Science and Healthy Meals in the World: Nutritional Epigenomics and Nutrigenetics of the Mediterranean Diet
Abstract
1. Introduction
2. Finality, Criteria, Scheme, and Drafting Method of This Review
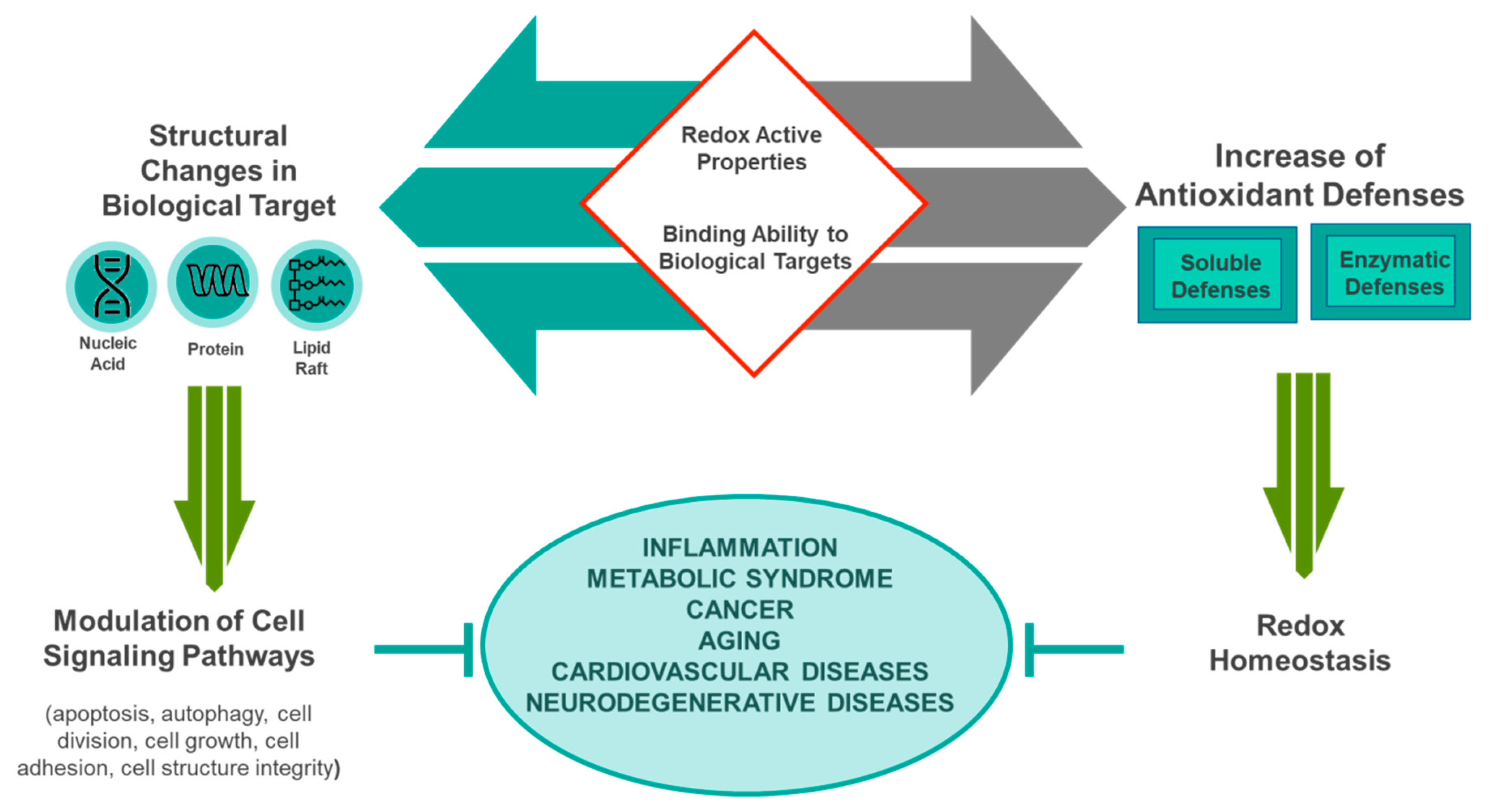
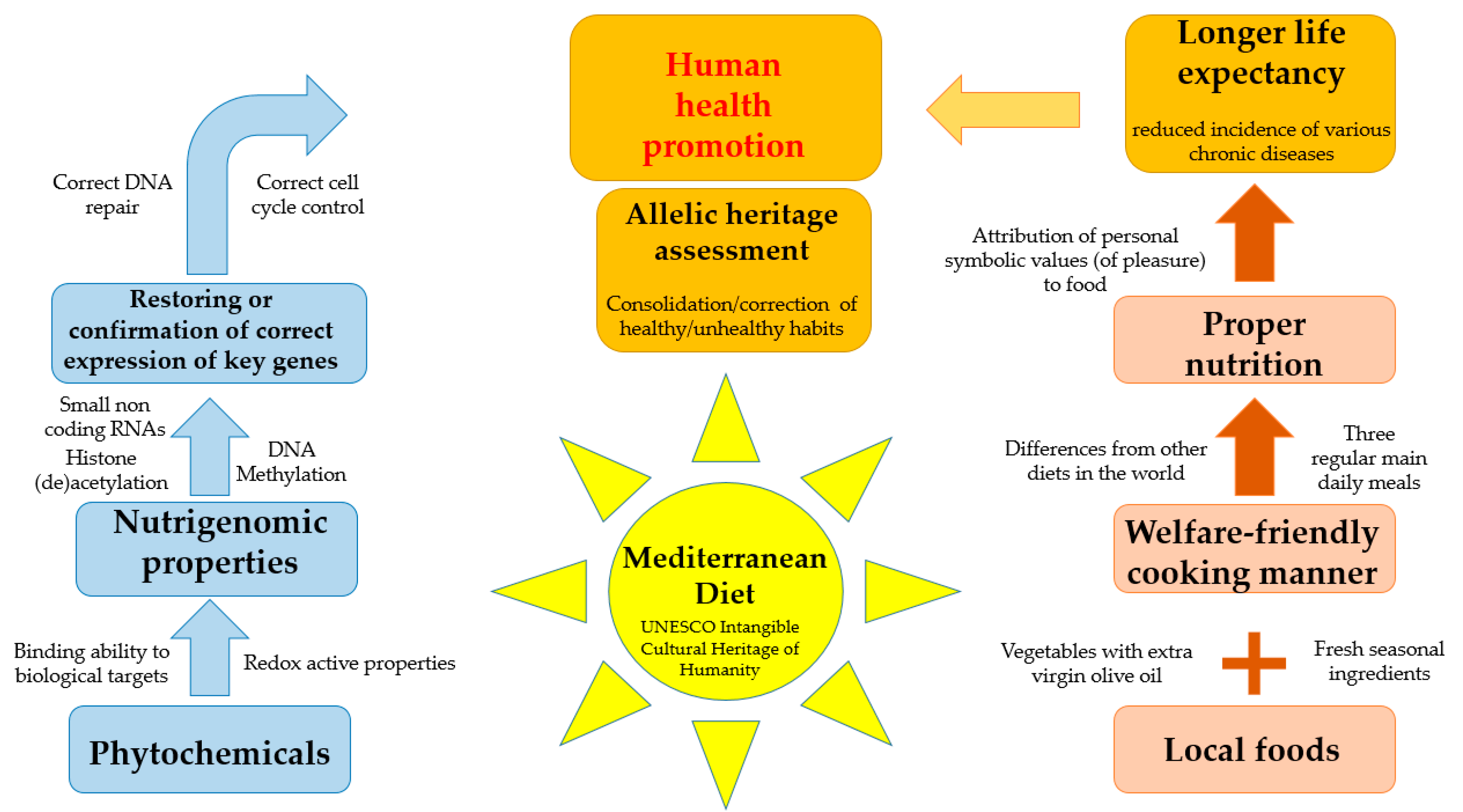
3. Healthy Properties of Mediterranean Diet
Antioxidative Potential of the Mediterranean Diet
4. The Epigenomics of Mediterranean Nutrients
4.1. Mediterranean Foods/Drinks Contained Molecules Which Act as Epigenetic Modulators
4.2. Main MD Cooking Methods and Their Implications in Variations of Nutrigenomic Effects
5. Genetic Variants, the Mediterranean Diet, and Human Health
5.1. Predisposing/Protective Genotypes, Main Human Diseases and the Mediterrranean Diet
5.2. The Importance of Considering the Allelic Diversity of CYP2E1 Gene in MD Consumers
5.3. The Ability to Assess TAS2R38 Allelic Variants in MD Consumers
6. Most Popular Foods in the World: Substantial Nutrigenomic Differences/Similarities from/to the Mediterranean Diet
6.1. American Foods
6.2. Indian Fruits and Soybean Oil
7. Conclusions and Future Perspectives
- dietary antioxidant → minor ROS → minor DNA damage → normalization of DNA methylation
- and the opposite one:
- endo/exo-oxidants → greater ROS → greater DNA damage → hypomethylation of DNA for defence
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fresán, U.; Martínez-González, M.A.; Sabaté, J.; Bes-Rastrollo, M. Global sustainability (health, environment and monetary costs) of three dietary patterns: Results from a Spanish cohort (the SUN project). BMJ Open 2019, 9, e021541. [Google Scholar] [CrossRef] [PubMed]
- Dernini, S.; Berry, E.M.; Serra-Majem, L.; La Vecchia, C.; Capone, R.; Medina, F.X.; Aranceta-Bartrina, J.; Belahsen, R.; Burlingame, B.; Calabrese, G.; et al. Med diet 4.0: The Mediterranean diet with four sustainable benefits. Public Health Nutr. 2017, 20, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Gerber, M.; Hoffman, R. The Mediterranean diet: Health, science and society. Br. J. Nutr. 2015, 113, S4–S10. [Google Scholar] [CrossRef] [PubMed]
- Palermo, M.; Pellegrini, N.; Fogliano, V. The effect of cooking on the phytochemical content of vegetables. J. Sci. Food Agric. 2014, 94, 1057–1070. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, M.A.; Hershey, M.S.; Zazpe, I.; Trichopoulou, A. Transferability of the Mediterranean diet to non-Mediterranean countries. What is and what is not the Mediterranean diet. Nutrients 2017, 9, 1226. [Google Scholar] [CrossRef]
- Saulle, R.; La Torre, G. The Mediterranean diet, recognized by UNESCO as a cultural heritage of humanity. Ital. J. Public Health 2010, 7, 414–415. [Google Scholar]
- Petersson, S.D.; Philippou, E. Mediterranean diet, cognitive function, and dementia: A Systematic review of the evidence. Adv. Nutr. 2016, 7, 889–904. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Schwedhelm, C.; Galbete, C.; Hoffmann, G. Adherence to Mediterranean diet and risk of cancer: An updated systematic review and meta-analysis. Nutrients 2017, 9, 1063. [Google Scholar] [CrossRef]
- Forsyth, C.; Kouvari, M.; D’Cunha, N.M.; Georgousopoulou, E.N.; Panagiotakos, D.B.; Mellor, D.D.; Kellett, J.; Naumovski, N. The effects of the Mediterranean diet on rheumatoid arthritis prevention and treatment: A systematic review of human prospective studies. Rheumatol. Int. 2018, 38, 737–747. [Google Scholar] [CrossRef]
- Sánchez-Muniz, F.J.; Macho-González, A.; Garcimartín, A.; Santos-López, J.A.; Benedí, J.; Bastida, S.; González-Muñoz, M.J. The nutritional components of beer and its relationship with neurodegeneration and Alzheimer’s disease. Nutrients 2019, 11, 1558. [Google Scholar] [CrossRef]
- Arranz, A.; Chiva-Blanch, G.; Valderas Martínez, P.; Casas, R.; Estruch, R. Beer: Beneficial aspects and contribution to the mediterranean diet. In The Mediterranean Diet: An Evidence-Based Approach; Preedy, V.R., Ross-Watson, R., Eds.; Academic Press: Amsterdam, The Netherlands, 2015; pp. 153–164. [Google Scholar]
- Mingot, L.D.; Gesteiro, E.; Bastida, S.; Sánchez-Muniz, F.J. Epigenetic effects of the pregnancy Mediterranean diet adherence on the offspring metabolic syndrome markers. J. Physiol. Biochem. 2017, 73, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Ros, E.; Covas, M.I.; Fiol, M.; Wärnberg, J.; Arós, F.; Ruíz-Gutiérrez, V.; Lamuela-Raventós, R.M. Cohort profile: Design and methods of the PREDIMED study. Int. J. Epidemiol. 2012, 41, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, A.; Johnson, L.; Toumpakari, Z.; England, C.; Rai, M.; Toms, S.; Penfold, C.; Zazpe, I.; Martínez-González, M.A.; Feder, G. Validation of the English version of the 14-item Mediterranean diet adherence screener of the PREDIMED study, in people at high cardiovascular risk in the UK. Nutrients 2018, 28, 138. [Google Scholar] [CrossRef]
- Fernández-García, J.C.; Araceli Muñoz-Garach, A.; Martínez-González, M.A.; Salas-Salvado, J.; Corella, D.; Hernáez, A.; Romaguera, D.; Vioque, J.; Alonso-Gómez, A.M.; Wärnberg, J. Association between lifestyle and hypertriglyceridemic waist phenotype in the PREDIMED-plus study. Obesity 2020, 28, 537–543. [Google Scholar] [CrossRef]
