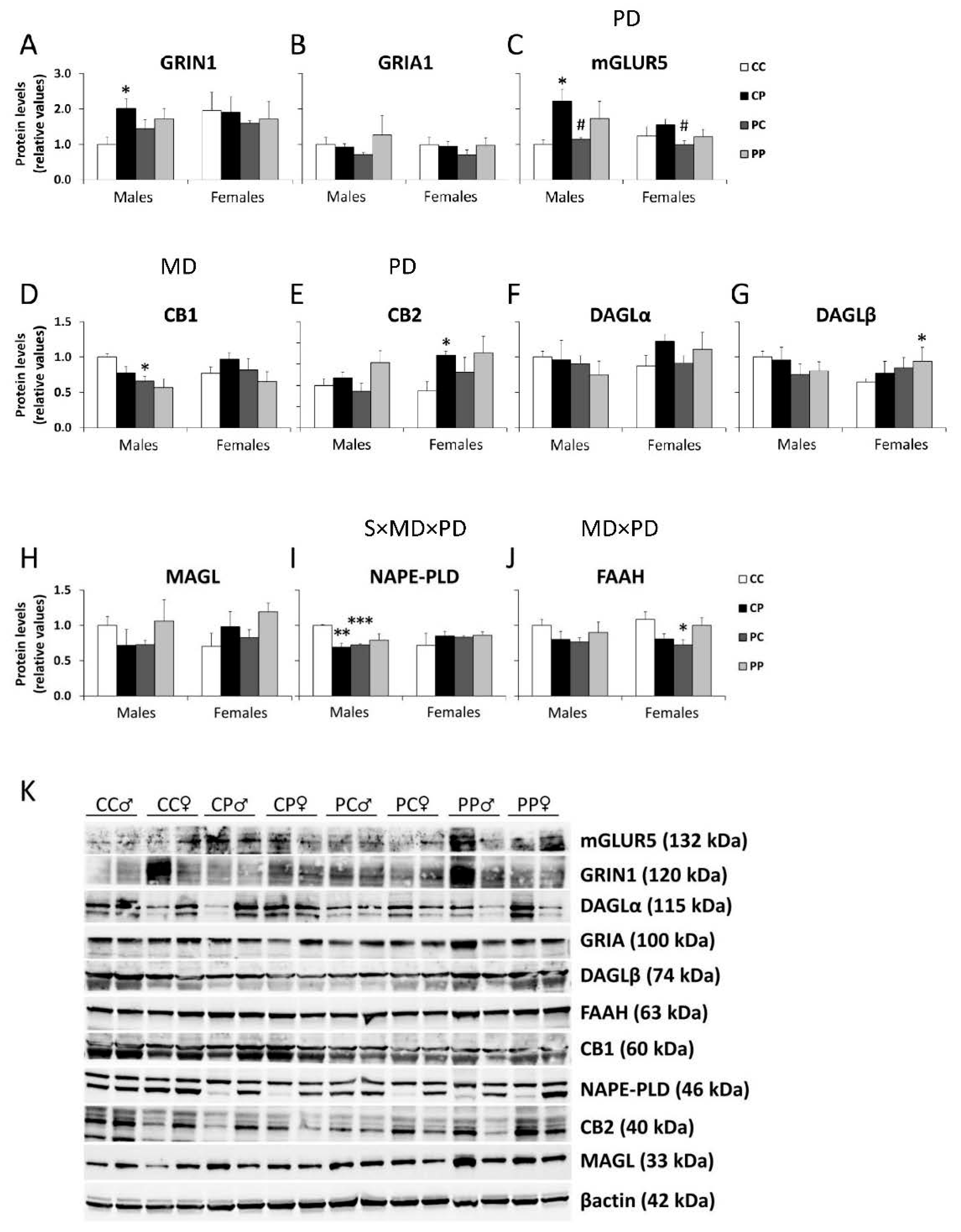
3.2. Effects of Maternal and/or Postnatal Hypercaloric Diet on Glutamatergic Signaling in Adult Offspring Prefrontal Cortex
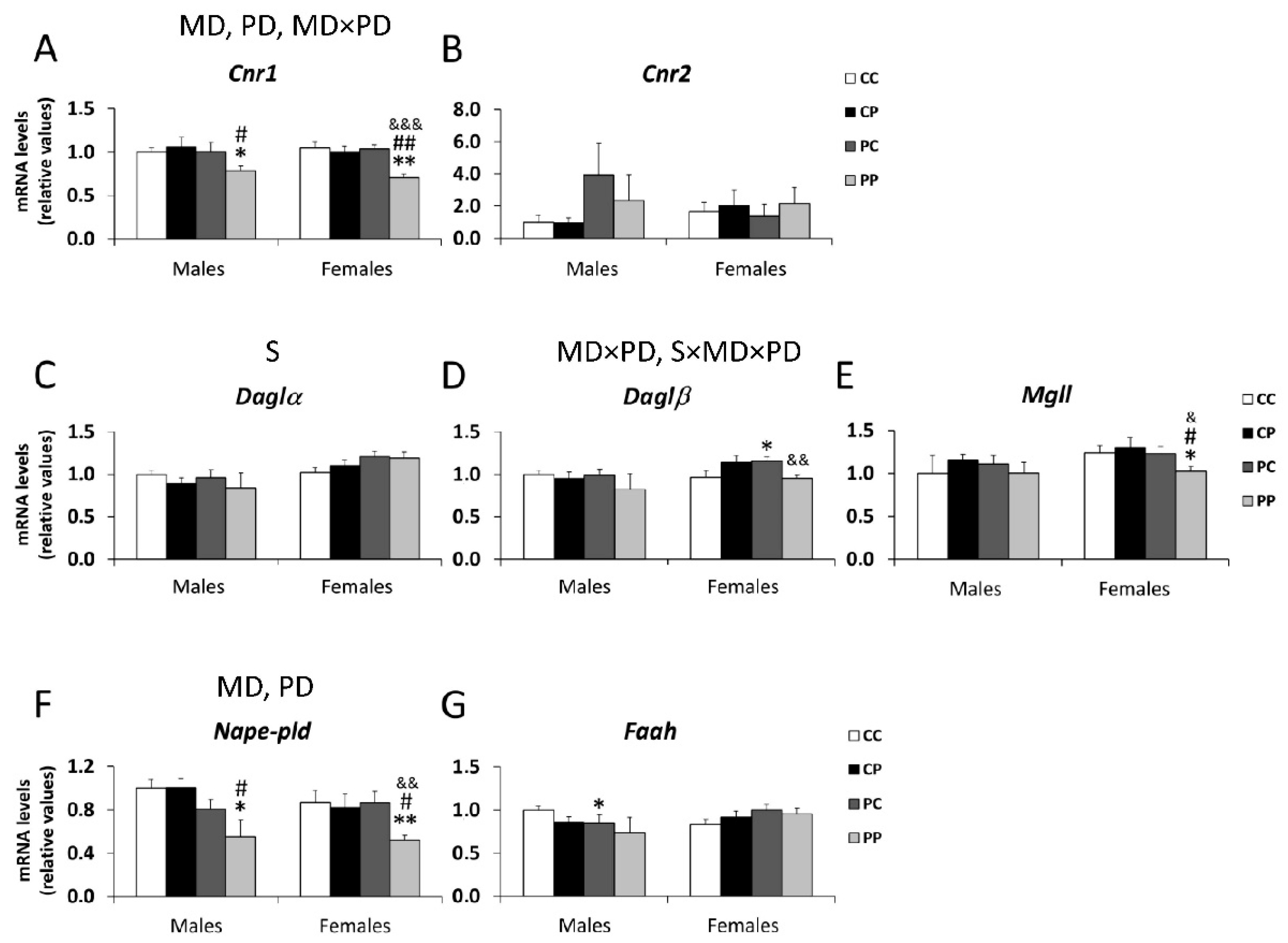
3.3. Effects of Maternal and/or Postnatal Hypercaloric Diet on Endocannabinoid Signaling in Adult Offspring Prefrontal Cortex
3.4. Effects of Maternal and/or Postnatal Hypercaloric Diet on ECS and Glutamatergic Signaling Protein Levels in Adult Offspring Prefrontal Cortex
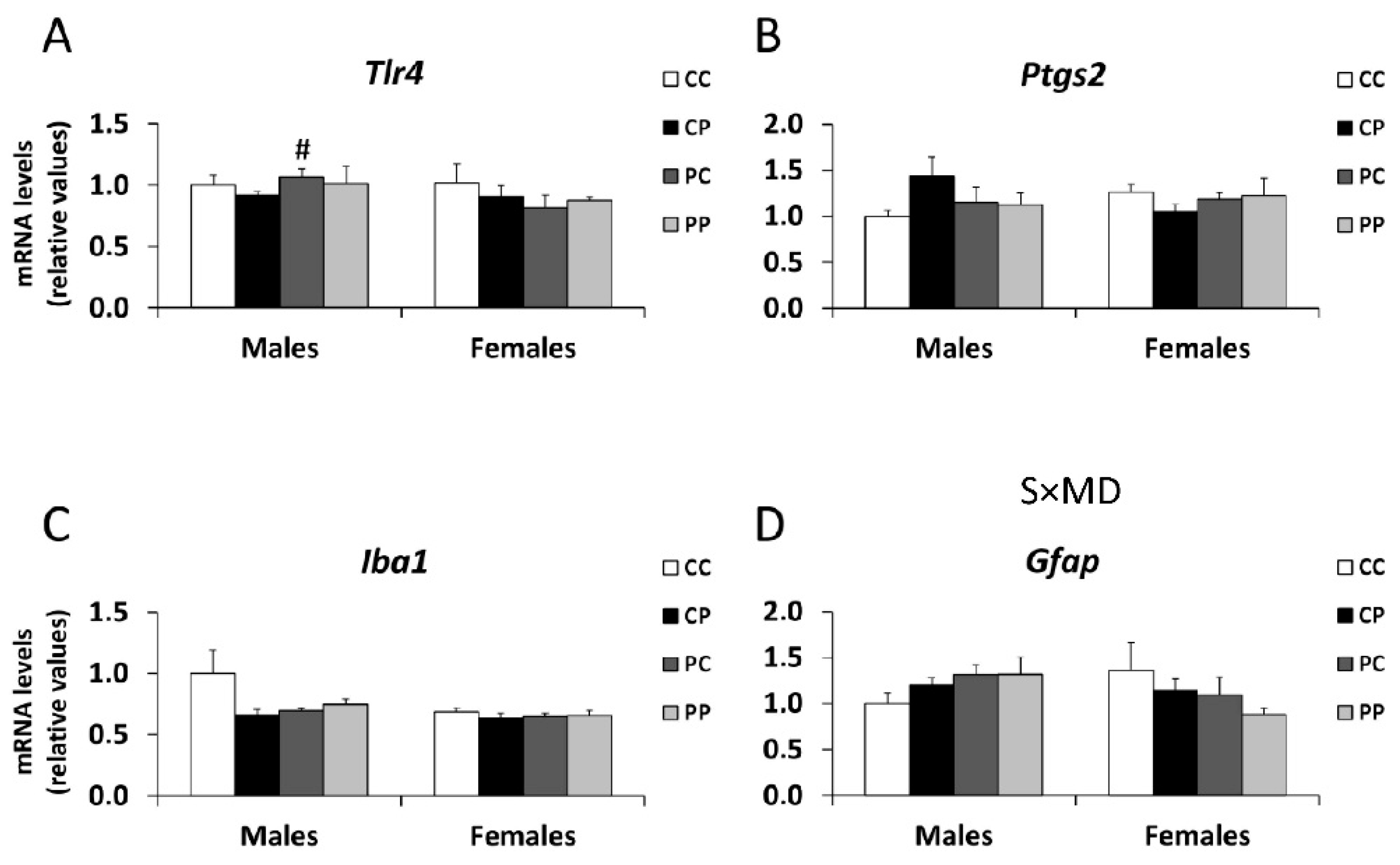
3.5. Effects of Maternal and/or Postnatal Hypercaloric Diet on Inflammation in Adult Offspring Prefrontal Cortex
3.6. Effects of Maternal and/or Postnatal Hypercaloric Diet on GABAergic Signaling in Adult Offspring Prefrontal Cortex
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chen, H.-F.; Su, H.-M. Exposure to a maternal n-3 fatty acid-deficient diet during brain development provokes excessive hypothalamic–pituitary–adrenal axis responses to stress and behavioral indices of depression and anxiety in male rat offspring later in life. J. Nutr. Biochem. 2013, 24, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Rajia, S.; Morris, M.J.; Chen, H. Maternal overnutrition impacts offspring adiposity and brain appetite markers-modulation by postweaning diet. J. Neuroendocr. 2010, 22. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-López, M.T.; Vázquez, M.; Bindila, L.; Lomazzo, E.; Hofmann, C.; Blanco, R.N.; Alén, F.; Anton, M.; Decara, J.; Arco, R.; et al. Maternal Caloric Restriction Implemented during the Preconceptional and Pregnancy Period Alters Hypothalamic and Hippocampal Endocannabinoid Levels at Birth and Induces Overweight and Increased Adiposity at Adulthood in Male Rat Offspring. Front. Behav. Neurosci. 2016, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Rivera, P.; Ramírez-López, M.T.; Vargas, A.; Decara, J.; Vázquez, M.; Arco, R.; De Heras, R.G.; Argente, J.; De Fonseca, F.R.; Chowen, J.A.; et al. Perinatal free-choice of a high-calorie low-protein diet affects leptin signaling through IRS1 and AMPK dephosphorylation in the hypothalami of female rat offspring in adulthood. Acta Physiol. 2019, 226, e13244. [Google Scholar] [CrossRef] [PubMed]
- Peleg-Raibstein, D.; Sarker, G.; Litwan, K.; Krämer, S.D.; Ametamey, S.M.; Schibli, R.; Wolfrum, C. Enhanced sensitivity to drugs of abuse and palatable foods following maternal overnutrition. Transl. Psychiatry 2016, 6, e911. [Google Scholar] [CrossRef]
