Lutein Supplementation for Eye Diseases
Abstract
1. Introduction
1.1. Dietary Lutein and Its Uptake
- (i) the nature of the food matrix (natural form or supplementation);
- (ii) the amount and nature of dietary fat, which promote the circulation of carotenoids;
- (iii) presence of phospholipids;
- (iv) presence of dietary fibers;
- (v) properties of dietary carotenoids.
1.2. Distribution of Lutein in the Human Body
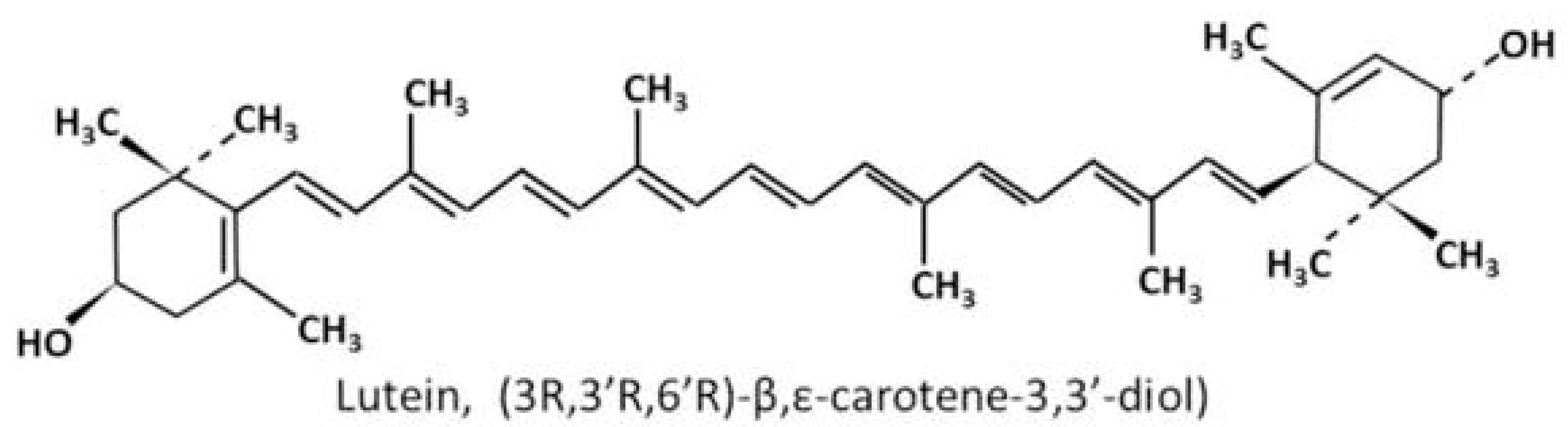
1.3. Chemical Structures and Properties of Lutein
2. Safety Profile of Lutein as Eye Health Supplement
3. Lutein Supplementation and Age-Related Macular Degeneration
3.1. Age-Related Macular Degeneration: Background
3.2. Age-Related Macular Degeneration: Pathogenesis and Current Treatments
3.3. Lutein and AMD (Clinical Studies)
3.4. Lutein and AMD (Experimental Studies)
4. Lutein Supplementation and Diabetic Retinopathy
4.1. Diabetic Retinopathy: Background
4.2. Diabetic Retinopathy: Pathogenesis and Current Treatments
4.3. Lutein and Diabetic Retinopathy (Clinical Studies)
4.4. Lutein and Diabetic Retinopathy (Animal Studies)
5. Lutein Supplementation and Retinopathy of Prematurity
5.1. Retinopathy of Prematurity: Background
5.2. Retinopathy of Prematurity: Pathogenesis and Current Treatments
5.3. Lutein and ROP (Clinical Studies)
5.4. Lutein and ROP (Animal Studies)
6. Lutein Supplementation and Myopia
6.1. Myopia: Background
6.2. Myopia: Pathogenesis and Current Treatments
6.3. Lutein and Myopia
7. Lutein Supplementation and Cataract
7.1. Cataract: Background and Treatment
7.2. Lutein and Cataract
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AGEs | Advanced glycation end products |
| AL | Axial length |
| AMD | Age-related macular degeneration |
| BDNF | Brain-derived neurotrophic factor |
| COX-2 | Cyclooxygenase-2 |
| CRN | Council for Responsible Nutrition |
| DR | Diabetic retinopathy |
| ERG | Electroretinogram |
| ERK | Extracellular signal-regulated kinase |
| FDA | US Food and Drug Administration |
| GGPP | Geranylgeranyl pyrophosphate |
| GPx | Glutathione peroxidase |
| GRAS | Generally Regarded as Safe |
| GSH | Glutathione |
| HbA1c | Hemoglobin A1c |
| HDL | High-density lipoproteins |
| IGF-1 | Insulin-like growth factor-1 |
| IL-1β | Interleukin 1β |
| iNOS | Inducible nitric oxide synthase |
| IRMA | Intraretinal microvascular abnormalities |
| LASIK | Laser Assisted in situ Keratomileusis |
| LDL | Low-density lipoproteins |
| mfERG | Multifocal electroretinography |
| MPOD | Macular pigment optical density |
| NF-κB | Nuclear factor-kappa B |
| NPDR | Non-proliferative diabetic retinopathy |
| OCT | Optical coherence tomography |
| OIR | Oxygen-Induced Retinopathy |
| PDR | Proliferative diabetic retinopathy |
| ROP | Retinopathy of prematurity |
| ROS | Reactive oxygen species |
| RPE | Retinal pigment epithelium |
| SMILE | Small Incision Lenticule Extraction |
| VLDL | Very-low-density lipoproteins |
References
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.A.; Landrum, J.T.; Tarsis, S.L. Preliminary identification of the human macular pigment. Vis. Res. 1985, 25, 1531–1535. [Google Scholar] [CrossRef]
- Parker, R.S. Carotenoids in human blood and tissues. J. Nutr. 1989, 119, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Perry, A.; Rasmussen, H.; Johnson, E. Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. J. Food Compos. Anal. 2009, 22, 9–15. [Google Scholar] [CrossRef]
- Landrum, J.T.; Bone, R.A. Lutein, zeaxanthin, and the macular pigment. Arch. Biochem. Biophys. 2001, 385, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Humphries, J.M.; Khachik, F. Distribution of lutein, zeaxanthin, and related geometrical isomers in fruit, vegetables, wheat, and pasta products. J. Agric. Food Chem. 2003, 51, 1322–1327. [Google Scholar] [CrossRef] [PubMed]
- Mangels, A.R.; Holden, J.M.; Beecher, G.R.; Forman, M.R.; Lanza, E. Carotenoid content of fruits and vegetables: An evaluation of analytic data. J. Am. Diet. Assoc. 1993, 93, 284–296. [Google Scholar] [CrossRef]
- Schaeffer, J.L.; Tyczkowski, J.K.; Parkhurst, C.R.; Hamilton, P.B. Carotenoid composition of serum and egg yolks of hens fed diets varying in carotenoid composition. Poult. Sci. 1988, 67, 608–614. [Google Scholar] [CrossRef]
- Bohn, T. Bioavailability of non-provitamin A carotenoids. Curr. Nutr. Food Sci. 2008, 4, 240–258. [Google Scholar] [CrossRef]
- van Het Hof, K.H.; West, C.E.; Weststrate, J.A.; Hautvast, J.G. Dietary factors that affect the bioavailability of carotenoids. J. Nutr. 2000, 130, 503–506. [Google Scholar] [CrossRef]