- Gavrilas, L.I.; Ionescu, C.; Tudoran, O.; Lisencu, C.; Balacescu, O.; Miere, D. The role of bioactive dietary components in modulating miRNA expression in colorectal cancer. Nutrients 2016, 8, 590. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C.; Reig, C.; Corona, O.; Todaro, A.; Mazzaglia, A.; Perrone, A.; Gianguzzi, G.; Agusti, M.; Farina, V. Pomological traits, sensory profile and nutraceutical properties of nine cultivars of loquat (Eriobotrya japonica Lindl.) fruits grown in Mediterranean area. Plant Foods Hum. Nutr. 2016, 71, 303–338. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C.; Di Gregorio, E.; Di Stefano, V.; Mannino, G.; Perrone, A.; Avellone, G.; Sortino, G.; Inglese, P.; Farina, V. Food quality and nutraceutical value of nine cultivars of mango (Mangifera indica L.) fruits grown in Mediterranean subtropical environment. Food Chem. 2019, 277, 471–479. [Google Scholar] [CrossRef]
- Slavin, J.L.; Lloyd, B. Health benefits of fruits and vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef]
- Tang, G.Y.; Meng, X.; Li, Y.; Zhao, C.N.; Liu, Q.; Li, H.B. Effects of vegetables on cardiovascular diseases and related mechanisms. Nutrients 2017, 9, 857. [Google Scholar] [CrossRef]
- Ros, E. Nuts and CVD. Br. J. Nutr. 2015, 113, S111–S120. [Google Scholar] [CrossRef]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean diet: A literature review. Nutrients 2015, 79, 9139–9153. [Google Scholar] [CrossRef] [PubMed]
- Farhangi, M.A.; Najafi, M.; Jafarabadi, M.A.; Jahangiry, L. Mediterranean dietary quality index and dietary phytochemical index among patients candidate for coronary artery bypass grafting (CABG) surgery. BMC Cardiovasc. Disord. 2017, 17, 114. [Google Scholar] [CrossRef] [PubMed]
- Ortega, R. R. Importance of functional foods in the Mediterranean diet. Public Health Nutr. 2006, 9, 1136–1140. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Functional foods and lifestyle approaches for diabetes prevention and management. Nutrients 2017, 9, 1310. [Google Scholar] [CrossRef]
- Mannino, G.; Gentile, C.; Maffei, M.E. Chemical partitioning and DNA fingerprinting of some pistachio (Pistacia vera L.) varieties of different geographical origin. Phytochemistry 2019, 160, 40–47. [Google Scholar] [CrossRef]
- Ros, E. Health benefits of nut consumption. Nutrients 2010, 2, 652–682. [Google Scholar] [CrossRef]
- Gentile, C.; Allegra, M.; Angileri, F.; Pintaudi, A.M.; Livrea, M.A.; Tesoriere, L. Polymeric proanthocyanidins from Sicilian pistachio (Pistacia vera L.) nut extract inhibit lipopolysaccharide-induced inflammatory response in RAW 264.7 cells. Eur. J. Nutr. 2012, 51, 353–363. [Google Scholar] [CrossRef]
- Gentile, C.; Perrone, A.; Attanzio, A.; Tesoriere, L.; Livrea, M.A. Sicilian pistachio (Pistacia vera L.) nut inhibits expression and release of inflammatory mediators and reverts the increase of paracellular permeability in IL-1β-exposed human intestinal epithelial cells. Eur. J. Nutr. 2015, 54, 811–821. [Google Scholar] [CrossRef]
- Visioli, F.; Franco, M.; Toledo, E.; Luchsinger, J.; Willett, W.C.; Hu, F.B.; Martinez-Gonzalez, M.A. Olive oil and prevention of chronic diseases: Summary of an International conference. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 649–656. [Google Scholar] [CrossRef]
- Kok, C.R.; Hutkins, R. Yogurt and other fermented foods as sources of health-promoting bacteria. Nutr. Rev. 2018, 76, 4–15. [Google Scholar] [CrossRef]
- Barkallah, M.; Dammak, M.; Louati, I.; Hentati, F.; Hadrich, B.; Mechichi, T.; Ayadi, M.A.; Fendri, I.; Attia, H.; Abdelkafi, S. Effect of Spirulina platensis fortification on physicochemical, textural, antioxidant and sensory properties of yogurt during fermentation and storage. LWT Food Sci. Technol. 2017, 84, 323–330. [Google Scholar] [CrossRef]
- Fazilah, N.F.; Ariff, A.B.; Khayat, M.E.; Rios-Solis, L.; Halim, M. Influence of probiotics, prebiotics, synbiotics and bioactive phytochemicals on the formulation of functional yogurt. J. Funct. Foods 2018, 48, 387–399. [Google Scholar] [CrossRef]
- Gaglio, R.; Gentile, C.; Bonanno, A.; Vintaloro, L.; Perrone, A.; Mazza, F.; Settanni, L.; Di Grigoli, A. Effect of saffron addition on the microbiological, physicochemical, antioxidant and sensory characteristics of yoghurt. J. Dairy Technol. 2019, 72, 208–217. [Google Scholar] [CrossRef]
- Jiménez-López, J.; Ruiz-Medina, A.; Ortega-Barrales, P.; Llorent-Martínez, E.J. Phytochemical profile and antioxidant activity of caper berries (Capparis spinosa L.): Evaluation of the influence of the fermentation process. Food Chem. 2018, 250, 54–59. [Google Scholar]
- Tesoriere, L.; Butera, D.; Gentile, C.; Livrea, M.A. Bioactive components of caper (Capparis spinosa L.) from Sicily and antioxidant effects in a red meat simulated gastric digestion. J. Agric. Food Chem. 2007, 55, 8465–8471. [Google Scholar] [CrossRef]