- Ramírez-López, M.T.; Vázquez, M.; Bindila, L.; Lomazzo, E.; Hofmann, C.; Blanco, R.N.; Alen, F.; Anton, M.; Decara, J.; Ouro, D.; et al. Exposure to a Highly Caloric Palatable Diet during Pregestational and Gestational Periods Affects Hypothalamic and Hippocampal Endocannabinoid Levels at Birth and Induces Adiposity and Anxiety-Like Behaviors in Male Rat Offspring. Front. Behav. Neurosci. 2016, 9, 1640. [Google Scholar] [CrossRef]
- Camacho, A.; Montalvo-Martinez, L.; Cardenas-Perez, R.E.; Fuentes-Mera, L.; Garza-Ocañas, L. Obesogenic diet intake during pregnancy programs aberrant synaptic plasticity and addiction-like behavior to a palatable food in offspring. Behav. Brain Res. 2017, 330, 46–55. [Google Scholar] [CrossRef]
- Grissom, N.M.; Herdt, C.T.; Desilets, J.; Lidsky-Everson, J.; Reyes, T.M. Dissociable Deficits of Executive Function Caused by Gestational Adversity Are Linked to Specific Transcriptional Changes in the Prefrontal Cortex. Neuropsychopharmacology 2014, 40, 1353–1363. [Google Scholar] [CrossRef]
- Musazzi, L.; Treccani, G.; Mallei, A.; Popoli, M. The Action of Antidepressants on the Glutamate System: Regulation of Glutamate Release and Glutamate Receptors. Biol. Psychiatry 2013, 73, 1180–1188. [Google Scholar] [CrossRef]
- Labouesse, M.A.; Dong, E.; Grayson, D.R.; Guidotti, A.; Meyer, U. Maternal immune activation induces GAD1 and GAD2 promoter remodeling in the offspring prefrontal cortex. Epigenetics 2015, 10, 1143–1155. [Google Scholar] [CrossRef]
- Cao, Y.J.; Wang, Q.; Zheng, X.X.I.; Heng, Y.C.; Zhang, Y. Involvement of SNARE complex in the hippocampus and prefrontal cortex of offspring with depression induced by prenatal stress. J. Affect. Disord. 2018, 235, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Nutt, D. Role of GABA in anxiety and depression. Depress. Anxiety 2007, 24, 495–517. [Google Scholar] [CrossRef] [PubMed]
- Treccani, G.; Musazzi, L.; Perego, C.; Milanese, M.; Nava, N.; Bonifacino, T.; Lamanna, J.; Malgaroli, A.; Drago, F.; Racagni, G.; et al. Stress and corticosterone increase the readily releasable pool of glutamate vesicles in synaptic terminals of prefrontal and frontal cortex. Mol. Psychiatry 2014, 19, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Chattarji, S.; Tomar, A.; Suvrathan, A.; Ghosh, S.; Rahman, M.M. Neighborhood matters: Divergent patterns of stress-induced plasticity across the brain. Nat. Neurosci. 2015, 18, 1364–1375. [Google Scholar] [CrossRef]