- Castenmiller, J.J.; West, C.E. Bioavailability and bioconversion of carotenoids. Annu. Rev. Nutr. 1998, 18, 19–38. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, O.F.; Ryan, L.; O’Brien, N.M. Xanthophyll carotenoids are more bioaccessible from fruits than dark green vegetables. Nutr. Res. 2007, 27, 258–264. [Google Scholar] [CrossRef]
- Thurnham, D.I. Macular zeaxanthins and lutein—A review of dietary sources and bioavailability and some relationships with macular pigment optical density and age-related macular disease. Nutr. Res. Rev. 2007, 20, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Nagao, A. Absorption and metabolism of dietary carotenoids. Biofactors 2011, 37, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Clevidence, B.A.; Bieri, J.G. Association of carotenoids with human plasma lipoproteins. Methods Enzym.. 1993, 214, 33–46. [Google Scholar]
- Goulinet, S.; Chapman, M.J. Plasma LDL and HDL subspecies are heterogenous in particle content of tocopherols and oxygenated and hydrocarbon carotenoids. Relevance to oxidative resistance and atherogenesis. Arter. Thromb. Vasc. Biol. 1997, 17, 786–796. [Google Scholar] [CrossRef]
- Renzi, L.M.; Hammond, B.R., Jr.; Dengler, M.; Roberts, R. The relation between serum lipids and lutein and zeaxanthin in the serum and retina: Results from cross-sectional, case-control and case study designs. Lipids Health Dis. 2012, 11, 33. [Google Scholar] [CrossRef]
- Bernstein, P.S.; Li, B.; Vachali, P.P.; Gorusupudi, A.; Shyam, R.; Henriksen, B.S.; Nolan, J.M. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog. Retin. Eye Res. 2016, 50, 34–66. [Google Scholar] [CrossRef]
- Nolan, J.M.; Meagher, K.; Kashani, S.; Beatty, S. What is meso-zeaxanthin, and where does it come from? Eye (Lond.) 2013, 27, 899–905. [Google Scholar] [CrossRef]
- Bone, R.A.; Landrum, J.T.; Mayne, S.T.; Gomez, C.M.; Tibor, S.E.; Twaroska, E.E. Macular pigment in donor eyes with and without AMD: A case-control study. Investig. Ophthalmol. Vis. Sci. 2001, 42, 235–240. [Google Scholar]
- Li, B.; George, E.W.; Rognon, G.T.; Gorusupudi, A.; Ranganathan, A.; Chang, F.Y.; Shi, L.; Frederick, J.M.; Bernstein, P.S. Imaging lutein and zeaxanthin in the human retina with confocal resonance Raman microscopy. Proc. Natl. Acad. Sci. USA 2020, 117, 12352–12358. [Google Scholar] [CrossRef] [PubMed]
- Manayi, A.; Abdollahi, M.; Raman, T.; Nabavi, S.F.; Habtemariam, S.; Daglia, M.; Nabavi, S.M. Lutein and cataract: From bench to bedside. Crit. Rev. Biotechnol. 2016, 36, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Broekmans, W.M.; Berendschot, T.T.; Klöpping-Ketelaars, I.A.; de Vries, A.J.; Goldbohm, R.A.; Tijburg, L.B.; Kardinaal, A.F.; van Poppel, G. Macular pigment density in relation to serum and adipose tissue concentrations of lutein and serum concentrations of zeaxanthin. Am. J. Clin. Nutr. 2002, 76, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Landrum, J.T.; Bone, R.A.; Joa, H.; Kilburn, M.D.; Moore, L.L.; Sprague, K.E. A one year study of the macular pigment: The effect of 140 days of a lutein supplement. Exp. Eye Res. 1997, 65, 57–62. [Google Scholar] [CrossRef]
- Yoshizako, H.; Hara, K.; Takai, Y.; Kaidzu, S.; Obana, A.; Ohira, A. Comparison of macular pigment and serum lutein concentration changes between free lutein and lutein esters supplements in Japanese subjects. Acta Ophthalmol 2016, 94, e411–e416. [Google Scholar] [CrossRef]
- Li, B.; Vachali, P.P.; Gorusupudi, A.; Shen, Z.; Sharifzadeh, H.; Besch, B.M.; Nelson, K.; Horvath, M.M.; Frederick, J.M.; Baehr, W.; et al. Inactivity of human beta,beta-carotene-9’,10’-dioxygenase (BCO2) underlies retinal accumulation of the human macular carotenoid pigment. Proc. Natl. Acad. Sci. USA 2014, 111, 10173–10178. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J.; Hammond, B.R.; Yeum, K.J.; Qin, J.; Wang, X.D.; Castaneda, C.; Snodderly, D.M.; Russell, R.M. Relation among serum and tissue concentrations of lutein and zeaxanthin and macular pigment density. Am. J. Clin. Nutr. 2000, 71, 1555–1562. [Google Scholar] [CrossRef]
- Arunkumar, R.; Gorusupudi, A.; Bernstein, P.S. The macular carotenoids: A biochemical overview. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 158617. [Google Scholar] [CrossRef]
- Nian, S.; Lo, A.C. Protecting the Aging Retina; Raymond, N., Chuen-Chung, C., Yuen-Shan, H., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Johnson, E.J. Carotenoid actions and their relation to health and disease. Mol. Asp. Med. 2005, 26, 459–516. [Google Scholar] [CrossRef]