- Perez-Gregorio, R.; Simal-Gandara, J. A critical review of bioactive food components, and of their functional mechanisms, biological effects and health outcomes. Curr. Pharm. Des. 2017, 23, 2731–2741. [Google Scholar] [CrossRef]
- Ghiselli, A.; D’Amicis, A.; Giacosa, A. The antioxidant potential of the Mediterranean diet. Eur. J. Cancer Prev. 1997, 6, S15–S19. [Google Scholar] [CrossRef]
- Kavouras, S.A.; Panagiotakos, D.B.; Pitsavos, C.; Chrysohoou, C.; Arnaoutis, G.; Skoumas, Y.; Stefanadis, C. Physical activity and adherence to Mediterranean diet increase total antioxidant capacity: The ATTICA study. Cardiol. Res. Pr. 2010, 2011, 248626. [Google Scholar] [CrossRef]
- Hadžiabdić, O.M.; Vitali-Čepo, D.; Rahelić, D.; Božikov, V. The effect of the Mediterranean diet on serum total antioxidant capacity in obese patients: A randomized controlled trial. J. Am. Coll. Nutr. 2016, 35, 224–235. [Google Scholar] [CrossRef]
- Mannino, G.; Perrone, A.; Campobenedetto, C.; Schittone, A.; Bertea, M.C.; Gentile, C. Phytochemical profile and antioxidative properties of Plinia trunciflora fruits: A new source of nutraceuticals. Food Chem. 2020, 307. [Google Scholar] [CrossRef]
- Janssen-Heininger, Y.M.; Mossman, B.T.; Heintz, N.H.; Forman, H.J.; Kalyanaraman, B.; Finkel, T.; Stamler, J.S.; Rhee, S.G.; Van der Vliet, A. Redox-based regulation of signal transduction: Principles, pitfalls, and promises. Free Radic. Biol. Med. 2008, 45, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Caradonna, F.; Cruciata, I.; Lauria, A.; Perrone, A.; Gentile, C. Melatonin reduces inflammatory response in human intestinal epithelial cells stimulated by interleukin-1β. J. Pineal. Res. 2019, 67, e12598. [Google Scholar] [CrossRef] [PubMed]
- Verstraeten, S.V.; Fraga, C.G.; Oteiza, P. Interactions of flavan-3-ols and procyanidins with membranes: Mechanisms and the physiological relevance. Food Funct. 2015, 6, 32–41. [Google Scholar] [CrossRef]
- Bordoni, A.; Capozzi, F. Foodomics for healthy nutrition. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Herrick, K.; Phillips, D.I.; Haselden, S.; Shiell, A.W.; Campbell-Brown, M.; Godfrey, K.M. Maternal consumption of a high-meat, low-carbohydrate diet in late pregnancy: Relation to adult cortisol concentrations in the offspring. J. Clin. Endocrinol. Metab. 2003, 88, 3554–3560. [Google Scholar] [CrossRef] [PubMed]
- Drake, A.J.; McPherson, R.C.; Godfrey, K.M.; Cooper, C.; Lillycrop, K.A.; Hanson, M.A.; Meehan, R.R.; Seckl, J.R.; Reynolds, R.M. An unbalanced maternal diet in pregnancy associates with offspring epigenetic changes in genes controlling glucocorticoid action and foetal growth. Clin. Endocrinol. 2012, 77, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Gesteiro, E.; Sánchez-Muniz, F.J.; Bastida, S. Hypercortisolaemia and hyperinsulinaemia interaction and their impact upon insulin resistance/sensitivity markers at birth. In Umbilical Cord Blood Banking for Clinical Application and Regenerative Medicine; Maurício, A.C., Ed.; Intechopen: Rijeka, Croatia, 2017; pp. 69–98. [Google Scholar]
- O’Connor, D.B.; Green, J.A.; Ferguson, E.; O’Carroll, R.E.; O’Connor, R.C. Effects of childhood trauma on cortisol levels in suicide attempters and ideators. Psychoneuroendocrinology 2018, 88, 9–16. [Google Scholar] [CrossRef]
- Gut, P.; Verdin, E. The nexus of chromatin regulation and intermediary metabolism. Nature 2013, 502, 489–498. [Google Scholar] [CrossRef]
- Stefanska, B.; Karlic, H.; Varga, F.; Fabianowska-Majewska, K.; Haslberger, A. Epigenetic mechanisms in anti-cancer actions of bioactive food components-the implications in cancer prevention. Br. J. Pharm. 2012, 167, 279–297. [Google Scholar] [CrossRef]
- Dominguez-Salas, P.; Cox, S.E.; Prentice, A.M.; Hennig, B.J.; Moore, S.E. Maternal nutritional status, C(1) metabolism and offspring DNA methylation: A review of current evidence in human subjects. Proc. Nutr. Soc. 2012, 71, 154–165. [Google Scholar] [CrossRef]
- Ferguson, L.R.; Philpott, M.; Karunasinghe, N. Dietary cancer and prevention using antimutagens. Toxicology 2004, 198, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Navarro, E.; Funtikova, A.N.; Fíto, M.; Schröder, H. Prenatal nutrition and the risk of adult obesity: Long-term effects of nutrition on epigenetic mechanisms regulating gene expression. J. Nutr. Biochem. 2017, 39, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Agodi, A.; Barchitta, M.; Quattrocchi, A.; Maugeri, A.; Canto, C.; Marchese, A.E.; Vinciguerra, M. Low fruit consumption and folate deficiency are associated with LINE-1 hypomethylation in women of a cancer-free population. Genes Nutr. 2015, 10, 480. [Google Scholar] [CrossRef] [PubMed]
- Karunamuni, G.; Sheehan, M.M.; Doughman, Y.Q.; Gu, S.; Sun, J.; Li, Y.; Strainic, J.P.; Rollins, A.M.; Jenkins, M.W.; Watanabe, M. Supplementation with the methyl donor betaine prevents congenital defects induced by prenatal alcohol exposure. Alcohol Clin. Exp. Res. 2017, 41, 1917–1927. [Google Scholar] [CrossRef] [PubMed]
