Dietary Red Meat Adversely Affects Disease Severity in a Pig Model of DSS-Induced Colitis Despite Reduction in Colonic Pro-Inflammatory Gene Expression
Abstract
:1. Introduction
2. Materials and Methods
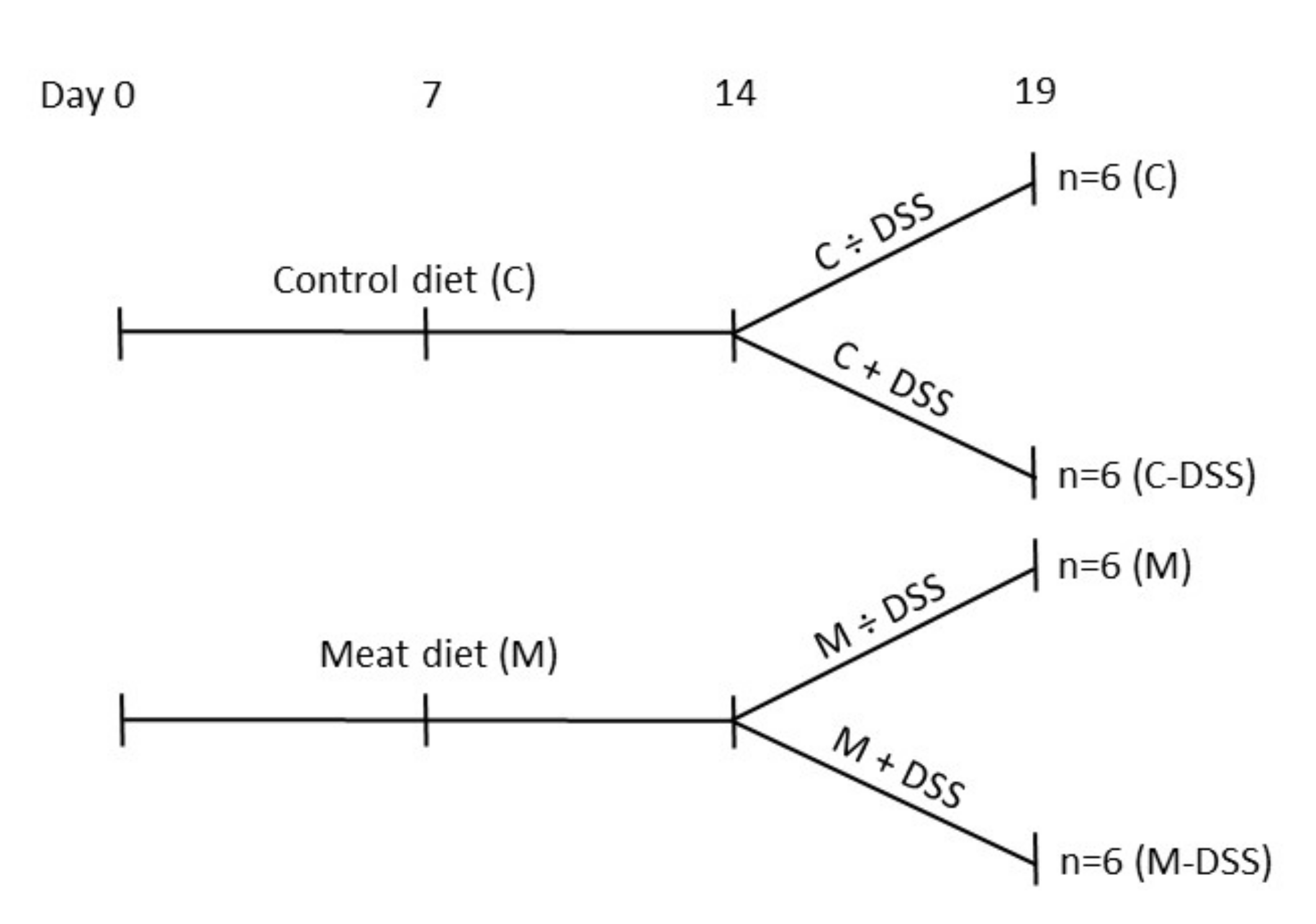
2.1. Animals and Experimental Design
2.2. Registrations and Samples
2.3. In Vivo Intestinal Permeability Test
2.4. Plasma and Mucosal Analysis
2.5. Histopathology
2.6. Colonic Gene Expression (qRT-PCR)
2.7. Statistical Analysis
3. Results
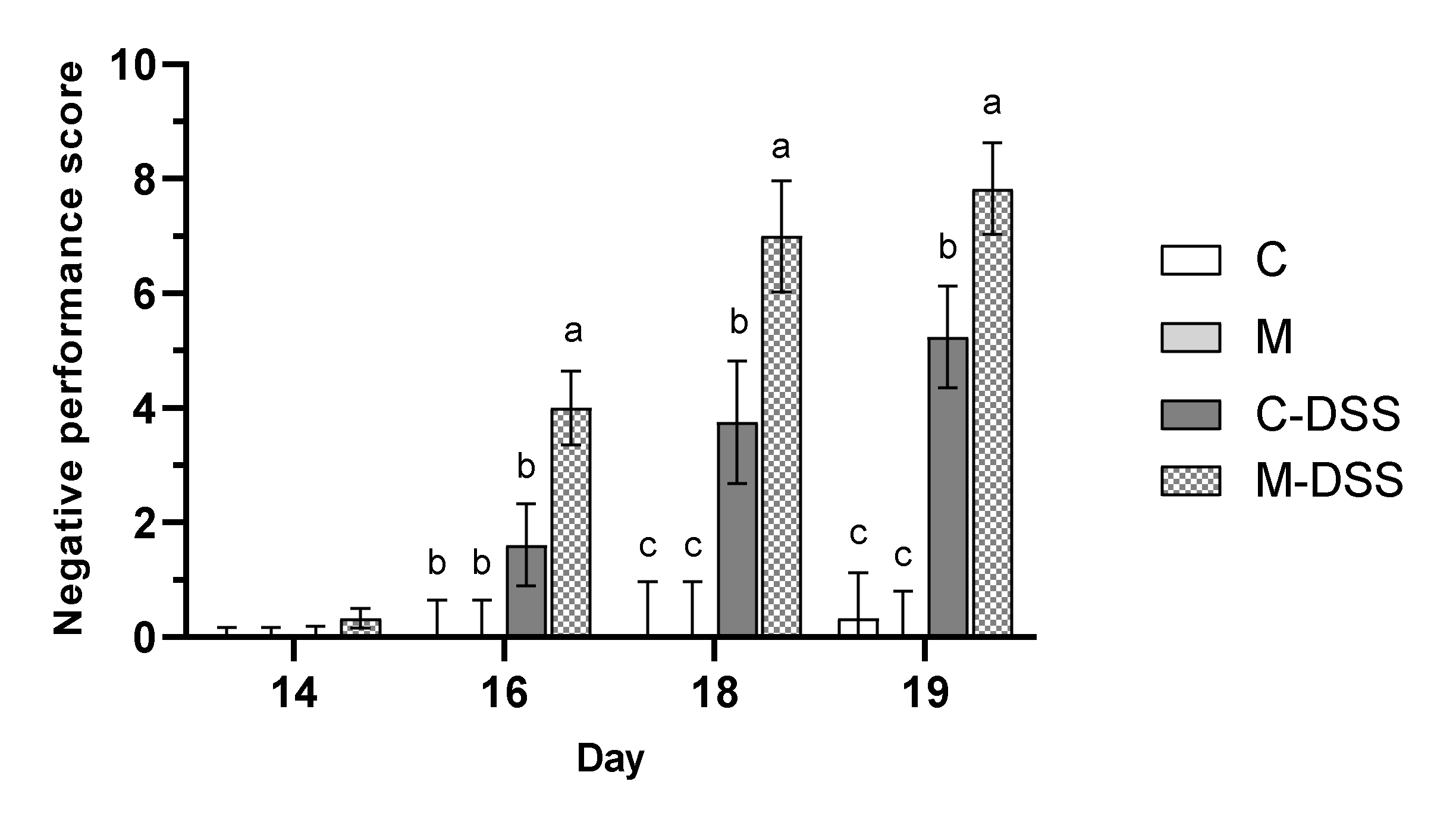
3.1. Body Weight, Feed Intake and Negative Performance Score
3.2. In Vivo Gut Permeability
3.3. Macroscopic and Histopathological Evaluation
3.4. Colonic Gene Expression
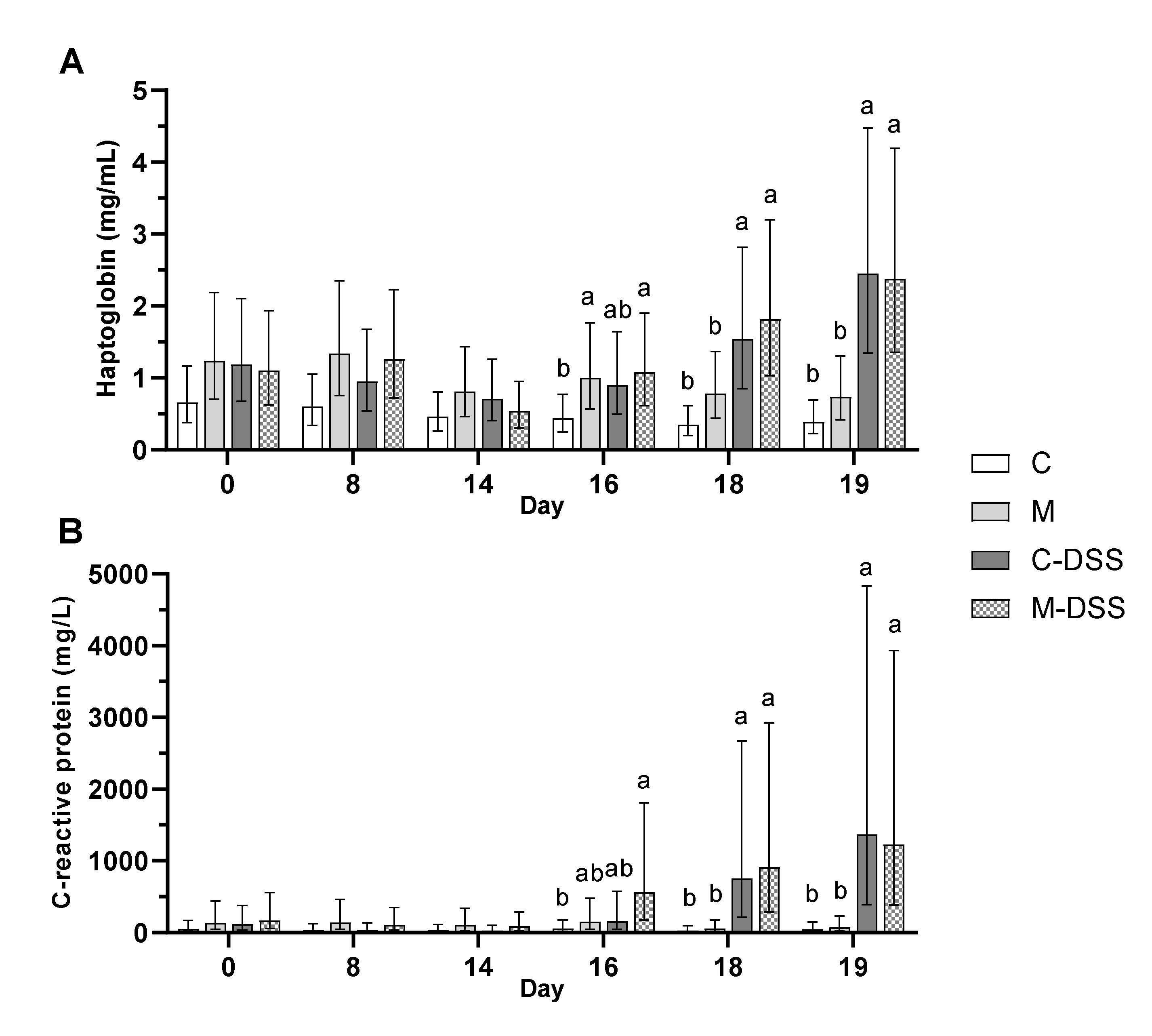
3.5. Immune Response Parameters in Blood and Intestinal Mucosa
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Granlund, A.V.B.; Flatberg, A.; Østvik, A.E.; Drozdov, I.; Gustafsson, B.I.; Kidd, M.; Beisvag, V.; Torp, S.H.; Waldum, H.L.; Martinsen, T.C.; et al. Whole genome gene expression meta-analysis of inflammatory bowel disease colon mucosa demonstrates lack of major differences between Crohn’s disease and ulcerative colitis. PLoS ONE 2013, 8, e56818. [Google Scholar] [CrossRef] [Green Version]
- Sartor, R.B. Mechanisms of disease: Pathogenesis of Crohn’s disease and ulcerative colitis. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006, 3, 390–407. [Google Scholar] [CrossRef]
- Ford, A.C.; Moayyedi, P.; Hanauer, S.B. Ulcerative colitis. Br. Med. J. 2013, 346, f432. [Google Scholar] [CrossRef] [Green Version]
- Ananthakrishnan, A.N.; Bernstein, C.N.; Iliopoulos, D.; Macpherson, A.; Neurath, M.F.; Ali, R.A.R.; Vavricka, S.R.; Fiocchi, C. Environmental triggers in IBD: A review of progress and evidence. Nat. Clin. Pract. Gastroenterol. Hepatol. 2017, 15, 39–49. [Google Scholar] [CrossRef]
- Andersen, V.; Olsen, A.; Carbonnel, F.; Tjønneland, A.; Vogel, U. Diet and risk of inflammatory bowel disease. Dig. Liver Dis. 2012, 44, 185–194. [Google Scholar] [CrossRef]