- Li, S.Y.; Fu, Z.J.; Lo, A.C. Hypoxia-induced oxidative stress in ischemic retinopathy. Oxid. Med. Cell. Longev. 2012, 2012, 426769. [Google Scholar] [CrossRef]
- Junghans, A.; Sies, H.; Stahl, W. Macular pigments lutein and zeaxanthin as blue light filters studied in liposomes. Arch. Biochem. Biophys. 2001, 391, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Renard, G.; Leid, J. The dangers of blue light: True story! J. Fr. Ophtalmol. 2016, 39, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Krinsky, N.I.; Yeum, K.J. Carotenoid-radical interactions. Biochem. Biophys. Res. Commun. 2003, 305, 754–760. [Google Scholar] [CrossRef]
- Landrum, J.T.; Bone, R.A.; Krinsky, N.I.; Mayne, S.T.; Sies, H. Carotenoids in Health and Disease; Marcel Dekker: New York, NY, USA, 2004; pp. 445–472. [Google Scholar]
- Snodderly, D.M.; Auran, J.D.; Delori, F.C. The macular pigment. II. Spatial distribution in primate retinas. Investig. Ophthalmol. Vis. Sci. 1984, 25, 674–685. [Google Scholar]
- Kijlstra, A.; Tian, Y.; Kelly, E.R.; Berendschot, T.T. Lutein: More than just a filter for blue light. Prog. Retin. Eye Res. 2012, 31, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Fung, F.K.; Fu, Z.J.; Wong, D.; Chan, H.H.; Lo, A.C. Anti-inflammatory effects of lutein in retinal ischemic/hypoxic injury: In vivo and in vitro studies. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5976–5984. [Google Scholar] [CrossRef]
- Bian, Q.; Gao, S.; Zhou, J.; Qin, J.; Taylor, A.; Johnson, E.J.; Tang, G.; Sparrow, J.R.; Gierhart, D.; Shang, F. Lutein and zeaxanthin supplementation reduces photooxidative damage and modulates the expression of inflammation-related genes in retinal pigment epithelial cells. Free Radic. Biol. Med. 2012, 53, 1298–1307. [Google Scholar] [CrossRef]
- Tian, Y.; Kijlstra, A.; Webers, C.A.B.; Berendschot, T. Lutein and Factor D: Two intriguing players in the field of age-related macular degeneration. Arch. Biochem. Biophys. 2015, 572, 49–53. [Google Scholar] [CrossRef]
- Fernandez-Robredo, P.; Sadaba, L.M.; Salinas-Alaman, A.; Recalde, S.; Rodriguez, J.A.; Garcia-Layana, A. Effect of lutein and antioxidant supplementation on VEGF expression, MMP-2 activity, and ultrastructural alterations in apolipoprotein E-deficient mouse. Oxid. Med. Cell. Longev. 2013, 2013, 213505. [Google Scholar] [CrossRef]
- Zhang, P.C.; Wu, C.R.; Wang, Z.L.; Wang, L.Y.; Han, Y.; Sun, S.L.; Li, Q.S.; Ma, L. Effect of lutein supplementation on visual function in nonproliferative diabetic retinopathy. Asia Pac. J. Clin. Nutr. 2017, 26, 406–411. [Google Scholar]
- Li, S.Y.; Fu, Z.J.; Ma, H.; Jang, W.C.; So, K.F.; Wong, D.; Lo, A.C. Effect of lutein on retinal neurons and oxidative stress in a model of acute retinal ischemia/reperfusion. Investig. Ophthalmol. Vis. Sci. 2009, 50, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Fung, F.K.; Law, B.Y.; Lo, A.C. Lutein Attenuates Both Apoptosis and Autophagy upon Cobalt (II) Chloride-Induced Hypoxia in Rat Muller Cells. PLoS ONE 2016, 11, e0167828. [Google Scholar] [CrossRef] [PubMed]
- Mares, J. Lutein and Zeaxanthin Isomers in Eye Health and Disease. Annu. Rev. Nutr. 2016, 36, 571–602. [Google Scholar] [CrossRef]
- Vishwanathan, R.; Schalch, W.; Johnson, E.J. Macular pigment carotenoids in the retina and occipital cortex are related in humans. Nutr. Neurosci. 2016, 19, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Ranard, K.M.; Jeon, S.; Mohn, E.S.; Griffiths, J.C.; Johnson, E.J.; Erdman, J.W., Jr. Dietary guidance for lutein: Consideration for intake recommendations is scientifically supported. Eur. J. Nutr. 2017, 56 (Suppl. 3), 37–42. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W. Macular carotenoids: Lutein and zeaxanthin. Dev. Ophthalmol. 2005, 38, 70–88. [Google Scholar]
- Institute of Medicine Panel on Dietary, A.; Related, C. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academy of Sciences: Washington, DC, USA, 2000. [Google Scholar]
- Chew, E.Y.; Clemons, T.E.; SanGiovanni, J.P.; Danis, R.; Ferris, F.L.; Elman, M.; Antoszyk, A.; Ruby, A.; Orth, D.; Bressler, S.; et al. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013, 309, 2005–2015. [Google Scholar]
- Shao, A.; Hathcock, J.N. Risk assessment for the carotenoids lutein and lycopene. Regul. Toxicol. Pharm. 2006, 45, 289–298. [Google Scholar] [CrossRef]
- Dagnelie, G.; Zorge, I.S.; McDonald, T.M. Lutein improves visual function in some patients with retinal degeneration: A pilot study via the Internet. Optometry 2000, 71, 147–164. [Google Scholar]
- Wenzel, A.J.; Sheehan, J.P.; Gerweck, C.; Stringham, J.M.; Fuld, K.; Curran-Celentano, J. Macular pigment optical density at four retinal loci during 120 days of lutein supplementation. Ophthalmic Physiol. Opt. 2007, 27, 329–335. [Google Scholar] [CrossRef]