- Mesías, M.; Seiquer, I.; Navarro, M.P. The Mediterranean diet and mineral composition. In The Mediterranean Diet: An Evidence-Based Approach; Preedy, V., Watson, R.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 185–198. [Google Scholar]
- Naselli, F.; Belshaw, N.J.; Gentile, C.; Tutone, M.; Tesoriere, L.; Livrea, M.A.; Caradonna, F. Phytochemical indicaxanthin inhibits colon cancer cell growth and affects the DNA methylation status by influencing epigenetically modifying enzyme expression and activity. J. Nutr. 2015, 8, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Myzak, M.C.; Tong, P.; Dashwood, W.M.; Dashwood, R.H.; Ho, E. Sulforaphane retards the growth of human PC-3 xenografts and inhibits HDAC activity in human subjects. Exp. Biol. Med. 2007, 232, 227–234. [Google Scholar]
- Juengel, E.; Erb, H.H.H.; Haferkamp, A.; Rutz, J.; Chun, F.K.; Blaheta, R.A. Relevance of the natural HDAC inhibitor sulforaphane as a chemopreventive agent in urologic tumors. Cancer Lett. 2018, 435, 121–126. [Google Scholar] [CrossRef]
- Kalea, A.Z.; Drosatos, K.; Buxton, J.L. Nutriepigenetics and cardiovascular disease. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 252–259. [Google Scholar] [CrossRef]
- Debnath, T.; Nath, D.N.C.; Kim, E.K.; Lee, K.G. Role of phytochemicals in the modulation of miRNA expression in cancer. Food Funct. 2017, 8, 3432–3442. [Google Scholar] [CrossRef]
- Parasramka, M.A.; Ho, E.; Williams, D.E.; Dashwood, R.H. MicroRNAs, diet, and cancer: New mechanistic insights on the epigenetic actions of phytochemicals. Mol. Carcinog. 2012, 51, 213–230. [Google Scholar] [CrossRef]
- Shah, M.S.; Schwartz, S.L.; Zhao, C.; Davidson, L.A.; Zhou, B.; Lupton, J.R.; Ivanov, I.; Chapkin, R.S. Integrated microRNA and mRNA expression profiling in a rat colon carcinogenesis model: Effect of a chemo-protective diet. Physiol. Genomics 2011, 43, 640–654. [Google Scholar] [CrossRef] [PubMed]
- Palmero, E.I.; De Campos, S.G.; Campos, M.; De Souza, N.C.; Guerreiro, I.D.; Carvalho, A.L.; Marques, M.M. Mechanisms and role of microRNA deregulation in cancer onset and progression. Genet Mol. Biol. 2011, 34, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, F.; Capri, M.; Bonafè, M.; Morsiani, C.; Jung, H.J.; Spazzafumo, L.; Viña, J.; Suh, Y. Circulating miRNAs and miRNA shuttles as biomarkers: Perspective trajectories of healthy and unhealthy aging. Mech. Ageing Dev. 2017, 165, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Ferrières, J. The French paradox: Lessons for other countries. Heart 2004, 90, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Ndlovu, T.; Van Jaarsveld, F.; Caleb, O.J. French and Mediterranean-style diets: Contradictions, misconceptions and scientific facts—A review. Food Res. Int. 2019, 116, 840–858. [Google Scholar] [CrossRef]
- Basso, E.; Leone, S.; Polticelli, F.; Cozzi, R. The anticancer effects of resveratrol in glioma cells. Eur. J. Nutr. 2011, 50, 489–498. [Google Scholar]
- Kocic, H.; Damiani, G.; Stamenkovic, B.; Tirant, M.; Jovic, A.; Tiodorovic, D.; Peris, K. Dietary compounds as potential modulators of microRNA expression in psoriasis. Adv. Chronic Dis. 2019. [Google Scholar] [CrossRef]
- Petyaev, I.M.; Bashmakov, Y.K. Could cheese be the missing piece in the French paradox puzzle? Med. Hypotheses 2012, 79, 746–749. [Google Scholar] [CrossRef]
- Baldwin, R.L.; Li, R.W.; Li, C.J.; Thomson, J.M.; Bequette, B.J. Characterization of the longissimus lumborum transcriptome response to adding propionate to the diet of growing angus beef steers. Physiol. Genom. 2012, 44, 543–550. [Google Scholar] [CrossRef][Green Version]
- Song, S.; Hooiveld, G.J.E.J.; Li, M.; Zhao, F.; Zhang, W.; Xu, X.; Muller, M.; Li, C.; Zhou, G. Distinct physiological, plasma amino acid, and liver transcriptome responses to purified dietary beef, chicken, fish, and pork proteins in young rats. Mol. Nutr. Food Res. 2016, 60, 1199–1205. [Google Scholar] [CrossRef]
- Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef]
- Nguyen, Q.V.; Malau-Aduli, B.S.; Cavalieri, J.; Malau-Aduli, A.E.O.; Nichols, P.D. Enhancing omega-3 long-chain polyunsaturated fatty acid content of dairy-derived foods for human consumption. Nutrients 2019, 11, 743. [Google Scholar] [CrossRef]
- Kortner, T.M.; Skugor, S.; Penn, M.H.; Mydland, T.L.; Djordjevic, B.; Hillestad, M.; Krasnov, A.; Krogdahl, A. Dietary soyasaponin supplementation to pea protein concentrate reveals nutrigenomic interactions underlying enteropathy in Atlantic salmon (Salmo salar). BMC Vet. Res. 2012, 8, 101. [Google Scholar] [CrossRef]
- Mileo, A.M.; Di Venere, D.; Abbruzzese, C.; Miccadei, S. Long term exposure to polyphenols of artichoke (Cynara scolymus L.) exerts induction of senescence driven growth arrest in the MDA-MB231 human breast cancer cell line. Oxid. Med. Cell Longev. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Sheoran, N.; Kumar, R.; Kumar, A.; Batra, K.; Sihag, S.; Maan, S.; Maan, N.S. Nutrigenomic evaluation of garlic (Allium sativum) and holy basil (Ocimum sanctum) leaf powder supplementation on growth performance and immune characteristics in broilers. Vet. World 2017, 10, 121–129. [Google Scholar] [CrossRef]