- Burisch, J.; Jess, T.; Martinato, M.; Lakatos, P.L. The burden of inflammatory bowel disease in Europe. J. Crohns Colitis 2013, 7, 322–337. [Google Scholar]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef]
- D’Souza, S.; Levy, E.; Mack, D.; Israel, D.; Lambrette, P.; Ghadirian, P.; Deslandres, C.; Morgan, K.; Seidman, E.G.; Amre, D.K. Dietary patterns and risk for Crohn’s disease in children. Inflamm. Bowel Dis. 2007, 14, 367–373. [Google Scholar] [CrossRef]
- Cohen, A.B.; Lee, D.; Long, M.D.; Kappelman, M.D.; Martin, C.F.; Sandler, R.S.; Lewis, J.D. Dietary patterns and self-reported associations of diet with symptoms of inflammatory bowel disease. Dig. Dis. Sci. 2013, 58, 1322–1328. [Google Scholar] [CrossRef]
- Jantchou, P.; Morois, S.; Clavel-Chapelon, F.; Boutron-Ruault, M.-C.; Carbonnel, F. Animal protein intake and risk of inflammatory bowel disease: The e3n prospective study. Am. J. Gastroenterol. 2010, 105, 2195–2201. [Google Scholar] [CrossRef]
- Pieczyńska, J.; Prescha, A.; Zabłocka-Słowińska, K.; Neubauer, K.; Smereka, A.; Grajeta, H.; Biernat, J.; Paradowski, L. Occurrence of dietary risk factors in inflammatory bowel disease: Influence on the nutritional status of patients in clinical remission. Adv. Clin. Exp. Med. 2018, 28, 587–592. [Google Scholar] [CrossRef]
- Jowett, S.L.; Seal, C.J.; Pearce, M.S.; Phillips, E.; Gregory, W.; Barton, J.R.; Welfare, M.R. Influence of dietary factors on the clinical course of ulcerative colitis: A prospective cohort study. Gut 2004, 53, 1479–1484. [Google Scholar] [CrossRef]
- Sakamoto, N.; Kono, S.; Wakai, K.; Fukuda, Y.; Satomi, M.; Shimoyama, T.; Inaba, Y.; Miyake, Y.; Sasaki, S.; Okamoto, K.; et al. Dietary risk factors for inflammatory bowel disease: A multicenter case-control study in japan. Inflamm. Bowel Dis. 2005, 11, 154–163. [Google Scholar] [CrossRef]
