Faster Short-Chain Fatty Acid Absorption from the Cecum Following Polydextrose Ingestion Increases the Salivary Immunoglobulin A Flow Rate in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Diets
2.3. Sampling
2.4. Collection of Saliva
2.5. Measurement of IgA Concentration
2.6. Measurement of IFN-γ Concentration
2.7. Measurement of IL-17A Concentration
2.8. Measurement of Cecal Digesta Water Content
2.9. Measurement of Cecal Digesta Viscosity
2.10. RNA Extraction, cDNA Synthesis, and Quantitative Real-Time PCR Measurement of pIgR mRNA in Submandibular Glands
2.11. Measurement of SCFA in Cecal Digesta
2.12. Portal Vein Blood Preparation
2.13. Measurement of SCFA in Portal Vein Blood
2.14. Bayesian Network
2.15. Statistical Analysis
3. Results
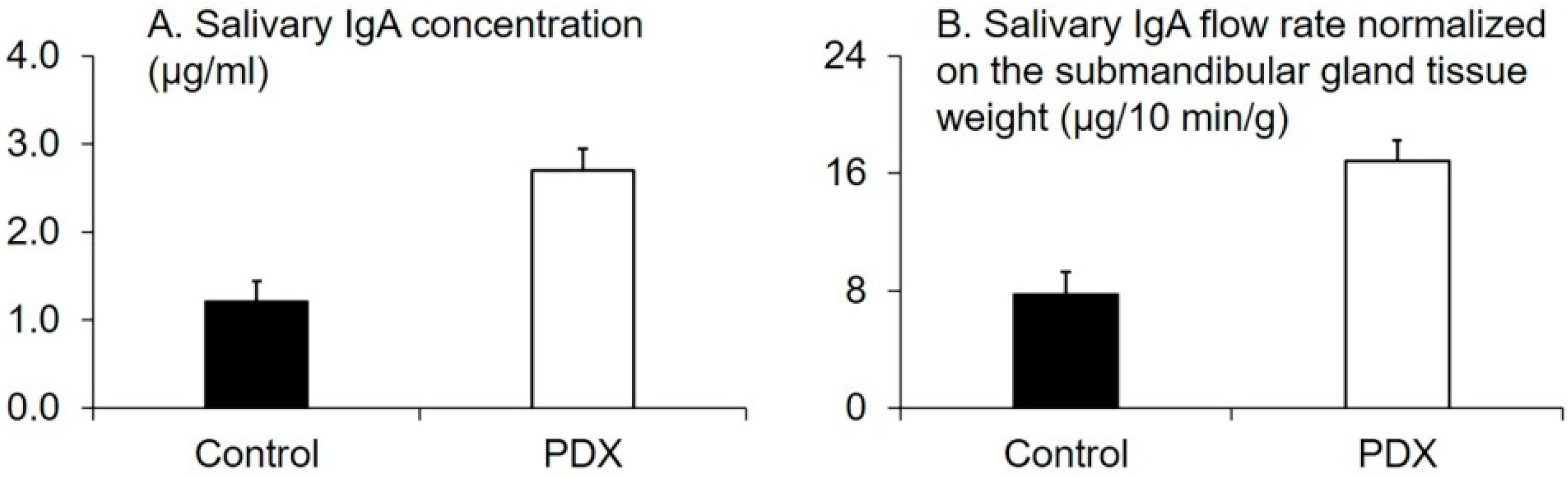
3.1. Effect of PDX Ingestion on Salivary IgA Production and SCFA-Induced Changes
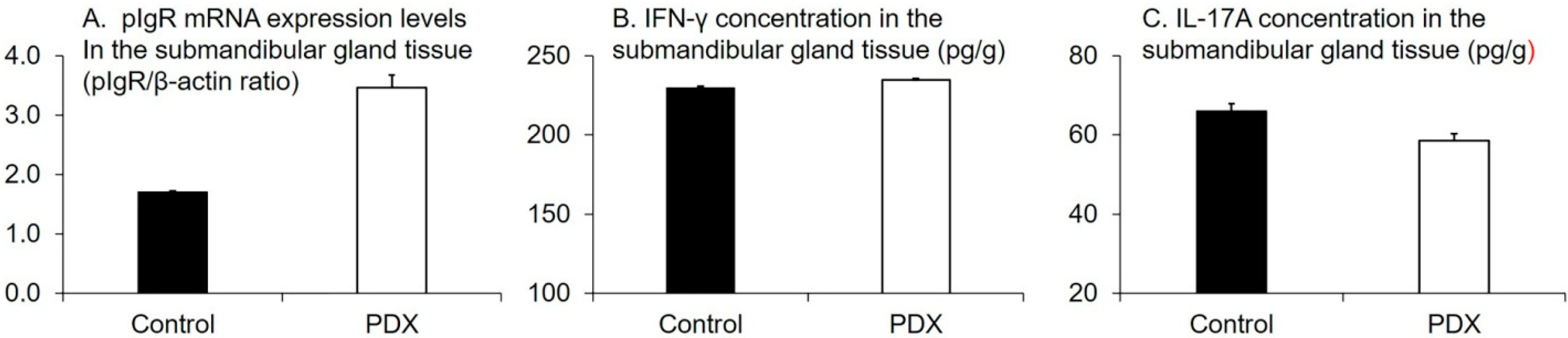
3.2. Correlation between SCFAs, IgA, and pIgR Parameters
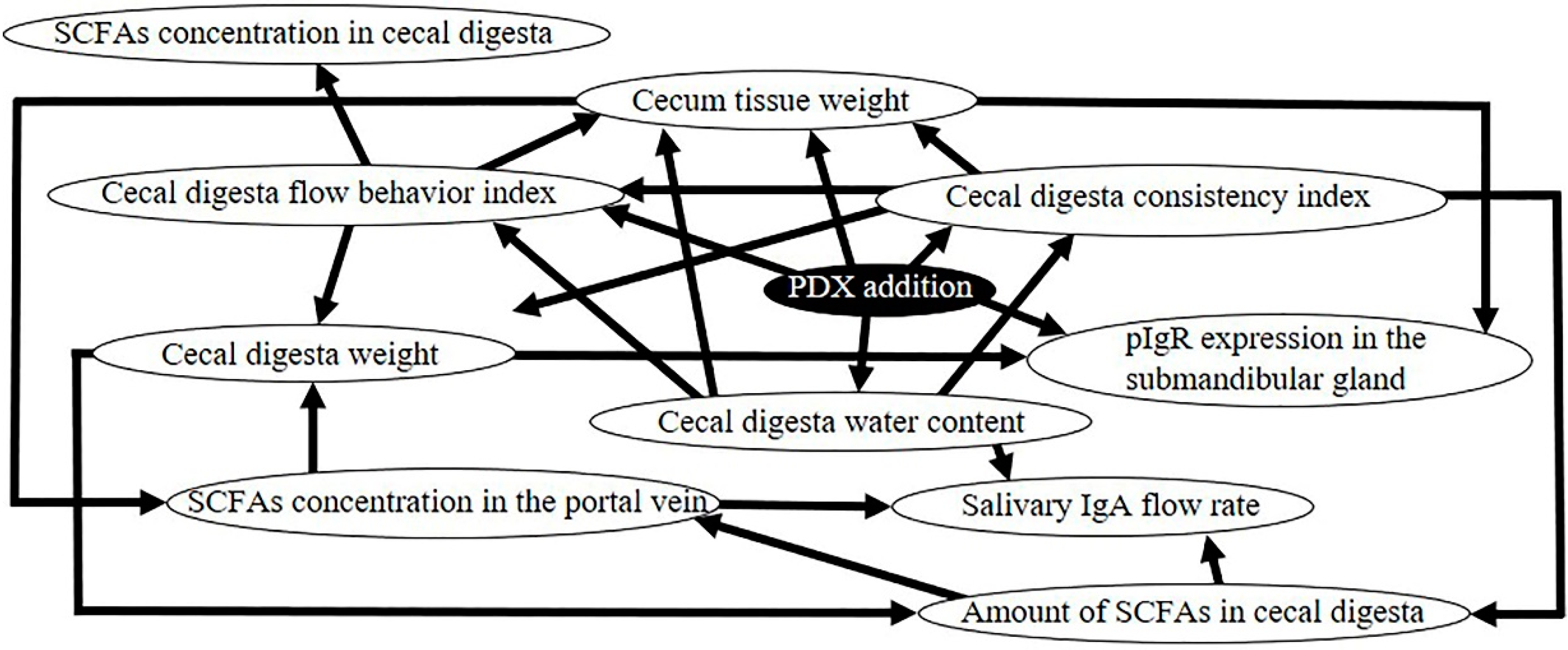
3.3. Bayesian Network of the Causal Effects Induced by PDX Ingestion
3.4. Factors Related to the Increase of SCFA Concentration in Portal Blood
4. Discussion
4.1. Production of SCFAs in the Cecum by PDX Ingestion
4.2. SCFAs’ Role in Salivary IgA Activation
4.3. pIgR and Sympathetic and Parasympathetic Nerves
4.4. Differences Between Regulatory Mechanisms of pIgR Expression in Salivary Glands and pIgR Expression in the Intestinal Tract
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Morris, I.R. Functional anatomy of the upper airway. Emerg. Med. Clin. N. Am. 1988, 6, 639–669. [Google Scholar] [PubMed]
- Diamond, G.; Beckloff, N.; Ryan, L.K. Host defense peptides in the oral cavity and the lung: Similarities and differences. J. Dent. Res. 2008, 87, 915–927. [Google Scholar] [CrossRef] [PubMed]
- Kiyono, H.; Azegami, T. The mucosal immune system: From dentistry to vaccine development. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2015, 91, 423–439. [Google Scholar] [CrossRef]
- Pedersen, A.M.L.; Sørensen, C.E.; Proctor, G.B.; Carpenter, G.H.; Ekström, J. Salivary secretion in health and disease. J. Oral Rehabil. 2018, 45, 730–746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Z.; Cheng, X.Q.; Li, J.Y.; Zhang, P.; Yi, P.; Xu, X.; Zhou, X.D. Saliva in the diagnosis of diseases. Int. J. Oral Sci. 2016, 8, 133–137. [Google Scholar] [CrossRef]
- Shimizu, K.; Sato, H.; Suga, Y.; Yamahira, S.; Toba, M.; Hamuro, K.; Kakumoto, K.; Kohda, N.; Akama, T.; Kono, I.; et al. The effects of Lactobacillus pentosus strain b240 and appropriate physical training on salivary secretory IgA levels in elderly adults with low physical fitness: A randomized, double-blind, placebo-controlled trial. J. Clin. Biochem. Nutr. 2014, 54, 61–66. [Google Scholar] [CrossRef]