- Kruger, C.L.; Murphy, M.; DeFreitas, Z.; Pfannkuch, F.; Heimbach, J. An innovative approach to the determination of safety for a dietary ingredient derived from a new source: Case study using a crystalline lutein product. Food Chem. Toxicol. 2002, 40, 1535–1549. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.Y.; Chortkoff, S.C.; Gorusupudi, A.; Bernstein, P.S. Crystalline Maculopathy Associated With High-Dose Lutein Supplementation. JAMA Ophthalmol. 2016, 134, 1445–1448. [Google Scholar] [CrossRef] [PubMed]
- Hammond, B.R.; Johnson, B.A.; George, E.R. Oxidative photodegradation of ocular tissues: Beneficial effects of filtering and exogenous antioxidants. Exp. Eye Res. 2014, 129, 135–150. [Google Scholar] [CrossRef]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Bird, A.C.; Bressler, N.M.; Bressler, S.B.; Chisholm, I.H.; Coscas, G.; Davis, M.D.; de Jong, P.T.; Klaver, C.C.; Klein, B.E.; Klein, R.; et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv. Ophthalmol. 1995, 39, 367–374. [Google Scholar] [CrossRef]
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study Research Group. Risk factors associated with age-related macular degeneration: A case-control study in the age-related eye disease study: Age-Related Eye Disease Study Report Number 3. Ophthalmology 2000, 107, 2224–2232. [Google Scholar] [CrossRef]
- Curcio, C.A.; Presley, J.B.; Malek, G.; Medeiros, N.E.; Avery, D.V.; Kruth, H.S. Esterified and unesterified cholesterol in drusen and basal deposits of eyes with age-related maculopathy. Exp. Eye Res. 2005, 81, 731–741. [Google Scholar] [CrossRef]
- Freund, K.B.; Ho, I.V.; Barbazetto, I.A.; Koizumi, H.; Laud, K.; Ferrara, D.; Matsumoto, Y.; Sorenson, J.A.; Yannuzzi, L. Type 3 neovascularization: The expanded spectrum of retinal angiomatous proliferation. Retina 2008, 28, 201–211. [Google Scholar] [CrossRef]
- Wei, Y.H. Oxidative stress and mitochondrial DNA mutations in human aging. Proc. Soc. Exp. Biol. Med. 1998, 217, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Rubio, M.A.; Davalos, A.R.; Campisi, J. Telomere length mediates the effects of telomerase on the cellular response to genotoxic stress. Exp. Cell Res. 2004, 298, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Catala, A. An overview of lipid peroxidation with emphasis in outer segments of photoreceptors and the chemiluminescence assay. Int. J. Biochem. Cell Biol. 2006, 38, 1482–1495. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, C.J.; Rakoczy, P.E.; Constable, I.J. Lipofuscin of the retinal pigment epithelium: A review. Eye (Lond.) 1995, 9 (Pt. 6), 763–771. [Google Scholar] [CrossRef]
- Feeney-Burns, L.; Hilderbrand, E.S.; Eldridge, S. Aging human RPE: Morphometric analysis of macular, equatorial, and peripheral cells. Investig. Ophthalmol. Vis. Sci. 1984, 25, 195–200. [Google Scholar]
- Shamsi, F.A.; Boulton, M. Inhibition of RPE lysosomal and antioxidant activity by the age pigment lipofuscin. Investig. Ophthalmol. Vis. Sci. 2001, 42, 3041–3046. [Google Scholar]
- Green, W.R.; McDonnell, P.J.; Yeo, J.H. Pathologic features of senile macular degeneration. Ophthalmology 1985, 92, 615–627. [Google Scholar] [CrossRef]
- Biesemeier, A.; Taubitz, T.; Julien, S.; Yoeruek, E.; Schraermeyer, U. Choriocapillaris breakdown precedes retinal degeneration in age-related macular degeneration. Neurobiol. Aging 2014, 35, 2562–2573. [Google Scholar] [CrossRef]
- Johnson, L.V.; Leitner, W.P.; Staples, M.K.; Anderson, D.H. Complement activation and inflammatory processes in Drusen formation and age related macular degeneration. Exp. Eye Res. 2001, 73, 887–896. [Google Scholar] [CrossRef]
- Martin, D.F.; Maguire, M.G.; Fine, S.L.; Ying, G.S.; Jaffe, G.J.; Grunwald, J.E.; Toth, C.; Redford, M.; Ferris, F.L., 3rd. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: Two-year results. Ophthalmology 2012, 119, 1388–1398. [Google Scholar] [CrossRef]
- SanGiovanni, J.P.; Chew, E.Y.; Clemons, T.E.; Ferris, F.L., 3rd; Gensler, G.; Lindblad, A.S.; Milton, R.C.; Seddon, J.M.; Sperduto, R.D. The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS Report No. 22. Arch. Ophthalmol. 2007, 125, 1225–1232. [Google Scholar]