- Ge, J.; Han, T.J.; Liu, J.; Li, J.S.; Zhang, X.H.; Wang, Y.; Li, Q.Y.; Zhu, Q.; Yang, C.M. Meat intake and risk of inflammatory bowel disease: A meta-analysis. Turk. J. Gastroenterol. 2015, 26, 492–497. [Google Scholar] [CrossRef]
- Gilbert, M.S.; Ijssennagger, N.; Kies, A.K.; van Mil, S.W.C. Protein fermentation in the gut; implications for intestinal dysfunction in humans, pigs, and poultry. Am. J. Physiol. Gastroint. Liver Physiol. 2018, 315, G159–G170. [Google Scholar] [CrossRef]
- Rasmussen, N.F.; Rubin, K.H.; Stougaard, M.; Tjønneland, A.; Stenager, E.; Lund Hetland, M.; Glintborg, B.; Bygum, A.; Andersen, V. Impact of red meat, processed meat and fibre intake on risk of late-onset chronic inflammatory diseases: Prospective cohort study on lifestyle factors using the Danish ‘diet, cancer and health’ cohort (PROCID-DCH): Protocol. BMJ Open 2019, 9, e024555. [Google Scholar] [CrossRef] [Green Version]
- Ijssennagger, N.; van der Meer, R.; van Mil, S.W.C. Sulfide as a mucus barrier-breaker in inflammatory bowel disease? Trends Mol. Med. 2016, 22, 190–199. [Google Scholar] [CrossRef]
- Maloy, K.J.; Powrie, F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 2011, 474, 298–306. [Google Scholar] [CrossRef]
- Hou, J.K.; Abraham, B.; El-Serag, H. Dietary intake and risk of developing inflammatory bowel disease: A systematic review of the literature. Am. J. Gastroenterol. 2011, 106, 563–573. [Google Scholar] [CrossRef]
- Kawada, M.A.A.; Mizoguchi, E. Insights from advances in research of chemically induced experimental models of human inflammatory bowel disease. World J. Gastroenterol. 2007, 13, 5581–5593. [Google Scholar] [CrossRef] [PubMed]
- Eichele, D.D.; Kharbanda, K.K. Dextran sodium sulfate colitis murine model: An indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J. Gastroenterol. 2017, 23, 6016–6029. [Google Scholar] [CrossRef] [PubMed]
