In “High-Risk” Infants with Sufficient Vitamin D Status at Birth, Infant Vitamin D Supplementation Had No Effect on Allergy Outcomes: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
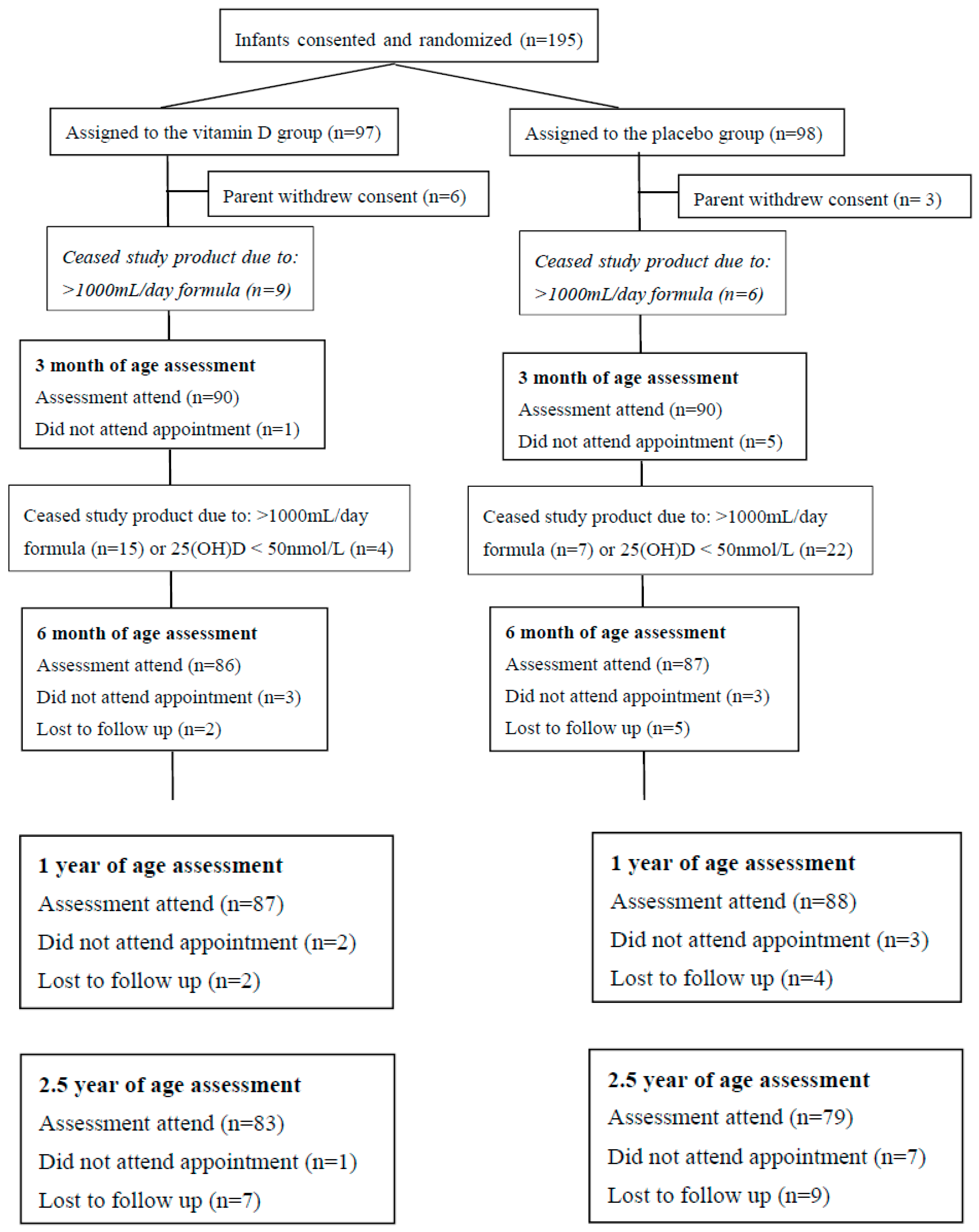
2.1. Study Population and Intervention
2.2. Allergic Disease Clinical Outcomes Assessments
2.3. Blood Collection and Measurement of 25-Hydroxyvitamin D (25(OH)D) Levels
2.4. Statistical Methods
3. Results
3.1. Study Population Characteristics
3.2. Intervention Period Trial Product Compliance and Infant Feeding
3.3. Longitudinal 25(OH)D Levels over the Course of the Study
3.4. Clinical Allergic Disease Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- D’Amato, G.; Holgate, S.T.; Pawankar, R.; Ledford, D.K.; Cecchi, L.; Al-Ahmad, M.; Baker, D.J. Meteorological conditions, climate change, new emerging factors, and asthma and related allergic disorders. A statement of the World Allergy Organization. World Allergy Organ. J. 2015, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Prescott, S.; Allen, K.J. Food allergy: Riding the second wave of the allergy epidemic. Pediatr. Allergy Immunol. 2011, 22, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Tooth, L.; Moss, K.; Hockey, R.; Mishra, G.D. Adherence to screen time recommendations for Australian children aged 0–12 years. Med. J. Aust. 2019, 211, 181–182. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. NEJM 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Saraf, R.; Morton, S.M.; Camargo, C.A.; Grant, C.C. Global summary of maternal and newborn vitamin D status–a systematic review. Matern Child. Nutr. 2016, 12, 647–668. [Google Scholar] [CrossRef] [PubMed]
- Bernard, J.J.; Gallo, R.L.; Krutmann, J. Photoimmunology: How ultraviolet radiation affects the immune system. Nat. Rev. Immunol. 2019, 19, 688–701. [Google Scholar] [CrossRef]
- Camargo, C.A.; Clark, S.; Kaplan, M.S.; Lieberman, P.; Wood, R.A. Regional differences in EpiPen prescriptions in the United States: The potential role of vitamin D. J. Allergy Clin. Immunol. 2007, 120, 131–136. [Google Scholar] [CrossRef]
- Mullins, R.J.; Camargo, C.A. Latitude, sunlight, vitamin D, and childhood food allergy/anaphylaxis. Curr. Allergy Asthma Rep. 2012, 12, 64–71. [Google Scholar] [CrossRef]
- Osborne, N.J.; Ukoumunne, O.C.; Wake, M.; Allen, K.J. Prevalence of eczema and food allergy is associated with latitude in Australia. J. Allergy Clin. Immunol. 2012, 129, 865–867. [Google Scholar] [CrossRef] [PubMed]
- Baiz, N.; Dargent-Molina, P.; Wark, J.D.; Souberbielle, J.C.; Annesi-Maesano, I. Group EM-CCS. Cord serum 25-hydroxyvitamin D and risk of early childhood transient wheezing and atopic dermatitis. J. Allergy Clin. Immunol. 2014, 133, 147–153. [Google Scholar] [CrossRef]