- Joseph, R.J.; Alonso-Alonso, M.; Bond, D.S.; Pascual-Leone, A.; Blackburn, G.L. The neurocognitive connection between physical activity and eating behaviour. Obes. Rev. 2011, 12, 800–812. [Google Scholar] [CrossRef]
- Wolfrum, C.; Peleg-Raibstein, D. Maternal overnutrition leads to cognitive and neurochemical abnormalities in C57BL/6 mice. Nutr. Neurosci. 2018, 22, 688–699. [Google Scholar] [CrossRef]
- Viveros, M.-P.; Llorente, R.; Suárez, J.; Llorente-Berzal, Á.; Gallardo, M.L.; De Fonseca, F.R. The endocannabinoid system in critical neurodevelopmental periods: Sex differences and neuropsychiatric implications. J. Psychopharmacol. 2011, 26, 164–176. [Google Scholar] [CrossRef]
- Lutz, B.; Marsicano, G.; Maldonado, R.; Hillard, C.J. The endocannabinoid system in guarding against fear, anxiety and stress. Nat. Rev. Neurosci. 2015, 16, 705–718. [Google Scholar] [CrossRef]
- Hill, M.N.; McLaughlin, R.J.; Pan, B.; Fitzgerald, M.L.; Roberts, C.J.; Lee, T.T.-Y.; Karatsoreos, I.N.; Mackie, K.; Viau, V.; Pickel, V.M.; et al. Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J. Neurosci. 2011, 31, 10506–10515. [Google Scholar] [CrossRef]
- De Fonseca, F.R.; Ramos, J.A.; Bonnin, A.; Fernández-Ruiz, J.J. Presence of cannabinoid binding sites in the brain from early postnatal ages. NeuroReport 1993, 4, 135–138. [Google Scholar] [CrossRef]
- McLaughlin, R.J.; Hill, M.N.; Gorzalka, B.B. A critical role for prefrontocortical endocannabinoid signaling in the regulation of stress and emotional behavior. Neurosci. Biobehav. Rev. 2014, 42, 116–131. [Google Scholar] [CrossRef] [PubMed]
- Scheyer, A.F.; Borsoi, M.; Wager-Miller, J.; Pelissier-Alicot, A.-L.; Murphy, M.N.; Mackie, K.; Manzoni, O.J.J. Cannabinoid Exposure via Lactation in Rats Disrupts Perinatal Programming of the Gamma-Aminobutyric Acid Trajectory and Select Early-Life Behaviors. Biol. Psychiatry 2020, 87, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-López, M.T.; Vázquez, M.; Lomazzo, E.; Hofmann, C.; Blanco, R.N.; Alen, F.; Anton, M.; Decara, J.; Arco, R.; Orio, L.; et al. A moderate diet restriction during pregnancy alters the levels of endocannabinoids and endocannabinoid-related lipids in the hypothalamus, hippocampus and olfactory bulb of rat offspring in a sex-specific manner. PLoS ONE 2017, 12, e0174307. [Google Scholar] [CrossRef] [PubMed]