- Spurlock, M.E.; Gabler, N.K. The development of porcine models of obesity and the metabolic syndrome. J. Nutr. 2008, 138, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Hou, Y.; Yin, Y.; Qiu, Y.; Wu, G.; Hu, C.-A.A. Roles of amino acids in preventing and treating intestinal diseases: Recent studies with pig models. Amino Acids 2017, 49, 1277–1291. [Google Scholar] [CrossRef] [PubMed]
- Clouard, C.; Meunier-Salaün, M.C.; Val-Laillet, D. Food preferences and aversions in human health and nutrition: How can pigs help the biomedical research? Animal 2012, 6, 118–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, E.R.; Ullrey, D.E. The pig as a model for human nutrition. Annu. Rev. Nutr. 1987, 7, 361–382. [Google Scholar] [CrossRef]
- Verma, N.; Rettenmeier, A.W.; Schmitz-Spanke, S. Recent advances in the use of sus scrofa (pig) as a model system for proteomic studies. Proteomics 2011, 11, 776–793. [Google Scholar] [CrossRef]
- Kararli, T.T. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm. Drug Dispos. 1995, 16, 351–380. [Google Scholar] [CrossRef]
- Kim, C.J.; Kovacs-Nolan, J.A.; Yang, C.; Archbold, T.; Fan, M.Z.; Mine, Y. L-tryptophan exhibits therapeutic function in a porcine model of dextran sodium sulfate (DSS)-induced colitis. J. Nutr. Biochem. 2010, 21, 468–475. [Google Scholar] [CrossRef]
- Kim, C.J.; Kovacs-Nolan, J.; Yang, C.; Archbold, T.; Fan, M.Z.; Mine, Y. L-cysteine supplementation attenuates local inflammation and restores gut homeostasis in a porcine model of colitis. Biochim. Biophys. Acta 2009, 1790, 1161–1169. [Google Scholar] [CrossRef]
- Bassaganya-Riera, J.; Hontecillas, R. CLA and n-3 PUFA differentially modulate clinical activity and colonic ppar-responsive gene expression in a pig model of experimental IBD. Clin. Nutr. 2006, 25, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Ibuki, M.; Fukui, K.; Kanatani, H.; Mine, Y. Anti-inflammatory effects of mannanase-hydrolyzed copra meal in a porcine model of colitis. J. Vet. Med. Sci. 2014, 76, 645–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, D.; Ibuki, M.; Nakamori, T.; Fan, M.; Mine, Y. Soy-derived di- and tripeptides alleviate colon and ileum inflammation in pigs with dextran sodium sulfate-induced colitis. J. Nutr. 2012, 142, 363–368. [Google Scholar] [CrossRef] [Green Version]