- Sgorlon, S.; Stefanon, B.; Sandri, M.; Colitti, M. Nutrigenomic activity of plant derived compounds in health and disease: Results of a dietary intervention study in dog. Res. Vet. Sci. 2016, 109, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Kim, M.; Kim, M.; Kwak, J.H.; Chang, D.H.; Yu, W.K.; Lee, S.H.; Lee, J.H. Consumption of dairy yogurt with the polysaccharide rhamnogalacturonan from the peel of the korean citrus hallabong enhances immune function and attenuates the inflammatory response. Food Funct. 2016, 7, 2833–2839. [Google Scholar] [CrossRef] [PubMed]
- Kubatka, P.; Uramova, S.; Kello, M.; Kajo, K.; Kruzliak, P.; Mojzis, J.; Vybohova, D.; Adamkov, M.; Jasek, K.; Lasabova, Z.; et al. Antineoplastic effects of clove buds (Syzygium Aromaticum, L.) in the model of breast carcinoma. J. Cell Mol. Med. 2017, 21, 2837–2851. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.; Sur, S.; Roy, R.; Mandal, S.; Panda, C.K. Epigallocatechin gallate in combination with eugenol or amarogentin shows synergistic chemotherapeutic potential in cervical cancer cell line. J. Cell. Physiol. 2018, 234, 825–836. [Google Scholar] [CrossRef]
- Hoshyar, R.; Mollaei, H. A comprehensive review on anticancer mechanisms of the main carotenoid of saffron, crocin. J. Pharm. Pharm. 2017, 69, 1419–1427. [Google Scholar] [CrossRef]
- Nirumand, M.C.; Hajialyani, M.; Rahimi, R.; Farzaei, M.H.; Zingue, S.; Nabavi, S.M.; Bishayee, A. Dietary plants for the prevention and management of kidney stones: Preclinical and clinical evidence and molecular mechanisms. Int. J. Mol. Sci. 2018, 19, 765. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Liu, Y.; Lan, Y.; Li, X.; Luo, L.; Duan, X.; Lei, M.; Liu, G.; Yang, Z.; Mai, X.; et al. Dietary vinegar prevents kidney stone recurrence via epigenetic regulations. EBioMedicine 2019, 45, 231–250. [Google Scholar] [CrossRef] [PubMed]
- Tauber, A.L.; Schweiker, S.S.; Levonis, S.M. From tea to treatment; epigallocatechin gallate and its potential involvement in minimizing the metabolic changes in cancer. Nutr. Res. 2020, 74, 23–36. [Google Scholar] [CrossRef]
- Basu, A.; Masek, E.; Ebersole, J.L. Dietary polyphenols and periodontitis—A mini-review of literature. Molecules 2018, 23, 1786. [Google Scholar] [CrossRef]
- Vacca, R.A.; Valenti, D.; Caccamese, S.; Daglia, M.; Braidy, N.; Nabavi, S.M. Plant polyphenols as natural drugs for the management of Down syndrome and related disorders. Neurosci. Biobehav. Rev. 2016, 71, 865–877. [Google Scholar] [CrossRef]
- Casanova, E.; Salvadó, J.; Crescenti, A.; Gibert-Ramos, A. Epigallocatechin gallate modulates muscle homeostasis in type 2 Diabetes and obesity by targeting energetic and redox pathways: A narrative review. Int. J. Mol. Sci. 2019, 20, 532. [Google Scholar] [CrossRef]
- Barchitta, M.; Maugeri, A.; San-Lio, M.R.; Favara, G.; La Rosa, M.C.; La Mastra, C.; Quattrocchi, A.; Agodi, A. Dietary patterns are associated with leukocyte LINE-1 methylation in women: A cross-sectional study in southern Italy. Nutrients 2019, 11, 1843. [Google Scholar] [CrossRef]
- Tripathy, A.; Ara, F.; Ghosh, P.; Ghosh, D. Effect of lycopene on testicular androgenic and anti-oxidative enzymes in cyproterone acetate-induced male infertile Wistar strain albino rats: An in vitro study. Andrologia 2020, 52, e13494. [Google Scholar] [CrossRef]
- Trivedi, M.S.; Hodgson, N.W.; Walker, S.J.; Trooskens, G.; Nair, V.; Deth, R.C. Epigenetic effects of casein-derived opioid peptides in SH-SY5Y human neuroblastoma cells. Nutr. Metab. 2015, 12, 54–64. [Google Scholar] [CrossRef]
- Lim, J.Y.; Wang, X.D. Mechanistic understanding of β-cryptoxanthin and lycopene in cancer prevention in animal models. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 5, 158652. [Google Scholar] [CrossRef]
- Tomas-Hernandez, S.; Garcia-Vallvé, S.; Pujadas, G.; Valls, C.; Ojeda-Montes, M.; Gimeno, A.; Cereto-Massagué, A.; Roca-Martinez, J.; Suárez, M.; Arola, L.; et al. Anti-inflammatory and proapoptotic properties of the natural compound o-Orsellinaldehyde. J. Agric. Food Chem. 2018, 66, 10952–10963. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, Y.Y.; Sun, Y.S.; Ma, R.H.; Thakur, K.; Zhang, J.G.; Wei, Z.J. Asparanin A from Asparagus officinalis L. induces G0/G1 cell cycle arrest and apoptosis in human endometrial carcinoma ishikawa cells via mitochondrial and PI3K/AKT signalling pathways. J. Agric. Food Chem. 2020, 68, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lim, W.; Bazer, F.W.; Whang, K.Y.; Song, G. Quercetin inhibits proliferation of endometriosis regulating cyclin D1 and its target microRNAs in vitro and in vivo. J. Nutr. Biochem. 2019, 63, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Gentile, L.; Uccella, N.A.; Sivakumar, G. Soft-MS and computational mapping of oleuropein. Int. J. Mol. Sci. 2017, 18, 992. [Google Scholar] [CrossRef]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Gondal, A.T.; Saeed, F.; Imran, A.; Shahbaz, M.; Tsouh-Fokou, P.V.; Arshad, U.M.; Khan, H.; et al. Kaempferol: A key emphasis to its anticancer potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef]