- Palmer, D.J.; Sullivan, T.R.; Skeaff, C.M.; Smithers, L.G.; Makrides, M. Team DOAF-u. Higher cord blood 25-hydroxyvitamin D concentrations reduce the risk of early childhood eczema: In children with a family history of allergic disease. World Allergy Organ. J. 2015, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.P.; Palmer, D.; Zhang, G.; Prescott, S.L. Cord blood 25-hydroxyvitamin D3 and allergic disease during infancy. Pediatrics 2012, 130, e1128–e1135. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.J.; Koplin, J.J.; Ponsonby, A.L.; Gurrin, L.C.; Wake, M.; Vuillermin, P.; Tey, D. Vitamin D insufficiency is associated with challenge-proven food allergy in infants. J. Allergy Clin. Immunol. 2013, 131, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Aryan, Z.; Rezaei, N.; Camargo, C.A. Vitamin D status, aeroallergen sensitization, and allergic rhinitis: A systematic review and meta-analysis. Int. Rev. Immunol. 2017, 36, 41–53. [Google Scholar] [CrossRef]
- Chawes, B.L.; Bønnelykke, K.; Stokholm, J.; Vissing, N.H.; Bjarnadóttir, E.; Schoos, A.M.M.; Arianto, L. Effect of Vitamin D3 Supplementation During Pregnancy on Risk of Persistent Wheeze in the Offspring: A Randomized Clinical Trial. JAMA 2016, 315, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Litonjua, A.A.; Carey, V.J.; Laranjo, N.; Harshfield, B.J.; McElrath, T.F.; O’Connor, G.T.; Bacharier, L.B. Effect of Prenatal Supplementation With Vitamin D on Asthma or Recurrent Wheezing in Offspring by Age 3 Years: The VDAART Randomized Clinical Trial. JAMA 2016, 315, 362–370. [Google Scholar] [CrossRef]
- Grant, C.C.; Crane, J.; Mitchell, E.A.; Sinclair, J.; Stewart, A.; Milne, T.; Camargo, C.A. Vitamin D supplementation during pregnancy and infancy reduces aeroallergen sensitisation: A randomized controlled trial. Allergy 2016, 71, 1325–1334. [Google Scholar] [CrossRef]
- Goldring, S.T.; Griffiths, C.J.; Martineau, A.R.; Robinson, S.; Yu, C.; Poulton, S.; Warner, J.O. Prenatal vitamin D supplementation and child respiratory health: A randomized controlled trial. PLoS ONE 2013, 8, e66627. [Google Scholar] [CrossRef]
- Garcia-Larsen, V.; Ierodiakonou, D.; Jarrold, K.; Cunha, S.; Chivinge, J.; Robinson, Z.; Leonardi-Bee, J. Diet during pregnancy and infancy and risk of allergic or autoimmune disease: A systematic review and meta-analysis. PLoS Med. 2018, 15, e1002507. [Google Scholar] [CrossRef]
- Hollis, B.W.; Wagner, C.L. Vitamin D requirements during lactation: High-dose maternal supplementation as therapy to prevent hypovitaminosis D for both the mother and the nursing infant. Am. J. Clin. Nutr. 2004, 80, 1752S–1758S. [Google Scholar] [CrossRef]