- Lackeyram, D.; Young, D.; Kim, C.J.; Yang, C.; Archbold, T.L.; Mine, Y.; Fan, M.Z. Interleukin-10 is differentially expressed in the small intestine and the colon experiencing chronic inflammation and ulcerative colitis induced by dextran sodium sulfate in young pigs. Physiol. Res. 2017, 66, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.S.; Toft, N. Intra- and inter-observer agreement when using a descriptive classification scale for clinical assessment of faecal consistency in growing pigs. Prev. Vet. Med. 2011, 98, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.; Fernández, C. Enzymatic-fluorometric analyses for glutamine, glutamate and free amino groups in protein-free plasma and milk. J. Dairy Res. 2017, 84, 32–35. [Google Scholar] [CrossRef]
- Keshteli, A.H.; Madsen, K.L.; Dieleman, L.A. Diet in the pathogenesis and management of ulcerative colitis; a review of randomized controlled dietary interventions. Nutrients 2019, 11, 1498. [Google Scholar] [CrossRef] [Green Version]
- Khalili, H.; Chan, S.S.M.; Lochhead, P.; Ananthakrishnan, A.N.; Hart, A.R.; Chan, A.T. The role of diet in the aetiopathogenesis of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 525–535. [Google Scholar] [CrossRef] [Green Version]
- O’Shea, C.J.; O’Doherty, J.V.; Callanan, J.J.; Doyle, D.; Thornton, K.; Sweeney, T. The effect of algal polysaccharides laminarin and fucoidan on colonic pathology, cytokine gene expression and enterobacteriaceae in a dextran sodium sulfate-challenged porcine model. J. Nutr. Sci. 2016, 5, e15. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, L.E.; Taddeo, S.S.; Weeks, B.R.; Carroll, R.J.; Dykes, L.; Rooney, L.W.; Turner, N.D. Impact of novel sorghum bran diets on DSS-induced colitis. Nutrients 2017, 9, 4. [Google Scholar] [CrossRef] [Green Version]