- Brandtzaeg, P. Secretory immunity with special reference to the oral cavity. J. Oral Microbiol. 2013, 5, 20401. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Sugimoto, N.; Islam, R.; Hossain, M.E.; Sumiyoshi, E.; Katakura, M.; Shido, O. Salivary immunoglobulin a secretion and polymeric ig receptor expression in the submandibular glands are enhanced in heat-acclimated rats. Int. J. Mol. Sci. 2020, 21, 815. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.; Mortatti, A.L.; Arruda, A.F.; Freitas, C.G.; de Arruda, M.; Aoki, M.S. Salivary IgA response and upper respiratory tract infection symptoms during a 21-week competitive season young soccer players. J. Strength Cond. Res. 2014, 28, 467–473. [Google Scholar] [CrossRef]
- McFarlin, B.K.; Carpenter, K.C.; Davidson, T.; McFarlin, M.A. Baker’s yeast beta glucan supplementation increases salivary IgA and decreases cold/flu symptomatic days after intense exercise. J. Diet. Suppl. 2013, 10, 171–183. [Google Scholar] [CrossRef]
- Nieman, D.C.; Henson, D.A.; Gusewitch, G.; Warren, B.J.; Dotson, R.C.; Butterworth, D.E.; Nehlsen-Cannarella, S.L. Physical activity and immune function in elderly women. Med. Sci. Sports Exerc. 1993, 25, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Miao, Y.; Zeng, J.; Jiang, W.; Shen, Y.M.; Deng, Q. Prenatal exposure to ambient temperature variation increases the risk of common cold in children. Ecotoxicol. Environ. Saf. 2018, 154, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Akash, M.S.H.; Rehman, K.; Fiayyaz, F.; Sabir, S.; Khurshid, M. Diabetes-associated infections: Development of antimicrobial resistance and possible treatment strategies. Arch. Microbiol. 2020, 3, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; McKenzie, C.; Vuillermin, P.J.; Goverse, G.; Vinuesa, C.G.; Mebius, R.E.; Macia, L.; Mackay, C.R. Dietary fiber and bacterial scfa enhance oral tolerance and protect against food allergy through diverse cellular pathways. Cell Rep. 2016, 15, 2809–2824. [Google Scholar] [CrossRef]
- Bai, G.; Ni, K.; Tsuruta, T.; Nishino, N. Dietary casein and soy protein isolate modulate the effects of raffinose and fructooligosaccharides on the composition and fermentation of gut microbiota in rats. J. Food Sci. 2016, 81, H2093–H2098. [Google Scholar] [CrossRef]