- Tan, J.S.; Wang, J.J.; Flood, V.; Rochtchina, E.; Smith, W.; Mitchell, P. Dietary antioxidants and the long-term incidence of age-related macular degeneration: The Blue Mountains Eye Study. Ophthalmology 2008, 115, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Eye Disease Case-Control Study Group. Antioxidant status and neovascular age-related macular degeneration. Arch Ophthalmol. 1993, 111, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Delcourt, C.; Carriere, I.; Delage, M.; Barberger-Gateau, P.; Schalch, W. Plasma lutein and zeaxanthin and other carotenoids as modifiable risk factors for age-related maculopathy and cataract: The POLA Study. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2329–2335. [Google Scholar] [CrossRef] [PubMed]
- Richer, S.; Devenport, J.; Lang, J.C. LAST II: Differential temporal responses of macular pigment optical density in patients with atrophic age-related macular degeneration to dietary supplementation with xanthophylls. Optometry 2007, 78, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Weigert, G.; Kaya, S.; Pemp, B.; Sacu, S.; Lasta, M.; Werkmeister, R.M.; Dragostinoff, N.; Simader, C.; Garhofer, G.; Schmidt-Erfurth, U.; et al. Effects of lutein supplementation on macular pigment optical density and visual acuity in patients with age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8174–8178. [Google Scholar] [CrossRef] [PubMed]
- Murray, I.J.; Makridaki, M.; van der Veen, R.L.; Carden, D.; Parry, N.R.; Berendschot, T.T. Lutein supplementation over a one-year period in early AMD might have a mild beneficial effect on visual acuity: The CLEAR study. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1781–1788. [Google Scholar] [CrossRef]
- Cho, E.; Hankinson, S.E.; Rosner, B.; Willett, W.C.; Colditz, G.A. Prospective study of lutein/zeaxanthin intake and risk of age-related macular degeneration. Am. J. Clin. Nutr. 2008, 87, 1837–1843. [Google Scholar] [CrossRef]
- VandenLangenberg, G.M.; Mares-Perlman, J.A.; Klein, R.; Klein, B.E.; Brady, W.E.; Palta, M. Associations between antioxidant and zinc intake and the 5-year incidence of early age-related maculopathy in the Beaver Dam Eye Study. Am. J. Epidemiol. 1998, 148, 204–214. [Google Scholar] [CrossRef]
- Moeller, S.M.; Parekh, N.; Tinker, L.; Ritenbaugh, C.; Blodi, B.; Wallace, R.B.; Mares, J.A. Associations between intermediate age-related macular degeneration and lutein and zeaxanthin in the Carotenoids in Age-related Eye Disease Study (CAREDS): Ancillary study of the Women’s Health Initiative. Arch. Ophthalmol. 2006, 124, 1151–1162. [Google Scholar] [CrossRef]
- Richer, S.; Stiles, W.; Statkute, L.; Pulido, J.; Frankowski, J.; Rudy, D.; Pei, K.; Tsipursky, M.; Nyland, J. Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: The Veterans LAST study (Lutein Antioxidant Supplementation Trial). Optometry 2004, 75, 216–230. [Google Scholar] [CrossRef]
- Neelam, K.; Hogg, R.E.; Stevenson, M.R.; Johnston, E.; Anderson, R.; Beatty, S.; Chakravarthy, U. Carotenoids and co-antioxidants in age-related maculopathy: Design and methods. Ophthalmic Epidemiol. 2008, 15, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.; van Leeuwen, R.; Witteman, J.C.; van Duijn, C.M.; Uitterlinden, A.G.; Hofman, A.; de Jong, P.T.; Vingerling, J.R.; Klaver, C.C. Reducing the genetic risk of age-related macular degeneration with dietary antioxidants, zinc, and omega-3 fatty acids: The Rotterdam study. Arch. Ophthalmol. 2011, 129, 758–766. [Google Scholar] [CrossRef]
- Cho, E.; Seddon, J.M.; Rosner, B.; Willett, W.C.; Hankinson, S.E. Prospective study of intake of fruits, vegetables, vitamins, and carotenoids and risk of age-related maculopathy. Arch. Ophthalmol. 2004, 122, 883–892. [Google Scholar] [CrossRef]
- Fernandez-Robredo, P.; Recalde, S.; Arnaiz, G.; Salinas-Alaman, A.; Sadaba, L.M.; Moreno-Orduna, M.; Garcia-Layana, A. Effect of zeaxanthin and antioxidant supplementation on vascular endothelial growth factor (VEGF) expression in apolipoprotein-E deficient mice. Curr. Eye Res. 2009, 34, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, H.L.; Tuo, J.; Shen, D.F.; Zhang, J.; Cao, X.; Chew, E.Y.; Chan, C.C. Nutrient supplementation with n3 polyunsaturated fatty acids, lutein, and zeaxanthin decrease A2E accumulation and VEGF expression in the retinas of Ccl2/Cx3cr1-deficient mice on Crb1rd8 background. J. Nutr. 2013, 143, 1129–1135. [Google Scholar] [CrossRef]
- Liu, H.; Liu, W.; Zhou, X.; Long, C.; Kuang, X.; Hu, J.; Tang, Y.; Liu, L.; He, J.; Huang, Z.; et al. Protective effect of lutein on ARPE-19 cells upon H2O2-induced G2/M arrest. Mol. Med. Rep. 2017, 16, 2069–2074. [Google Scholar] [CrossRef]
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef]
- Leasher, J.L.; Bourne, R.R.; Flaxman, S.R.; Jonas, J.B.; Keeffe, J.; Naidoo, K.; Pesudovs, K.; Price, H.; White, R.A.; Wong, T.Y.; et al. Global Estimates on the Number of People Blind or Visually Impaired by Diabetic Retinopathy: A Meta-analysis From 1990 to 2010. Diabetes Care 2016, 39, 1643–1649. [Google Scholar] [CrossRef]