- Panasevich, M.R.; Allen, J.M.; Wallig, M.A.; Woods, J.A.; Dilger, R.N. Moderately fermentable potato fiber attenuates signs and inflammation associated with experimental colitis in mice. J. Nutr. 2015, 145, 2781–2788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pistol, G.C.; Marin, D.E.; Rotar, M.C.; Ropota, M.; Taranu, I. Bioactive compounds from dietary whole grape seed meal improved colonic inflammation via inhibition of mapks and nf-kb signaling in pigs with DSS-induced colitis. J. Funct. Foods 2020, 66, 103708. [Google Scholar] [CrossRef]
- Matsuda, R.; Koide, T.; Tokoro, C.; Yamamoto, T.; Godai, T.; Morohashi, T.; Fujita, Y.; Takahashi, D.; Kawana, I.; Suzuki, S.; et al. Quantitive cytokine mRNA expression profiles in the colonic mucosa of patients with steroid naive ulcerative colitis during active and quiescent disease. Inflamm. Bowel Dis. 2009, 15, 328–334. [Google Scholar] [CrossRef]
- Del Valle-Pinero, A.Y.; Van Deventer, H.E.; Fourie, N.H.; Martino, A.C.; Patel, N.S.; Remaley, A.T.; Henderson, W.A. Gastrointestinal permeability in patients with irritable bowel syndrome assessed using a four probe permeability solution. Clin. Chim. Acta 2013, 418, 97–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schepens, M.A.; Vink, C.; Schonewille, A.J.; Dijkstra, G.; van der Meer, R.; Bovee-Oudenhoven, I.M. Dietary heme adversely affects experimental colitis in rats, despite heat-shock protein induction. Nutrition 2011, 27, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Otaka, M.; Odashima, M.; Watanabe, S. Role of heat shock proteins (molecular chaperones) in intestinal mucosal protection. Biochem. Biophys. Res. Com. 2006, 348, 1–5. [Google Scholar] [CrossRef]
- Manichanh, C.; Borruel, N.; Casellas, F.; Guarner, F. The gut microbiota in IBD. Nature Rev. Gastroenterol. Hepatol. 2012, 9, 599–608. [Google Scholar] [CrossRef]