- Dias-Rocha, C.P.; Almeida, M.M.; Santana, E.M.; Costa, J.C.; Franco, J.G.; Pazos-Moura, C.C.; Trevenzoli, I.H. Maternal high-fat diet induces sex-specific endocannabinoid system changes in newborn rats and programs adiposity, energy expenditure and food preference in adulthood. J. Nutr. Biochem. 2018, 51, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Heyne, A.; Kiesselbach, C.; Sahún, I.; McDonald, J.; Gaiffi, M.; Dierssen, M.; Wolffgramm, J. An animal model of compulsive food-taking behaviour. Addict. Biol. 2009, 14, 373–383. [Google Scholar] [CrossRef] [PubMed]
- McClintock, M. Estrous synchrony: Modulation of ovarian cycle length by female pheromones. Physiol. Behav. 1984, 32, 701–705. [Google Scholar] [CrossRef]
- Paxinos, G.; Charles, W. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: Sidney, OH, USA, 2007; ISBN 9780125476126. [Google Scholar]
- Ramírez-López, M.T.; Arco, R.; Decara, J.; Vázquez, M.; Blanco, R.N.; Alen, F.; Suarez, J.; De Heras, R.G.; De Fonseca, F.R. Exposure to a Highly Caloric Palatable Diet during the Perinatal Period Affects the Expression of the Endogenous Cannabinoid System in the Brain, Liver and Adipose Tissue of Adult Rat Offspring. PLoS ONE 2016, 11, e0165432. [Google Scholar] [CrossRef]
- Decara, J.; Rivera, P.; Arrabal, S.; Vargas, A.; Serrano, A.; Pavón, F.J.; Dieguez, C.; Nogueiras, R.; De Fonseca, F.R.; Suarez, J. Cooperative role of the glucagon-like peptide-1 receptor and β3-adrenergic-mediated signalling on fat mass reduction through the downregulation of PKA/AKT/AMPK signalling in the adipose tissue and muscle of rats. Acta Physiol. 2017, 222, e13008. [Google Scholar] [CrossRef]
- Boney, C.M. Metabolic Syndrome in Childhood: Association with Birth Weight, Maternal Obesity, and Gestational Diabetes Mellitus. Pediatrics 2005, 115. [Google Scholar] [CrossRef]
- Block, T.; El-Osta, A. Epigenetic programming, early life nutrition and the risk of metabolic disease. Atherosclerosis 2017, 266, 31–40. [Google Scholar] [CrossRef]
- Decapo, M.; Thompson, J.R.; Dunn, G.; Sullivan, E.L. Perinatal Nutrition and Programmed Risk for Neuropsychiatric Disorders: A Focus on Animal Models. Biol. Psychiatry 2019, 85, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Nivoit, P.; Morens, C.; Van Assche, F.A.; Jansen, E.; Poston, L.; Remacle, C.; Reusens, B. Established diet-induced obesity in female rats leads to offspring hyperphagia, adiposity and insulin resistance. Diabetologia 2009, 52, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Samuelsson, A.