- Lecerf, J.M.; Dépeint, F.; Clerc, E.; Dugenet, Y.; Niamba, C.N.; Rhazi, L.; Cayzeele, A.; Abdelnour, G.; Jaruga, A.; Younes, H.; et al. Xylo-oligosaccharide (XOS) in combination with inulin modulates both the intestinal environment and immune status in healthy subjects, while XOS alone only shows prebiotic properties. Br. J. Nutr. 2012, 108, 1847–1858. [Google Scholar] [CrossRef]
- Komura, M.; Fukuta, T.; Genda, T.; Hino, S.; Aoe, S.; Kawagishi, H.; Morita, T. A short-term ingestion of fructo-oligosaccharides increases immunoglobulin A and mucin concentrations in the rat cecum, but the effects are attenuated with the prolonged ingestion. Biosci. Biotechnol. Biochem. 2014, 78, 1592–1602. [Google Scholar] [CrossRef]
- Genda, T.; Sasaki, Y.; Kondo, T.; Hino, S.; Nishimura, N.; Tsukahara, T.; Sonoyama, K.; Morita, T. Fructo-oligosaccharide-induced transient increases in cecal immunoglobulin A concentrations in rats are associated with mucosal inflammation in response to increased gut permeability. J. Nutr. 2017, 147, 1900–1908. [Google Scholar] [CrossRef]
- Scalabrin, D.M.; Mitmesser, S.H.; Welling, G.W.; Harris, C.L.; Marunycz, J.D.; Walker, D.C.; Bos, N.A.; Tölkkö, S.; Salminen, S.; Vanderhoof, J.A. New prebiotic blend of polydextrose and galacto-oligosaccharides has a bifidogenic effect in young infants. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 343–352. [Google Scholar] [CrossRef]
- Peuranen, S.; Tiihonen, K.; Apajalahti, J.; Kettunen, A.; Saarinen, M.; Rautonen, N. Combination of polydextrose and lactitol affects microbial ecosystem and immune responses in rat gastrointestinal tract. Br. J. Nutr. 2004, 91, 905–914. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kubota, N.; Takahashi, T.; To, M.; Hayashi, T.; Shimizu, T.; Kamata, Y.; Saruta, J.; Tsukinoki, K. Continuous combined intake of polydextrose and lactitol stimulates cecal fermentation and salivary IgA secretion in rats. J. Oral Sci. 2017, 59, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; To, M.; Hayashi, T.; Shimizu, T.; Kamata, Y.; Saruta, J.; Takahashi, T.; Tsukinoki, K. Intake of indigestible carbohydrates influences IgA response and polymeric Ig receptor expression in the rat submandibular gland. Br. J. Nutr. 2015, 113, 1895–1902. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Takahashi, T.; To, M.; Nakagawa, Y.; Hayashi, T.; Shimizu, T.; Kamata, Y.; Saruta, J.; Tsukinoki, K. The salivary IgA flow rate is increased by high concentrations of short-chain fatty acids in the cecum of rats ingesting fructooligosaccharides. Nutrients 2016, 8, 500. [Google Scholar] [CrossRef] [PubMed]