- Kostic, A.D.; Xavier, R.J.; Gevers, D. The microbiome in inflammatory bowel disease: Current status and the future ahead. Gastroenterol. 2014, 146, 1489–1499. [Google Scholar] [CrossRef] [Green Version]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; LeLeiko, N.; Snapper, S.B.; et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Gen. Biol. 2012, 13, R79. [Google Scholar] [CrossRef]


| Weight Loss Rel. to the Day before | Fecal Texture | Blood in the Feces | Overall Condition |
|---|---|---|---|
| 0 = None | 0 = normal | 0 = normal | 0 = normal |
| 1 = 1–5% | 2 = thin/soft | 2 = little-moderate | 2 = slightly affected |
| 2 = >5–10% | 4 = diarrhea | 4 = moderate-heavy | 4 = highly affected |
| 3 = >10–15% | |||
| 4 = >15% |
| Treatment | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|
| C | M | C-DSS | M-DSS | SEM | Diet | DSS | D × DSS | |
| BW d14, kg | 14.7 b | 15.7 ab | 15.1 b | 16.9 a | 0.71 | 0.01 | 0.13 | 0.38 |
| BW d19, kg | 16.9 ab | 18.1 a | 16.2 b | 18.8 a | 0.80 | 0.01 | 0.99 | 0.35 |
| BW gain d14-18, g/day | 426 a | 481 a | 215 b | 366 ab | 74 | 0.12 | 0.03 | 0.50 |
| Feed intake d0-13, g/day | 502 | 488 | 518 | 428 | 30 | 0.08 | 0.60 | 0.19 |
| Meat intake d0-13, g/day | 0 c | 55 b | 0 c | 68 a | 3 | <0.001 | 0.02 | 0.01 |
| Feed intake d14-18, g/day | 443 ab | 550 a | 366 b | 401 ab | 53 | 0.21 | 0.05 | 0.52 |
| Meat intake d14-18, g/day | 0 c | 66 b | 0 c | 87 a | 9 | <0.001 | 0.23 | 0.22 |
| Treatment | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|
| C | M | C-DSS | M-DSS | SEM | Diet | DSS | D × DSS | |
| Co25 | ||||||||
| Cryptitis, % | 67 | 67 | 61 | 75 | 22 | 0.74 | 0.95 | 0.74 |
| Crypt abscess, % | 67 | 50 | 100 | 83 | 21 | 0.40 | 0.11 | 1.00 |
| Erosion/Ulceration, % | 0 b | 0 b | 37 a | 33 a | 16 | 0.90 | 0.03 | 0.90 |
| Inflammation score | 0.75 b | 0.58 b | 1.9 a | 1.9 a | 0.4 | 0.86 | <0.01 | 0.76 |
| Co50 | ||||||||