-M.; Matthews, P.A.; Argenton, M.; Christie, M.R.; McConnell, J.M.; Jansen, E.H.M.; Piersma, A.H.; Ozanne, S.E.; Fernandez-Twinn, D.S.; Remacle, C.; et al. Diet-Induced Obesity in Female Mice Leads to Offspring Hyperphagia, Adiposity, Hypertension, and Insulin Resistance: A Novel Murine Model of Developmental Programming. Hypertension 2008, 51, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Breton, C.; Lukaszewski, M.-A.; Risold, P.-Y.; Enache, M.; Guillemot, J.; Guillaume, R.; Delahaye, F.; Lesage, J.; Dutriez-Casteloot, I.; Laborie, C.; et al. Maternal prenatal undernutrition alters the response of POMC neurons to energy status variation in adult male rat offspring. Am. J. Physiol. Metab. 2009, 296, E462–E472. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.; De Vega, W.; Sivanathan, S.; St-Cyr, S.; McGowan, P.O. Maternal high-fat diet alters anxiety behavior and glucocorticoid signaling in adolescent offspring. Neuroscience 2014, 272, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, E.L.; Smith, M.S.; Grove, K.L. Perinatal exposure to high-fat diet programs energy balance, metabolism and behavior in adulthood. Neuroendocrinology 2010, 93, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Glendining, K.A.; Fisher, L.C.; Jasoni, C.L. Maternal high fat diet alters offspring epigenetic regulators, amygdala glutamatergic profile and anxiety. Psychoneuroendocrinology 2018, 96, 132–141. [Google Scholar] [CrossRef]
- Cheung, C.C.; Krause, W.C.; Edwards, R.H.; Yang, C.; Shah, N.M.; Hnasko, T.S.; Ingraham, H.A. Sex-dependent changes in metabolism and behavior, as well as reduced anxiety after eliminating ventromedial hypothalamus excitatory output. Mol. Metab. 2015, 4, 857–866. [Google Scholar] [CrossRef]
- De Noronha, S.I.S.R.; Lima, P.; Campos, G.; Chírico, M.; Abreu, A.; Figueiredo, A.; Silva, F.; Jr, D.C.; Lowry, C.; De Menezes, R.C.A. Association of high-fat diet with neuroinflammation, anxiety-like defensive behavioral responses, and altered thermoregulatory responses in male rats. Brain Behav. Immun. 2019, 80, 500–511. [Google Scholar] [CrossRef]
- Baker, K.D.; Reichelt, A.C. Impaired fear extinction retention and increased anxiety-like behaviours induced by limited daily access to a high-fat/high-sugar diet in male rats: Implications for diet-induced prefrontal cortex dysregulation. Neurobiol. Learn. Mem. 2016, 136, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Abuaish, S.; Spinieli, R.L.; McGowan, P.O. Perinatal high fat diet induces early activation of endocrine stress responsivity and anxiety-like behavior in neonates. Psychoneuroendocrinology 2018, 98, 11–21. [Google Scholar] [CrossRef]