- Duh, E.J.; Sun, J.K.; Stitt, A.W. Diabetic retinopathy: Current understanding, mechanisms, and treatment strategies. JCI Insight 2017, 2, e93751. [Google Scholar] [CrossRef]
- Wu, L.; Fernandez-Loaiza, P.; Sauma, J.; Hernandez-Bogantes, E.; Masis, M. Classification of diabetic retinopathy and diabetic macular edema. World J. Diabetes 2013, 4, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Viswanath, K.; McGavin, D.D. Diabetic retinopathy: Clinical findings and management. Community Eye Health 2003, 16, 21–24. [Google Scholar]
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998, 352, 854–865. [Google Scholar] [CrossRef]
- Zavrelova, H.; Hoekstra, T.; Alssema, M.; Welschen, L.M.; Nijpels, G.; Moll, A.C.; de Vet, H.C.; Polak, B.C.; Dekker, J.M. Progression and regression: Distinct developmental patterns of diabetic retinopathy in patients with type 2 diabetes treated in the diabetes care system west-friesland, the Netherlands. Diabetes Care 2011, 34, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Genuth, S.; Sun, W.; Cleary, P.; Gao, X.; Sell, D.R.; Lachin, J.; Monnier, V.M. Skin advanced glycation end products glucosepane and methylglyoxal hydroimidazolone are independently associated with long-term microvascular complication progression of type 1 diabetes. Diabetes 2015, 64, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Neelam, K.; Goenadi, C.J.; Lun, K.; Yip, C.C.; Au Eong, K.G. Putative protective role of lutein and zeaxanthin in diabetic retinopathy. Br. J. Ophthalmol. 2017, 101, 551–558. [Google Scholar] [CrossRef]
- Early Treatment Diabetic Retinopathy Study research group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch. Ophthalmol. 1985, 103, 1796–1806. [Google Scholar] [CrossRef]
- Mohamed, Q.; Gillies, M.C.; Wong, T.Y. Management of diabetic retinopathy: A systematic review. JAMA 2007, 298, 902–916. [Google Scholar] [CrossRef]
- Wu, L.; Martinez-Castellanos, M.A.; Quiroz-Mercado, H.; Arevalo, J.F.; Berrocal, M.H.; Farah, M.E.; Maia, M.; Roca, J.A.; Rodriguez, F.J. Twelve-month safety of intravitreal injections of bevacizumab (Avastin): Results of the Pan-American Collaborative Retina Study Group (PACORES). Graefe’s Arch. Clin. Exp. Ophthalmol. 2008, 246, 81–87. [Google Scholar] [CrossRef]
- Moschos, M.M.; Dettoraki, M.; Tsatsos, M.; Kitsos, G.; Kalogeropoulos, C. Effect of carotenoids dietary supplementation on macular function in diabetic patients. Eye Vis. (Lond.) 2017, 4, 23. [Google Scholar] [CrossRef]
- Davies, N.P.; Morland, A.B. Color matching in diabetes: Optical density of the crystalline lens and macular pigments. Investig. Ophthalmol. Vis. Sci. 2002, 43, 281–289. [Google Scholar]
- Lima, V.C.; Rosen, R.B.; Maia, M.; Prata, T.S.; Dorairaj, S.; Farah, M.E.; Sallum, J. Macular pigment optical density measured by dual-wavelength autofluorescence imaging in diabetic and nondiabetic patients: A comparative study. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5840–5845. [Google Scholar] [CrossRef] [PubMed]
- Brazionis, L.; Rowley, K.; Itsiopoulos, C.; O’Dea, K. Plasma carotenoids and diabetic retinopathy. Br. J. Nutr. 2009, 101, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.J.; Hu, Y.N.; Lin, S.; Ma, W.J.; Li, X.R. Application of Lutein and Zeaxanthin in nonproliferative diabetic retinopathy. Int. J. Ophthalmol. 2011, 4, 303–306. [Google Scholar]
- Lenzen, S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 2008, 51, 216–226. [Google Scholar] [CrossRef]
- Sasaki, M.; Ozawa, Y.; Kurihara, T.; Kubota, S.; Yuki, K.; Noda, K.; Kobayashi, S.; Ishida, S.; Tsubota, K. Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes. Diabetologia 2010, 53, 971–979. [Google Scholar] [CrossRef]
- Muriach, M.; Bosch-Morell, F.; Alexander, G.; Blomhoff, R.; Barcia, J.; Arnal, E.; Almansa, I.; Romero, F.J.; Miranda, M. Lutein effect on retina and hippocampus of diabetic mice. Free Radic. Biol. Med. 2006, 41, 979–984. [Google Scholar] [CrossRef]
- Arnal, E.; Miranda, M.; Johnsen-Soriano, S.; Alvarez-Nolting, R.; Diaz-Llopis, M.; Araiz, J.; Cervera, E.; Bosch-Morell, F.; Romero, F.J. Beneficial effect of docosahexanoic acid and lutein on retinal structural, metabolic, and functional abnormalities in diabetic rats. Curr. Eye Res. 2009, 34, 928–938. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Zhong, Q.; Santos, J.M.; Thandampallayam, M.; Putt, D.; Gierhart, D.L. Beneficial effects of the nutritional supplements on the development of diabetic retinopathy. Nutr. Metab. (Lond.) 2014, 11, 8. [Google Scholar] [CrossRef]