| Cryptitis, % | 67 ab | 33 b | 63 ab | 100 a | 19 | 0.90 | 0.08 | 0.05 |
| Crypt abscess, % | 83 | 83 | 100 | 100 | 14 | 0.97 | 0.22 | 0.97 |
| Erosion/Ulceration, % | 0 c | 0 c | 40 b | 83 a | 13 | 0.10 | <0.001 | 0.10 |
| Inflammation, score | 0.75 c | 0.50 c | 1.9 b | 2.7 a | 0.2 | 0.18 | <0.001 | 0.01 |
| Co75 | ||||||||
| Cryptitis, % | 33 | 50 | 74 | 83 | 20 | 0.49 | 0.06 | 0.84 |
| Crypt abscess, % | 50 b | 67 b | 94 a | 100 a | 14 | 0.39 | <0.01 | 0.70 |
| Erosion/Ulceration, % | 0 c | 0 c | 40 b | 83 a | 13 | 0.10 | <0.001 | 0.10 |
| Inflammation, score | 0.50 c | 0.58 c | 1.7 b | 2.5 a | 0.3 | 0.06 | <0.001 | 0.13 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nielsen, T.S.; Fredborg, M.; Theil, P.K.; Yue, Y.; Bruhn, L.V.; Andersen, V.; Purup, S. Dietary Red Meat Adversely Affects Disease Severity in a Pig Model of DSS-Induced Colitis Despite Reduction in Colonic Pro-Inflammatory Gene Expression. Nutrients 2020, 12, 1728. https://doi.org/10.3390/nu12061728
Nielsen TS, Fredborg M, Theil PK, Yue Y, Bruhn LV, Andersen V, Purup S. Dietary Red Meat Adversely Affects Disease Severity in a Pig Model of DSS-Induced Colitis Despite Reduction in Colonic Pro-Inflammatory Gene Expression. Nutrients. 2020; 12(6):1728. https://doi.org/10.3390/nu12061728
Chicago/Turabian StyleNielsen, Tina S., Marlene Fredborg, Peter K. Theil, Yuan Yue, Lærke V. Bruhn, Vibeke Andersen, and Stig Purup. 2020. "Dietary Red Meat Adversely Affects Disease Severity in a Pig Model of DSS-Induced Colitis Despite Reduction in Colonic Pro-Inflammatory Gene Expression" Nutrients 12, no. 6: 1728. https://doi.org/10.3390/nu12061728
APA StyleNielsen, T. S., Fredborg, M., Theil, P. K., Yue, Y., Bruhn, L. V., Andersen, V., & Purup, S. (2020). Dietary Red Meat Adversely Affects Disease Severity in a Pig Model of DSS-Induced Colitis Despite Reduction in Colonic Pro-Inflammatory Gene Expression. Nutrients, 12(6), 1728. https://doi.org/10.3390/nu12061728