- Basta-Kaim, A.; Fijał, K.; Ślusarczyk, J.; Trojan, E.; Głombik, K.; Budziszewska, B.; Leśkiewicz, M.; Regulska, M.; Kubera, M.; Lasoń, W.; et al. Prenatal administration of lipopolysaccharide induces sex-dependent changes in glutamic acid decarboxylase and parvalbumin in the adult rat brain. Neuroscience 2015, 287, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Bluett, R.; Gamble-George, J.; Hermanson, D.J.; Hartley, N.D.; Marnett, L.J.; Patel, S. Central anandamide deficiency predicts stress-induced anxiety: Behavioral reversal through endocannabinoid augmentation. Transl. Psychiatry 2014, 4, e408. [Google Scholar] [CrossRef] [PubMed]
- Kaur, T.; Kaur, G. Withania somnifera as a potential candidate to ameliorate high fat diet-induced anxiety and neuroinflammation. J. Neuroinflamm. 2017, 14, 201. [Google Scholar] [CrossRef]
- Jones, P.J.H.; Lin, L.; Gillingham, L.G.; Yang, H.; Omar, J.M. Modulation of plasmaN-acylethanolamine levels and physiological parameters by dietary fatty acid composition in humans. J. Lipid Res. 2014, 55, 2655–2664. [Google Scholar] [CrossRef] [PubMed]
- Feria-Velasco, A.; Mena-Munguía, S.; Cárabez-Torres, J.; Gómez-Medrano, A.; Recéndiz-Hurtado, F.; Orozco-Suarez, S.; Beas-Zárate, C. Low Tryptophan and Protein in the Diet During Development Increase the Susceptibility to Convulsions in Adult Rats. Neurochem. Res. 2008, 33, 1484–1491. [Google Scholar] [CrossRef]
| Protein | Total Carbohydrate | Simple Carbohydrates | Total Fat | Saturated Fatty Acid | Unsaturated Fatty Acid | Fiber | Sodium | Energy | |
|---|---|---|---|---|---|---|---|---|---|
| Standard chow diet | 16.1% | 60.0% | 3.3% | 3.1% | 22.20% | 77.70% | 4.0% | 0.003% | 2.9 Kcal/g |
| Palatable diet | 6.0% | 60.4% | 89.0% | 24.45% | 56.2% | 43.88% | 1.45% | 0.17% | 4.88 Kcal/g |
| Gene Symbol | Assay ID | GenBank Accession Number | Amplicon Length (bp) |
|---|---|---|---|
| Actb | Rn00667869_m1 | NM_0311443 | 91 |
| Cnr1 (CB1) | Rn02758689_s1 | NM_012784.4 | 92 |
| Cnr2 (CB2) | Rn01637601_m1 | NM_020543.4 | 68 |
| Napepld | Rn01786262_m1 | NM_199381.1 | 71 |
| Faah | Rn00577086_m1 | NM_024132.3 | 63 |
| Daglα | Rn01454304_m1 | NM_001005886.1 | 67 |
| Daglβ | Rn01453771_m1 | NM_001107120.1 | 98 |
| Mgll (MAGL) | Rn00593297_m1 | NM_138502.2 | 78 |
| Gls (kidney-type) | Rn00561285_m1 | NM_001109968.1 | 80 |
| Gls2 (liver-type) | Rn00594296_m1 | NM_001270786.1 | 58 |