- Yu, H.; Wark, L.; Ji, H.; Willard, L.; Jaing, Y.; Han, J.; He, H.; Ortiz, E.; Zhang, Y.; Medeiros, D.M.; et al. Dietary wolfberry upregulates carotenoid metabolic genes and enhances mitochondrial biogenesis in the retina of db/db diabetic mice. Mol. Nutr. Food Res. 2013, 57, 1158–1169. [Google Scholar] [CrossRef]
- American Academy of Pediatrics Section on Ophthalmology; American Academy of Ophthalmology; American Association for Pediatric Ophthalmology and Strabismus and American Association of Certified Orthoptists. Screening examination of premature infants for retinopathy of prematurity. Pediatrics 2013, 131, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Blencowe, H.; Lawn, J.E.; Vazquez, T.; Fielder, A.; Gilbert, C. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr. Res. 2013, 74 (Suppl. 1), 35–49. [Google Scholar] [CrossRef] [PubMed]
- Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity: Preliminary results. Pediatrics 1988, 81, 697–706. [Google Scholar]
- Wang, Z.H.; Li, Y.Y.; Liu, Z.M. Birth weight and gestational age on retinopathy of prematurity in discordant twins in China. Int. J. Ophthalmol. 2014, 7, 663–667. [Google Scholar]
- Bashinsky, A.L. Retinopathy of Prematurity. North Carol. Med J. 2017, 78, 124–128. [Google Scholar] [CrossRef]
- Graven, S.N. Early visual development: implications for the neonatal intensive care unit and care. Clin. Perinatol. 2011, 38, 671–683. [Google Scholar] [CrossRef]
- Qanungo, S.; Mukherjea, M. Ontogenic profile of some antioxidants and lipid peroxidation in human placental and fetal tissues. Mol. Cell. Biochem. 2000, 215, 11–19. [Google Scholar] [CrossRef]
- Ates, O.; Alp, H.H.; Caner, I.; Yildirim, A.; Tastekin, A.; Kocer, I.; Baykal, O. Oxidative DNA damage in retinopathy of prematurity. Eur. J. Ophthalmol. 2009, 19, 80–85. [Google Scholar] [CrossRef]
- Mutlu, F.M.; Sarici, S.U. Treatment of retinopathy of prematurity: A review of conventional and promising new therapeutic options. Int. J. Ophthalmol. 2013, 6, 228–236. [Google Scholar]
- Walz, J.M.; Bemme, S.; Reichl, S.; Akman, S.; Breuss, H.; Susskind, D.; Glitz, B.; Muller, V.C.; Wagenfeld, L.; Gabel-Pfisterer, A.; et al. Treated cases of retinopathy of prematurity in Germany: 5-year data from the Retina.net ROP registry. Ophthalmologe 2018, 115, 476–488. [Google Scholar] [CrossRef]
- Stewart, M.W. The expanding role of vascular endothelial growth factor inhibitors in ophthalmology. Mayo Clin. Proc. 2012, 87, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Perrone, S.; Longini, M.; Marzocchi, B.; Picardi, A.; Bellieni, C.V.; Proietti, F.; Rodriguez, A.; Turrisi, G.; Buonocore, G. Effects of lutein on oxidative stress in the term newborn: A pilot study. Neonatology 2010, 97, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, C.; Tirone, C.; Persichilli, S.; Gervasoni, J.; Zuppi, C.; Barone, G.; Zecca, E. Lutein absorption in premature infants. Eur. J. Clin. Nutr. 2010, 64, 760–761. [Google Scholar] [CrossRef]
- Dani, C.; Lori, I.; Favelli, F.; Frosini, S.; Messner, H.; Wanker, P.; De Marini, S.; Oretti, C.; Boldrini, A.; Ciantelli, M.; et al. Lutein and zeaxanthin supplementation in preterm infants to prevent retinopathy of prematurity: A randomized controlled study. J. Matern. Fetal Neonatal Med. 2012, 25, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, C.; Giannantonio, C.; Cota, F.; Papacci, P.; Vento, G.; Valente, E.; Purcaro, V.; Costa, S. A prospective, randomized, double blind study comparing lutein to placebo for reducing occurrence and severity of retinopathy of prematurity. J. Matern. Fetal Neonatal Med. 2011, 24 (Suppl. 1), 147–150. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.P.; Chan, G.M.; Barrett-Reis, B.M.; Fulton, A.B.; Hansen, R.M.; Ashmeade, T.L.; Oliver, J.S.; Mackey, A.D.; Dimmit, R.A.; Hartmann, E.E.; et al. Effect of carotenoid supplementation on plasma carotenoids, inflammation and visual development in preterm infants. J. Perinatol. 2012, 32, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, P.; Guardione, R.; Bonetti, P.; Priolo, C.; Maestri, A.; Mansoldo, C.; Mostert, M.; Anselmetti, G.; Sardei, D.; Bellettato, M.; et al. Lutein and zeaxanthin supplementation in preterm very low-birth-weight neonates in neonatal intensive care units: A multicenter randomized controlled trial. Am. J. Perinatol. 2013, 30, 25–32. [Google Scholar] [CrossRef]
- Zielinska, M.A.; Wesolowska, A.; Pawlus, B.; Hamulka, J. Health Effects of Carotenoids during Pregnancy and Lactation. Nutrients 2017, 9, 838. [Google Scholar] [CrossRef]