- Batool, R.; Salahuddin, H.; Mahmood, T.; Ismail, M. Study of anticancer and antibacterial activities of Foeniculum vulgare, Justicia adhatoda and Urtica dioica as natural curatives. Cell. Mol. Biol. 2017, 63, 109–114. [Google Scholar] [CrossRef]
- Campestrini, L.H.; Melo, P.S.; Peres, L.E.P.; Calhelha, R.C.; Ferreira, I.C.F.R.; Alencar, S.M. A new variety of purple tomato as a rich source of bioactive carotenoids and its potential health benefits. Heliyon 2019, 5, e02831. [Google Scholar] [CrossRef]
- Kathirvel, P.; Ravi, S. Chemical composition of the essential oil from basil (Ocimum basilicum Linn.) and its in vitro cytotoxicity against HeLa and HEp-2 human cancer cell lines and NIH 3T3 mouse embryonic fibroblasts. Nat. Prod. Res. 2012, 26, 1112–1118. [Google Scholar] [CrossRef]
- De Santiago, E.; Gill, C.I.R.; Carafa, I.; Tuohy, K.M.; De Peña, M.P.; Cid, C. Digestion and colonic fermentation of raw and cooked Opuntia ficus-indica cladodes impacts bioaccessibility and bioactivity. J. Agric. Food Chem. 2019, 67, 2490–2499. [Google Scholar] [CrossRef]
- Vinha, A.F.; Alves, R.C.; Barreira, S.V.; Costa, A.S.; Oliveira, M.B. Impact of boiling on phytochemicals and antioxidant activity of green vegetables consumed in the Mediterranean diet. Food Funct. 2015, 6, 1157–1163. [Google Scholar] [CrossRef]
- Lanza, B.; Ninfali, P. Antioxidants in extra virgin olive oil and table olives: Connections between agriculture and processing for health choices. Antioxidants 2020, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Castellón, J.; Vallverdú-Queralt, A.; De Alvarenga, R.J.F.; Illán, M.; Torrado-Prat, X.; Lamuela-Raventós, R.M. Domestic sautéing with EVOO: Change in the phenolic profile. Antioxidants 2020, 9, 77. [Google Scholar] [CrossRef]
- Olivero-David, R.; Mena, C.; Pérez-Jimenez, M.A.; Sastre, B.; Bastida, S.; Márquez-Ruiz, G.; Sánchez-Muniz, F.J. Influence of Picual olive ripening on virgin olive oil alteration and stability during potato frying. J. Agric. Food Chem. 2014, 62, 11637–11646. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Gleize, B.; Zhang, L.; Caris-Veyrat, C.; Renard, C.M.G.C. A D-optimal mixture design of tomato-based sauce formulations: Effects of onion and EVOO on lycopene isomerization and bioaccessibility. Food Funct. 2019, 10, 3589–3602. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Liu, J. Effects of the cooking modes on commonly used pesticides residue in vegetables and their chronic dietary exposure risk in South China. Food Addit. Contam. Part A 2020, 37, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I.; Hausman-Cohen, S.; Pizano, J.; Schmidt, M.A.; Minich, D.M.; Joffe, Y.; Brandhorst, S.; Evans, S.; Brady, D.M. Personalized nutrition: Translating the science of nutrigenomics into practice: Proceedings from the 2018 American college of nutrition meeting. J. Am. Coll. Nutr. 2019, 38, 287–301. [Google Scholar] [CrossRef]
- De Toro-Martín, J.; Arsenault, B.J.; Després, J.P.; Vohl, M.C. Precision nutrition: A review of personalized nutritional approaches for the prevention and management of metabolic syndrome. Nutrients 2017, 9, 913. [Google Scholar] [CrossRef]
- Nasreddine, L.; Akika, R.; Mailhac, A.; Tamim, H.; Zgheib, N.K. The Interaction between genetic polymorphisms in FTO and TCF7L2 genes and dietary intake with regard to body mass and composition: An exploratory study. J. Pers. Med. 2019, 9, 11. [Google Scholar] [CrossRef]
- Xiang, L.; Wu, H.; Pan, A.; Patel, B.; Xiang, G.; Qi, L.; Kaplan, R.C.; Hu, F.; Wylie-Rosett, J.; Qi, Q. FTO genotype and weight loss in diet and lifestyle interventions: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2016, 103, 1162–1170. [Google Scholar] [CrossRef]
- Hosseini-Esfahani, F.; Koochakpoor, G.; Daneshpour, M.S.; Sedaghati-Khayat, B.; Mirmiran, P.; Azizi, F. Mediterranean dietary pattern adherence modify the association between FTO genetic variations and obesity phenotypes. Nutrients 2017, 9, 1064. [Google Scholar] [CrossRef]
- Rees, K.; Takeda, A.; Martin, N.; Ellis, L.; Wijesekara, D.; Vepa, A.; Das, A.; Hartley, L.; Stranges, S. Mediterranean-style diet for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2019, 3. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Delgado, F.; Alcala-Diaz, J.F.; Leon-Acuña, A.; Lopez-Moreno, J.; Delgado-Lista, J.; Gomez-Marin, B.; Roncero-Ramos, I.; Yubero-Serrano, E.M.; Rangel-Zuñiga, O.A.; Vals-Delgado, C.; et al. Apolipoprotein E genetic variants interact with Mediterranean diet to modulate postprandial hypertriglyceridemia in coronary heart disease patients, CORDIOPREV study. Eur. J. Clin. Invest. 2019, 49, e13146. [Google Scholar] [CrossRef] [PubMed]