- Sepehr, E.; Peace, R.W.; Storey, K.B.; Jee, P.; Lampi, B.J.; Brooks, S.P. Folate derived from cecal bacterial fermentation does not increase liver folate stores in 28-d folate-depleted male Sprague-Dawley rats. J. Nutr. 2003, 133, 1347–1354. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takahashi, T.; Sakata, T. Large particles increase viscosity and yield stress of pig cecal contents without changing basic viscoelastic properties. J. Nutr. 2002, 132, 1026–1030. [Google Scholar] [CrossRef][Green Version]
- Takahashi, T. Flow behavior of digesta and the absorption of nutrients in the gastrointestine. J. Nutr. Sci. Vitaminol. (Tokyo) 2011, 57, 265–273. [Google Scholar] [CrossRef]
- Tsukahara, T.; Matsukawa, N.; Tomonaga, S.; Inoue, R.; Ushida, K.; Ochiai, K. High-sensitivity detection of short-chain fatty acids in porcine ileal, cecal, portal and abdominal blood by gas chromatography-mass spectrometry. Anim. Sci. J. 2014, 85, 494–498. [Google Scholar] [CrossRef]
- Maglogiannis, I.; Zafiropoulos, E.; Platis, A.; Lambrinoudakis, C. Risk analysis of a patient monitoring system using Bayesian Network modeling. J. Biomed. Inf. 2006, 39, 637–647. [Google Scholar] [CrossRef]
- Nakatani, M.; Inoue, R.; Tomonaga, S.; Fukuta, K.; Tsukahara, T. Production, Absorption, and blood flow dynamics of short-chain fatty acids produced by fermentation in piglet hindgut during the suckling-weaning period. Nutrients 2018, 10, 1220. [Google Scholar] [CrossRef]
- Yu, B.; Chiou, W.S. The morphological changes of intestinal mucosa in growing rabbits. Lab. Anim. 1997, 31, 254–263. [Google Scholar] [CrossRef]
- Yoshioka, M.; Shimomura, Y.; Suzuki, M. Dietary polydextrose affects the large intestine in rats. J. Nutr. 1994, 124, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Lobo, A.R.; Colli, C.; Alvares, E.P.; Filisetti, T.M. Effects of fructans-containing yacon (Smallanthus sonchifolius Poepp and Endl.) flour on caecum mucosal morphometry, calcium and magnesium balance, and bone calcium retention in growing rats. Br. J. Nutr. 2007, 97, 776–785. [Google Scholar] [CrossRef] [PubMed]
- McRorie, J.; Brown, S.; Cooper, R.; Givaruangsawat, S.; Scruggs, D.; Boring, G. Effects of dietary fibre and olestra on regional apparent viscosity and water content of digesta residue in porcine large intestine. Aliment. Pharmacol. Ther. 2000, 14, 471–477. [Google Scholar] [CrossRef] [PubMed]
- McRorie, J.; Pepple, S.; Rudolph, C. Effects of fiber laxatives and calcium docusate on regional water content and viscosity of digesta in the large intestine of the pig. Dig. Dis. Sci. 1998, 43, 738–745. [Google Scholar] [CrossRef]
- Takahashi, T.; Karita, S.; Ogawa, N.; Goto, M. Crystalline cellulose reduces plasma glucose concentrations and stimulates water absorption by increasing the digesta viscosity in rats. J. Nutr. 2005, 135, 2405–2410. [Google Scholar] [CrossRef]