| Grin1 (GluN1) | Rn01436034_m1 | NM_001270602.1 | 73 |
| Grin2a | Rn00561341_m1 | NM_012573.3 | 68 |
| Grin2b | Rn00680474_m1 | NM_012574.1 | 79 |
| Grin2c | Rn00561359_m1 | NM_012575.3 | 57 |
| Gria1 (GluA1) | Rn00709588_m1 | NM_031608.1 | 85 |
| Gria2 | Rn00568514_m1 | NM_001083811.1 | 122 |
| Gria3 | Rn00583547_m1 | NM_001112742.1 | 74 |
| Gria4 | Rn00568544_m1 | NM_001113184.1 | 76 |
| Grm3 | Rn01755349_m1 | NM_001105712.1 | 71 |
| Grm5 (mGlu5) | Rn005666628_m1 | NM_017012.1 | 112 |
| Slc1a1 (EAAAC1) | Rn00564705_m1 | NM_013032.3 | 92 |
| Gabra1 | Rn00788315_m1 | NM_183326.2 | 75 |
| Gabra2 | Rn01413643_m1 | NM_001135779.1 | 123 |
| Gabrb1 | Rn00564146_m1 | NM_012956.1 | 81 |
| Gabrb2 | Rn00564149_m1 | NM_012957.2 | 64 |
| Gabrg1 | Rn00589841_m1 | NM_080586.1 | 73 |
| Gabrg2 | Rn01464079_m1 | NM_183327.1 | 78 |
| Gabbr1 | Rn00578911_m1 | NM_031028.3 | 113 |
| Gabbr2 | Rn00582550_m1 | NM_031802.1 | 87 |
| Tlr4 | Rn00569848_m1 | NM_019178.1 | 127 |
| Ptgs2 | Rn01483828_m1 | NM_017232.3 | 112 |
| Iba1 | Rn00574125_g1 | NM_017196.3 | 126 |
| Gfap | Rn01253033_m1 | NM_017009.2 | 75 |
| Antigen | Immunogen | Manufacturing Details | Dilution |
|---|---|---|---|
| βactin | Slightly modified β-cytoplasmic actin N-terminal peptide, Ac-Asp-Asp-Asp-Ile-Ala-Ala-Leu-Val-Ile-Asp-Asn-Gly-Ser-Gly-Lys, conjugated to KLH. | Sigma #2535L, Mouse monoclonal antibody. | 1:2000 |
| CB1 | Synthetic peptide corresponding to C terminal amino acids 461-472 of human cannabinoid receptor I (MSVSTDTSAEAL) | Abcam. Rabbit polyclonal antibody. Cat. nº: ab23703 | 1:200 |
| CB2 | Fusion protein, corresponding to aa 1-32 of rat cannabinoid receptor II | Abcam. Rabbit monoclonal antibody. Cat. nº: ab3561 | 1:200 |
| DAGLα | KLH conjugated synthetic peptide derived from the region aa121-195 of human diacylglycerol lipase alpha (DAGLA) | bioNova Científica (#orb156533). Rabbit polyclonal antibody | 1:100 |
| DAGLβ | KLH conjugated synthetic peptide derived between 61-140 amino acids of human diacylglycerol lipase beta (DAGLB) | Biorbyt (orb182976). Rabbit polyclonal antibody | 1:200 |
| MAGL | Recombinant fragment corresponding to Human Monoacylglycerol Lipase/MGL aa 1-14. | Abcam. Rabbit polyclonal antibody. (Ab24701) | 1:200 |
| FAAH | Synthetic peptide from rat Fatty acid amide hydrolase (FAAH), aa 561-579 (CLRFMREVEQLMTPQKQPS) | Cayman. Rabbit polyclonal antibody. Cat. Nº: 101600 | 1:200 |