- Caradonna, F.; Cruciata, I.; Schifano, I.; La Rosa, C.; Naselli, F.; Chiarelli, R.; Perrone, A.; Gentile, C. Methylation of cytokines gene promoters in IL-1β-treated human intestinal epithelial cells. Inflamm. Res. 2018, 67, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Roncero-Ramos, I.; Rangel-Zuñiga, O.A.; Lopez-Moreno, J.; Alcala-Diaz, J.F.; Perez-Martinez, P.; Jimenez-Lucena, R.; Castaño, J.P.; Roche, H.M.; Delgado-Lista, J.; Ordovas, J.M.; et al. Mediterranean diet, glucose homeostasis, and inflammasome genetic variants: The CORDIOPREV study. Mol. Nutr. Food Res. 2018, 62, e1700960. [Google Scholar] [CrossRef]
- Jaacks, L.M.; Siegel, K.R.; Gujral, U.P.; Narayan, K.M. Type 2 diabetes: A 21st century epidemic. Best Pr. Res. Clin. Endocrinol. Metab. 2016, 30, 331–343. [Google Scholar] [CrossRef]
- Corella, D.; Coltell, O.; Sorlí, J.V.; Estruch, R.; Quiles, L.; Martínez-González, M.Á.; Salas-Salvadó, J.; Castañer, O.; Arós, F.; Ortega-Calvo, M.; et al. Polymorphism of the transcription factor 7-like 2 gene (TCF7L2) interacts with obesity on type-2 Diabetes in the PREDIMED study emphasizing the heterogeneity of genetic variants in type-2 diabetes risk prediction: Time for obesity-specific genetic risk scores. Nutrients 2016, 8, 793–810. [Google Scholar]
- Moreno-Valdespino, C.A.; Luna-Vitalc, D.D.; Camacho-Ruizb, R.M.; Mojica, L. Bioactive proteins and phytochemicals from legumes: Mechanisms of action preventing obesity and type-2 diabetes. Food Res. Int. 2020, 130, 108905. [Google Scholar] [CrossRef]
- Ojeda-Rodríguez, A.; Zazpe, I.; Alonso-Pedrero, L.; Zalba, G.; Guillen-Grima, F.; Martinez-Gonzalez, M.A.; Marti, A. Association between diet quality indexes and the risk of short telomeres in an elderly population of the SUN project. Clin. Nutr. 2019, 33130–33139. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, I.; Naselli, F.; Saverini, M.; Giacalone, A.; Montalto, G.; Caradonna, F. Cytochrome P450 2E1 variable number tandem repeat polymorphisms and health risks: A genotype-phenotype study in cancers associated with drinking and/or smoking. Mol. Med. Rep. 2012, 6, 416–420. [Google Scholar] [CrossRef]
- Hogervorst, J.G.; Van den Brandt, P.A.; Godschalk, R.W.; Van Schooten, F.J.; Schouten, L.J. The influence of single nucleotide polymorphisms on the association between dietary acrylamide intake and endometrial cancer risk. Sci. Rep. 2016, 6, 34902. [Google Scholar] [CrossRef]
- Sciandrello, G.; Mauro, M.; Caradonna, F.; Catanzaro, I.; Saverini, M.; Barbata, G. Acrylamide catalytically inhibits topoisomerase II in V79 cells. Toxicol. Vitr. 2010, 24, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, J.G.; Castagnino, J.P.; Aidar, O.; Musella, R.M.; Frías, A.; Visca, M.; Nogueras, M.; Costa, L.; Perez, A.; Caradonna, F.; et al. Effect of gene-gene and gene-environment interactions associated with antituberculosis drug-induced hepatotoxicity. Pharm. Genom. 2017, 27, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Seol, J.E.; Kim, J.; Lee, B.H.; Hwang, D.Y.; Jeong, J.; Lee, H.J.; Ahn, Y.O.; Kim, D.H.; Lee, J.E. Red meat intake, CYP2E1 and PPARγ polymorphisms, and colorectal cancer risk. Eur. J. Cancer Prev. 2019, 28, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Negri, R.; Di Feola, M.; Di Domenico, S.; Scala, M.G.; Artesi, G.; Valente, S.; Smarrazzo, A.; Turco, F.; Morini, G.; Greco, L. Taste perception and food choices. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Larussa, T.; Imeneo, M.; Luzza, F. Olive tree biophenols in inflammatory bowel disease: When bitter is better. Int. J. Mol. Sci. 2019, 20, 1390. [Google Scholar] [CrossRef]
- Wang, J.C.; Hinrichs, A.L.; Bertelsen, S.; Stock, H.; Budde, J.P.; Dick, D.M.; Bucholz, K.K.; Rice, J.; Saccone, N.; Edenberg, H.J.; et al. Functional variants in TAS2R38 and TAS2R16 influence alcohol consumption in high-risk families of African American origin. Alcohol. Clin. Exp. Res 2007, 31, 209–215. [Google Scholar] [CrossRef]
- Keller, M.; Liu, X.; Wohland, T.; Rohde, K.; Gast, M.T.; Stumvoll, M.; Kovacs, P.; Tönjes, A.; Böttcher, Y. TAS2R38 and its influence on smoking behavior and glucose homeostasis in the German sorbs. PLoS ONE 2013, 8, e80512. [Google Scholar] [CrossRef]
- Coltell, O.; José, V.; Sorlí, J.V.; Asensio, E.M.; Fernández-Carrión, R.; Barragán, R.; Ortega-Azorín, C.; Estruch, R.; González, J.I.; Salas-Salvadó, J.; et al. Association between taste perception and adiposity in overweight or obese older subjects with metabolic syndrome and identification of novel taste-related genes. Am. J. Clin. Nutr. 2019, 109, 1709–1723. [Google Scholar] [CrossRef]
- Barragán, R.; Coltell, O.; Portolés, O.; Asensio, E.M.; Sorlí, J.V.; Ortega-Azorín, C.; González, J.I.; Sáiz, C.; Fernández-Carrión, R.; Ordovas, J.M.; et al. Bitter, sweet, salty, sour and umami taste reference associations in 18 to 80 year-old subjects. Nutrients 2018, 10, 1539. [Google Scholar] [CrossRef]
- Capurso, C.; Vendemiale, G. The Mediterranean diet reduces the risk and mortality of the prostate cancer: A narrative review. Front. Nutr. 2017, 4, 38. [Google Scholar] [CrossRef]
- Farinetti, A.; Zurlo, V.; Manenti, A.; Coppi, F.; Mattioli, A.V. Mediterranean diet and colorectal cancer: A systematic review. Nutrition 2017, 43, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health benefits of the Mediterranean diet: Metabolic and molecular mechanisms. J. Gerontol. Series A 2017. [Google Scholar] [CrossRef] [PubMed]