- Hara, H.; Suzuki, T.; Aoyama, Y. Ingestion of the soluble dietary fibre, polydextrose, increases calcium absorption and bone mineralization in normal and total-gastrectomized rats. Br. J. Nutr. 2000, 84, 655–661. [Google Scholar] [CrossRef]
- Probert, H.M.; Apajalahti, J.H.; Rautonen, N.; Stowell, J.; Gibson, G.R. Polydextrose, lactitol, and fructo-oligosaccharide fermentation by colonic bacteria in a three-stage continuous culture system. Appl. Environ. Microbiol. 2004, 70, 4505–4511. [Google Scholar] [CrossRef]
- Beards, E.; Tuohy, K.; Gibson, G. Bacterial, SCFA and gas profiles of a range of food ingredients following in vitro fermentation by human colonic microbiota. Anaerobe 2010, 16, 420–425. [Google Scholar] [CrossRef]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef]
- Fuller, M.; Priyadarshini, M.; Gibbons, S.M.; Angueira, A.R.; Brodsky, M.; Hayes, M.G.; Kovatcheva-Datchary, P.; Bäckhed, F.; Gilbert, J.A.; Lowe, W.L., Jr.; et al. The short-chain fatty acid receptor, FFA2, contributes to gestational glucose homeostasis. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E840–E851. [Google Scholar] [CrossRef]
- Onyszkiewicz, M.; Gawrys-Kopczynska, M.; Konopelski, P.; Aleksandrowicz, M.; Sawicka, A.; Koźniewska, E.; Samborowska, E.; Ufnal, M. Butyric acid, a gut bacteria metabolite, lowers arterial blood pressure via colon-vagus nerve signaling and GPR41/43 receptors. Pflugers Arch. 2019, 471, 1441–1453. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, G.H.; Garrett, J.R.; Hartley, R.H.; Proctor, G.B. The influence of nerves on the secretion of immunoglobulin A into submandibular saliva in rats. J. Physiol. 1998, 512, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, G.H.; Proctor, G.B.; Anderson, L.C.; Zhang, X.S.; Garrett, J.R. Immunoglobulin A secretion into saliva during dual sympathetic and parasympathetic nerve stimulation of rat submandibular glands. Exp. Physiol. 2000, 85, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, G.H.; Proctor, G.B.; Ebersole, L.E.; Garrett, J.R. Secretion of IgA by rat parotid and submandibular cells in response to autonomimetic stimulation in vitro. Int. Immunopharmacol. 2004, 4, 1005–1014. [Google Scholar] [CrossRef]
- Carpenter, G.H.; Proctor, G.B.; Garrett, J.R. Preganglionic parasympathectomy decreases salivary SIgA secretion rates from the rat submandibular gland. J. Neuroimmunol. 2005, 160, 4–11. [Google Scholar] [CrossRef]