| NAPE-PLD | Synthetic peptide:YMGPKRFRRSPCTI, corresponding to amino acids 159–172 of Human N-acyl phosphatidylethanolamine phospholipase D (NAPE-PLD) (UniProt: Q6IQ20) | Abcam. Rabbit polyclonal antibody. Ab95397 | 1:200 |
| NMDAR1 | Synthetic peptide corresponding to the C-terminus of rat N-methyl-D-aspartate (NMDA) receptor subunit (Catalog number AG344). | Sigma. Rabbit monoclonal antibody. AB9864 | 1:200 |
| GluR1 | Synthetic phospho-peptide corresponding to amino acid residues surrounding Ser845 of rat Glutamate Ionotropic Receptor AMPA Type Subunit 1 (GRIA1) conjugated to KLH | Thermo Fisher. Rabbit polyclonal antibody. OPA1-04118 | 1:200 |
| mGluR5 | Synthetic peptide corresponding to residues Y D R R L A Q H K S E I E of Metabotropic glutamate receptor 5 (mGluR5). | Thermo Fisher. Rabbit polyclonal antibody. (#PA1-24637) | 1:500 |
| Gls | Slc1a1 | Grin1 | Gria1 | Gria2 | Grm3 | Grm5 | |
|---|---|---|---|---|---|---|---|
| Time spent in central area | −0.622 ** | −0.653 ** | |||||
| Time spent in open arms | −0.424 * | −0.517 * | |||||
| Open arm entries | −0.513 * | −0.541 ** | −0.462 * | −0.443 * | −0.598 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera, P.; Tovar, R.; Ramírez-López, M.T.; Navarro, J.A.; Vargas, A.; Suárez, J.; Fonseca, F.R.d. Sex-Specific Anxiety and Prefrontal Cortex Glutamatergic Dysregulation Are Long-Term Consequences of Pre-and Postnatal Exposure to Hypercaloric Diet in a Rat Model. Nutrients 2020, 12, 1829. https://doi.org/10.3390/nu12061829
Rivera P, Tovar R, Ramírez-López MT, Navarro JA, Vargas A, Suárez J, Fonseca FRd. Sex-Specific Anxiety and Prefrontal Cortex Glutamatergic Dysregulation Are Long-Term Consequences of Pre-and Postnatal Exposure to Hypercaloric Diet in a Rat Model. Nutrients. 2020; 12(6):1829. https://doi.org/10.3390/nu12061829
Chicago/Turabian StyleRivera, Patricia, Rubén Tovar, María Teresa Ramírez-López, Juan Antonio Navarro, Antonio Vargas, Juan Suárez, and Fernando Rodríguez de Fonseca. 2020. "Sex-Specific Anxiety and Prefrontal Cortex Glutamatergic Dysregulation Are Long-Term Consequences of Pre-and Postnatal Exposure to Hypercaloric Diet in a Rat Model" Nutrients 12, no. 6: 1829. https://doi.org/10.3390/nu12061829
APA StyleRivera, P., Tovar, R., Ramírez-López, M. T., Navarro, J. A., Vargas, A., Suárez, J., & Fonseca, F. R. d. (2020). Sex-Specific Anxiety and Prefrontal Cortex Glutamatergic Dysregulation Are Long-Term Consequences of Pre-and Postnatal Exposure to Hypercaloric Diet in a Rat Model. Nutrients, 12(6), 1829. https://doi.org/10.3390/nu12061829