- Furtado, K.S.; De Oliveira-Andrade, F.; Campos, A.; Papaléo-Rosim, M.; Vargas-Mendez, E.; Henriques, A.; De Conti, A.; Scolastici, C.; Barbisan, L.F.; Carvalho, R.F.; et al. β-Ionone modulates the expression of mirnas and genes involved in the metastatic phenotype of microdissected persistent preneoplastic lesions in rats submitted to hepatocarcinogenesis. Mol. Carcinog. 2017, 56, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Bishayee, A.; Pandey, A.K. Targeting histone deacetylases with natural and synthetic agents: An emerging anticancer strategy. Nutrients 2018, 10, 731. [Google Scholar] [CrossRef]
- Mazzio, E.A.; Soliman, K.F.A. HTP nutraceutical screening for histone deacetylase inhibitors and effects of HDACis on tumor-suppressing miRNAs by trichostatin A and grapeseed (Vitis Vinifera) in HeLa cells. Cancer Genom. Proteom. 2017, 14, 17–33. [Google Scholar] [CrossRef][Green Version]
- Sabater, A.G.; Ribot, J.; Priego, T.; Vazquez, I.; Frank, S.; Palou, A.; Buchwald-Werner, S. Consumption of a mango fruit powder protects mice from high-fat induced insulin resistance and hepatic fat accumulation. Cell Physiol. Biochem. 2017, 42, 564–578. [Google Scholar] [CrossRef]
- Marotta, F.; Naito, Y.; Padrini, F.; Xuewei, X.; Jain, S.; Soresi, V.; Zhou, L.; Catanzaro, R.; Zhong, K.; Polimeni, A.; et al. Redox balance signalling in occupational stress: Modification by nutraceutical intervention. J. Biol. Regul. Homeost. Agents 2011, 25, 221–229. [Google Scholar]
- Menezo, Y.J.R.; Silvestris, E.; Dale, B.; Elder, K. Oxidative stress and alterations in DNA methylation: Two sides of the same coin in reproduction. Reprod. Biomed. Online 2016, 33, 668–683. [Google Scholar] [CrossRef]
| MD Food | Peculiar Contained Molecule(s) | Nutrigenomics Effect | Ref. | Notes |
|---|---|---|---|---|
| Whole meal bread | [Whole food] | Increase of genome-wide DNA methylation. | [90] | In vivo study (Leukocytes) in Caucasian women (Southern Italy) aged 12–87 years). |
| Rice | [Whole food] | Increase of genome-wide DNA methylation. | [90] | In vivo study (Leukocytes) in Caucasian women (Southern Italy) aged 12–87 years). |
| Tomato sauce | Lycopene |
| [91] | In vitro study (Wistar rats testis cells). |
| Parmigiano Reggiano or Grana Padano | β-casomorphin7 | Increasing of epigenetic-mediated expression of glutathione S transferase detoxifying enzyme. | [92] | |
| Pizza | [Whole food] | Increasing of genome-wide DNA methylation. | [90] | In vivo study (Leukocytes) in Caucasian women (Southern Italy) aged 12–87 years). |
| Peeled raw tomatoes | Lycopene | Anticancer activity related with p53, NF-κB, SIRT1 and with gut microbiome. | [93] | Both male and female animal model studies. |
| Mushrooms | o-orsellinaldehyde | Indirect inhibition of NF-κb, via IKK-2. | [94] | Present in Grifola frondosa Mushrooms. |
| Asparagus | Asparanin A | Induction of apoptosis via the PI3K/AKT/mTOR pathway. | [95] | Assessed in endometrial cells in vitro and in vivo. |
Typical salad:
| Quercetin (3,3′,4′,5,7-pentahydroxyflavone) |
| [96] | (a), (b) Assessed in VK2/E6E7 and End1/E6E7 human endometriosis cells; (c) Assessed in a mouse model. |
|
|
| [97] | Reported in glioblastoma cells. |
| Kaempferol | Inhibition of the proliferation of several cancer cell lines via the down-regulation of proteins involved in cancer progression, apoptosis induction, and cell cycle arrest. | [98] | In vitro studies. |
| [Whole food] | Significant inhibition of MCF-7 cancer cell proliferation. | [99] | In vitro study with an ethanol extract. |
| Carotenoids: lutein, lycopene, and β-carotene | Inhibition of the proliferation of some cancer cell lines at non-toxic concentrations. | [100] | In vitro study on MCF-7, NCI-H460, HeLa, and HepG2 cell lines. |
| Methyl cinnamate in essential oil | Cytotoxicity in HeLa, HEp-2, NIH, and 3T3 cell lines. | [101] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caradonna, F.; Consiglio, O.; Luparello, C.; Gentile, C. Science and Healthy Meals in the World: Nutritional Epigenomics and Nutrigenetics of the Mediterranean Diet. Nutrients 2020, 12, 1748. https://doi.org/10.3390/nu12061748
Caradonna F, Consiglio O, Luparello C, Gentile C. Science and Healthy Meals in the World: Nutritional Epigenomics and Nutrigenetics of the Mediterranean Diet. Nutrients. 2020; 12(6):1748. https://doi.org/10.3390/nu12061748
Chicago/Turabian StyleCaradonna, Fabio, Ornella Consiglio, Claudio Luparello, and Carla Gentile. 2020. "Science and Healthy Meals in the World: Nutritional Epigenomics and Nutrigenetics of the Mediterranean Diet" Nutrients 12, no. 6: 1748. https://doi.org/10.3390/nu12061748
APA StyleCaradonna, F., Consiglio, O., Luparello, C., & Gentile, C. (2020). Science and Healthy Meals in the World: Nutritional Epigenomics and Nutrigenetics of the Mediterranean Diet. Nutrients, 12(6), 1748. https://doi.org/10.3390/nu12061748