- Wada, M.; Orihara, K.; Kamagata, M.; Hama, K.; Sasaki, H.; Haraguchi, A.; Miyakawa, H.; Nakao, A.; Shibata, S. Circadian clock-dependent increase in salivary IgA secretion modulated by sympathetic receptor activation in mice. Sci. Rep. 2017, 7, 8802. [Google Scholar] [CrossRef]
- Sakata, T.; von Engelhardt, W. Stimulatory effect of short chain fatty acids on the epithelial cell proliferation in rat large intestine. Comp. Biochem. Physiol. A Comp. Physiol. 1983, 74, 459–462. [Google Scholar] [CrossRef]
- Nakamura, Y.; Nosaka, S.; Suzuki, M.; Nagafuchi, S.; Takahashi, T.; Yajima, T.; Takenouchi-Ohkubo, N.; Iwase, T.; Moro, I. Dietary fructooligosaccharides up-regulate immunoglobulin A response and polymeric immunoglobulin receptor expression in intestines of infant mice. Clin. Exp. Immunol. 2004, 137, 52–58. [Google Scholar] [CrossRef]
- Kaetzel, C.S. Cooperativity among secretory IgA, the polymeric immunoglobulin receptor, and the gut microbiota promotes host-microbial mutualism. Immunol. Lett. 2014, 162, 10–21. [Google Scholar] [CrossRef]
- Proctor, G.B. The physiology of salivary secretion. Periodontology 2000 2016, 70, 11–25. [Google Scholar] [CrossRef]
- Garrett, J.R. The proper role of nerves in salivary secretion: A review. J. Dent. Res. 1987, 66, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Freeman, K.; Foy, T.; Feste, A.S.; Reeds, P.J.; Lifschitz, C.H. Colonic acetate in the circulating acetate pool of the infant pig. Pediatr. Res. 1993, 34, 318–322. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vissink, A.; Kalicharan, D.; S-Gravenmade, E.J.; Jongebloed, W.L.; Ligeon, E.E.; Nieuwenhuis, P.; Konings, A.W. Acute Irradiation Effects on Morphology and Function of Rat Submandibular Glands. J. Oral. Pathol. Med. 1991, 20, 449–456. [Google Scholar] [CrossRef] [PubMed]


| Acids | Control | PDX * | p-Value † | ||
|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | ||
| Acetate | 23.3 | 1.19 | 15.7 | 1.59 | 0.004 |
| Propionate | 7.43 | 0.58 | 3.81 | 0.56 | 0.001 |
| n-Butyrate | 5.36 | 1.05 | 5.14 | 0.68 | 0.9 |
| SCFAs total | 36.1 | 1.97 | 24.7 | 2.75 | 0.008 |
| Acids | Control | PDX * | p-Value † | ||
|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | ||
| Acetate | 227.2 | 16.48 | 305.8 | 13.84 | 0.005 |
| Propionate | 43.62 | 3.270 | 42.80 | 6.722 | 0.9 |
| n-Butyrate | 27.93 | 3.318 | 46.90 | 6.221 | 0.03 |
| SCFAs total | 298.7 | 19.20 | 395.5 | 16.92 | 0.004 |
| Acids | Control | PDX * | p-Value † | ||
|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | ||
| Acetate | 41.2 | 2.78 | 77.8 | 9.42 | 0.005 |
| Propionate | 13.1 | 1.24 | 18.9 | 3.08 | 0.06 |
| n-Butyrate | 9.63 | 2.08 | 25.4 | 3.73 | 0.003 |
| SCFAs total | 63.9 | 5.04 | 122 | 15.9 | 0.007 |