- Norizoe, C.; Akiyama, N.; Segawa, T.; Tachimoto, H.; Mezawa, H.; Ida, H.; Urashima, M. Increased food allergy and vitamin D: Randomized, double-blind, placebo-controlled trial. Pediatr. Int. 2014, 56, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Rueter, K.; Jones, A.P.; Siafarikas, A.; Lim, E.M.; Bear, N.; Noakes, P.S.; Palmer, D.J. Direct infant UV light exposure is associated with eczema and immune development. J. Allergy Clin. Immunol. 2019, 143, 1012–1120. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.L.; Greer, F.R.; American Academy of Pediatrics Section on Breastfeeding; American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics 2008, 122, 1142–1152. [Google Scholar] [CrossRef] [PubMed]
- Hanifin, J.M. Atopic dermatitis: Broadening the perspective. J. Am. Acad. Dermatol. 2004, 51, S23–S24. [Google Scholar] [CrossRef]
- Rosendahl, J.; Pelkonen, A.S.; Helve, O.; Hauta-alus, H.; Holmlund-Suila, E.; Valkama, S.; Andersson, S. High-Dose Vitamin D Supplementation Does Not Prevent Allergic Sensitization of Infants. J. Pediatr. 2019, 209, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Paxton, G.A.; Teale, G.R.; Nowson, C.A.; Mason, R.S.; McGrath, J.J.; Thompson, M.J.; Munns, C.F. Vitamin D and health in pregnancy, infants, children and adolescents in Australia and New Zealand: A position statement. MJA 2013, 198, 142–143. [Google Scholar] [CrossRef]
- Munns, C.F.; Shaw, N.; Kiely, M.; Specker, B.L.; Thacher, T.D.; Ozono, K.; Ramos-Abad, L. Global consensus recommendations on prevention and management of nutritional rickets. J. Clin. Endocrinol. Metab. 2016, 101, 394–415. [Google Scholar] [CrossRef]
- Grossman, Z.; Hadjipanayis, A.; Stiris, T.; Del Torso, S.; Mercier, J.C.; Valiulis, A.; Shamir, R. Vitamin D in European children–Statement from the European Academy of Paediatrics. Eur. J. Pediatr. 2017, 176, 829–831. [Google Scholar] [CrossRef]
- Hibbs, A.M.; Ross, K.; Kerns, L.A.; Wagner, C.; Fuloria, M.; Groh-Wargo, S.; Tatsuoka, C. Effect of Vitamin D Supplementation on Recurrent Wheezing in Black Infants Who Were Born Preterm: The D-Wheeze Randomized Clinical Trial. JAMA 2018, 319, 2086–2094. [Google Scholar] [CrossRef]
- Zosky, G.R.; Berry, L.J.; Elliot, J.G.; James, A.L.; Gorman, S.; Hart, P.H. Vitamin D deficiency causes deficits in lung function and alters lung structure. Am. J. Respir. Crit. Care Med. 2011, 183, 1336–1343. [Google Scholar] [CrossRef]
- Been, J.V.; Lugtenberg, M.J.; Smets, E.; van Schayck, C.P.; Kramer, B.W.; Mommers, M.; Sheikh, A. Preterm birth and childhood wheezing disorders: A systematic review and meta-analysis. PLoS Med. 2014, 11, e1001596. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, A.; Navid, F.; Sparwasser, T.; Clausen, B.E.; Schwarz, T. 1,25-dihydroxyvitamin D exerts similar immunosuppressive effects as UVR but is dispensable for local UVR-induced immunosuppression. J. Investig. Dermatol. 2012, 132, 2762–2769. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, S.; Nishioka, A.; Kasuya, S.; Ohkura, N.; Hemmi, H.; Kaisho, T.; Morita, A. Homeostasis of thymus-derived Foxp3+ regulatory T cells is controlled by ultraviolet B exposure in the skin. J. Immunol. 2014, 193, 5488–5497. [Google Scholar] [CrossRef]
- Ng, R.L.; Scott, N.M.; Bisley, J.L.; Lambert, M.J.; Gorman, S.; Norval, M.; Hart, P.H. Characterization of regulatory dendritic cells differentiated from the bone marrow of UV-irradiated mice. Immunology 2013, 140, 399–412. [Google Scholar] [CrossRef]
- Schwarz, A.; Schwarz, T. UVR-induced regulatory T cells switch antigen-presenting cells from a stimulatory to a regulatory phenotype. J. Investig. Dermatol 2010, 130, 1914–1921. [Google Scholar] [CrossRef]
- Byrne, S.N.; Limon-Flores, A.Y.; Ullrich, S.E. Mast cell migration from the skin to the draining lymph nodes upon ultraviolet irradiation represents a key step in the induction of immune suppression. J. Immunol. 2008, 180, 4648–4655. [Google Scholar] [CrossRef]
- Byrne, S.N.; Halliday, G.M. B cells activated in lymph nodes in response to ultraviolet irradiation or by interleukin-10 inhibit dendritic cell induction of immunity. J. Investig. Dermatol. 2005, 124, 570–578. [Google Scholar] [CrossRef]
- Gorman, S.; Scott, N.M.; Tan, D.H.; Weeden, C.E.; Tuckey, R.C.; Bisley, J.L.; Hart, P.H. Acute erythemal ultraviolet radiation causes systemic immunosuppression in the absence of increased 25-hydroxyvitamin D3 levels in male mice. PLoS ONE 2012, 7, e46006. [Google Scholar] [CrossRef]
- Sleijffers, A.; Kammeyer, A.; de Gruijl, F.R.; Boland, G.J.; van Hattum, J.; van Vloten, W.A.; Garssen, J. Epidermal cis-urocanic acid levels correlate with lower specific cellular immune responses after hepatitis B vaccination of ultraviolet B-exposed humans. Photochem. Photobiol. 2003, 77, 271–275. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Hiromasa, K.; Kabashima-Kubo, R.; Yoshioka, M.; Nakamura, M. Galectin-7, induced by cis-urocanic acid and ultraviolet B irradiation, down-modulates cytokine production by T lymphocytes. Exp. Dermatol. 2013, 22, 840–842. [Google Scholar] [CrossRef]
- Nagy, G.; Clark, J.M.; Buzas, E.I.; Gorman, C.L.; Cope, A.P. Nitric oxide, chronic inflammation and autoimmunity. Immunol. Lett. 2007, 111, 1–5. [Google Scholar] [CrossRef] [PubMed]