| Salivary IgA Flow Rate Normalized on the Submandibular Gland Tissue Weight | |||
|---|---|---|---|
| rs * | p-Value | n | |
| Concentration of SCFAs in portal vein blood | 0.88 | 0.0002 | 12 |
| Weight of cecal tissue | 0.76 | 0.004 | 12 |
| Water content of cecal digesta | 0.76 | 0.005 | 12 |
| pIgR expression level in submandibular gland | 0.66 | 0.02 | 12 |
| Weight of cecal digesta | 0.60 | 0.04 | 12 |
| Concentration of SCFAs in cecal digesta | −0.53 | 0.09 | 12 |
| Concentration of SCFAs in the Portal Vein | |||
|---|---|---|---|
| rs * | p-Value | n | |
| Cecal tissue weight | 0.74 | 0.006 | 12 |
| Cecal digesta weight | 0.69 | 0.01 | 12 |
| Amount of SCFAs in cecal digesta | 0.66 | 0.02 | 12 |
| Cecal digesta water content | 0.63 | 0.03 | 12 |
| pIgR Expression Level in Submandibular Gland Tissue | |||
|---|---|---|---|
| rs * | p-Value | n | |
| Cecal tissue weight | 0.87 | 0.0003 | 12 |
| Cecal digesta weight | 0.77 | 0.003 | 12 |
| Concentration of SCFAs in the portal vein | 0.71 | 0.009 | 12 |
| Concentration of IFN-γ in the submandibular gland tissue | −0.046 | 0.9 | 12 |
| Concentration of IL-17A in the submandibular gland tissue | −0.028 | 0.9 | 12 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto, Y.; Morozumi, T.; Takahashi, T.; Saruta, J.; To, M.; Sakaguchi, W.; Shimizu, T.; Kubota, N.; Tsukinoki, K. Faster Short-Chain Fatty Acid Absorption from the Cecum Following Polydextrose Ingestion Increases the Salivary Immunoglobulin A Flow Rate in Rats. Nutrients 2020, 12, 1745. https://doi.org/10.3390/nu12061745
Yamamoto Y, Morozumi T, Takahashi T, Saruta J, To M, Sakaguchi W, Shimizu T, Kubota N, Tsukinoki K. Faster Short-Chain Fatty Acid Absorption from the Cecum Following Polydextrose Ingestion Increases the Salivary Immunoglobulin A Flow Rate in Rats. Nutrients. 2020; 12(6):1745. https://doi.org/10.3390/nu12061745
Chicago/Turabian StyleYamamoto, Yuko, Toshiya Morozumi, Toru Takahashi, Juri Saruta, Masahiro To, Wakako Sakaguchi, Tomoko Shimizu, Nobuhisa Kubota, and Keiichi Tsukinoki. 2020. "Faster Short-Chain Fatty Acid Absorption from the Cecum Following Polydextrose Ingestion Increases the Salivary Immunoglobulin A Flow Rate in Rats" Nutrients 12, no. 6: 1745. https://doi.org/10.3390/nu12061745
APA StyleYamamoto, Y., Morozumi, T., Takahashi, T., Saruta, J., To, M., Sakaguchi, W., Shimizu, T., Kubota, N., & Tsukinoki, K. (2020). Faster Short-Chain Fatty Acid Absorption from the Cecum Following Polydextrose Ingestion Increases the Salivary Immunoglobulin A Flow Rate in Rats. Nutrients, 12(6), 1745. https://doi.org/10.3390/nu12061745