| Time Point | Vitamin D Group | Placebo Group | p-Value |
|---|---|---|---|
| Cord Blood | 67.8 (17.5) | 61.1 (14.2) | 0.17 |
| 3 months of age | 83.2 (27.8) | 59.2 (22.7) | <0.01 |
| 6 months of age | 93.1 (28.7) | 82.0 (27.9) | 0.02 |
| 1 year of age | 78.4 (21.9) | 81.2 (23.5) | 0.45 |
| 2.5 years of age 1 | 79.0 (69.0–110.0) | 74.0 (60.0–86.5) | 0.19 |
| Allergic Disease | Vitamin D Group | Placebo Group | Relative Risk (95% CI) | p-Value |
|---|---|---|---|---|
| Eczema at 1 year | 30/87 (34.5) | 21/88 (23.9) | 1.45 (0.90–2.32) | 0.17 |
| Eczema at 2.5 years | 33/83 (39.8) | 26/78 (33.3) | 1.19 (0.79–1.80) | 0.50 |
| Food allergy at 1 year | 6/87 (6.9) | 5/88 (5.7) | 1.21 (0.39–3.83) | 0.98 |
| Food allergy at 2.5 years | 3/83 (3.6) | 6/79 (7.6) | 0.48 (0.12–1.84) | 0.45 |
| Wheeze at 1 year | 23/87 (26.4) | 14/88 (15.9) | 1.66 (0.92–3.01) | 0.13 |
| Asthma and/or wheeze at 2.5 years | 25/83 (30.1) | 18/79 (22.8) | 1.32 (0.79–2.23) | 0.38 |
| Allergic rhinitis at 2.5 years | 16/83(19.3) | 11/78 (14.1) | 1.37 (0.68–2.76) | 0.51 |
| Sensitization at 1 year | 10/85 (11.8) | 16/82 (19.5) | 0.60 (0.29–1.25) | 0.24 |
| Sensitization at 2.5 years | 17/69 (24.6) | 14/67 (20.9) | 1.18 (0.63–2.20) | 0.75 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rueter, K.; Jones, A.P.; Siafarikas, A.; Lim, E.-M.; Prescott, S.L.; Palmer, D.J. In “High-Risk” Infants with Sufficient Vitamin D Status at Birth, Infant Vitamin D Supplementation Had No Effect on Allergy Outcomes: A Randomized Controlled Trial. Nutrients 2020, 12, 1747. https://doi.org/10.3390/nu12061747
Rueter K, Jones AP, Siafarikas A, Lim E-M, Prescott SL, Palmer DJ. In “High-Risk” Infants with Sufficient Vitamin D Status at Birth, Infant Vitamin D Supplementation Had No Effect on Allergy Outcomes: A Randomized Controlled Trial. Nutrients. 2020; 12(6):1747. https://doi.org/10.3390/nu12061747
Chicago/Turabian StyleRueter, Kristina, Anderson P. Jones, Aris Siafarikas, Ee-Mun Lim, Susan L. Prescott, and Debra J. Palmer. 2020. "In “High-Risk” Infants with Sufficient Vitamin D Status at Birth, Infant Vitamin D Supplementation Had No Effect on Allergy Outcomes: A Randomized Controlled Trial" Nutrients 12, no. 6: 1747. https://doi.org/10.3390/nu12061747
APA StyleRueter, K., Jones, A. P., Siafarikas, A., Lim, E.-M., Prescott, S. L., & Palmer, D. J. (2020). In “High-Risk” Infants with Sufficient Vitamin D Status at Birth, Infant Vitamin D Supplementation Had No Effect on Allergy Outcomes: A Randomized Controlled Trial. Nutrients, 12(6), 1747. https://doi.org/10.3390/nu12061747