- Phelan, D.; Prado-Cabrero, A.; Nolan, J.M. Stability of Commercially Available Macular Carotenoid Supplements in Oil and Powder Formulations. Nutrients 2017, 9, 1133. [Google Scholar] [CrossRef]
- Smith, L.E.; Wesolowski, E.; McLellan, A.; Kostyk, S.K.; D’Amato, R.; Sullivan, R.; D’Amore, P.A. Oxygen-induced retinopathy in the mouse. Investig. Ophthalmol. Vis. Sci. 1994, 35, 101–111. [Google Scholar]
- Penn, J.S.; Tolman, B.L.; Henry, M.M. Oxygen-induced retinopathy in the rat: Relationship of retinal nonperfusion to subsequent neovascularization. Investig. Ophthalmol. Vis. Sci. 1994, 35, 3429–3435. [Google Scholar]
- Tsang, J.K.W.; Liu, J.; Lo, A.C.Y. Vascular and Neuronal Protection in the Developing Retina: Potential Therapeutic Targets for Retinopathy of Prematurity. Int. J. Mol. Sci. 2019, 20, 4321. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Meng, S.S.; Burnim, S.B.; Smith, L.E.; Lo, A.C. Lutein facilitates physiological revascularization in a mouse model of retinopathy of prematurity. Clin. Exp. Ophthalmol. 2017, 45, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Morgan, I.G.; Ohno-Matsui, K.; Saw, S.M. Myopia. Lancet 2012, 379, 1739–1748. [Google Scholar] [CrossRef]
- Saw, S.M.; Gazzard, G.; Shih-Yen, E.C.; Chua, W.H. Myopia and associated pathological complications. Ophthalmic Physiol. Opt. 2005, 25, 381–391. [Google Scholar] [CrossRef]
- EJ, T. The Optical Elements of the Refractive Power of the Eye; Hoeber Press: New York, NY, USA, 1940. [Google Scholar]
- Damgaard, I.B.; Reffat, M.; Hjortdal, J. Review of Corneal Biomechanical Properties Following LASIK and SMILE for Myopia and Myopic Astigmatism. Open Ophthalmol. J. 2018, 12, 164–174. [Google Scholar] [CrossRef]
- Williams, K.M.; Bentham, G.C.; Young, I.S.; McGinty, A.; McKay, G.J.; Hogg, R.; Hammond, C.J.; Chakravarthy, U.; Rahu, M.; Seland, J.; et al. Association Between Myopia, Ultraviolet B Radiation Exposure, Serum Vitamin D Concentrations, and Genetic Polymorphisms in Vitamin D Metabolic Pathways in a Multicountry European Study. JAMA Ophthalmol. 2017, 135, 47–53. [Google Scholar] [CrossRef]
- Tanito, M.; Obana, A.; Gohto, Y.; Okazaki, S.; Gellermann, W.; Ohira, A. Macular pigment density changes in Japanese individuals supplemented with lutein or zeaxanthin: Quantification via resonance Raman spectrophotometry and autofluorescence imaging. Jpn. J. Ophthalmol. 2012, 56, 488–496. [Google Scholar] [CrossRef]
- Benoudis, L.; Ingrand, P.; Jeau, J.; Lichtwitz, O.; Boissonnot, M.; Leveziel, N. Relationships between macular pigment optical density and lacquer cracks in high myopia. J. Fr. Ophtalmol. 2016, 39, 615–621. [Google Scholar] [CrossRef]
- Garcia, M.; Jha, A.K.; Healy, K.E.; Wildsoet, C. A soft hyaluronic acid-based hydrogel can control myopia progression in guinea pigs. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4632. [Google Scholar]
- Fagerholm, P.; Hamberg-Nystrom, H.; Tengroth, B. Wound healing and myopic regression following photorefractive keratectomy. Acta Ophthalmol. (Copenh.) 1994, 72, 229–234. [Google Scholar] [CrossRef]
- Sayo, T.; Sugiyama, Y.; Inoue, S. Lutein, a nonprovitamin A, activates the retinoic acid receptor to induce HAS3-dependent hyaluronan synthesis in keratinocytes. Biosci. Biotechnol. Biochem. 2013, 77, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Tong, N.; Zhang, W.; Zhang, Z.; Gong, Y.; Wooten, B.; Wu, X. Inverse relationship between macular pigment optical density and axial length in Chinese subjects with myopia. Graefes. Arch. Clin. Exp. Ophthalmol. 2013, 251, 1495–1500. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.H.; Gilbert, C.E.; Spector, T.D.; Mellerio, J.; Van Kuijk, F.J.; Beatty, S.; Fitzke, F.; Marshall, J.; Hammond, C.J. Central retinal thickness is positively correlated with macular pigment optical density. Exp. Eye Res. 2006, 82, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.R.; Liang, Y.B.; Friedman, D.S.; Sun, L.P.; Wong, T.Y.; Tao, Q.S.; Bao, L.; Wang, N.L.; Wang, J.J. Normal macular thickness measurements using optical coherence tomography in healthy eyes of adult Chinese persons: The Handan Eye Study. Ophthalmology 2010, 117, 1585–1594. [Google Scholar] [CrossRef]
- van der Veen, R.L.; Ostendorf, S.; Hendrikse, F.; Berendschot, T.T. Macular pigment optical density relates to foveal thickness. Eur. J. Ophthalmol. 2009, 19, 836–841. [Google Scholar] [CrossRef]
- Zhang, X.R.; Zhang, Z.Y. Comments on “Inverse relationship between macular pigment optical density and axial length in Chinese subjects with myopia”. Graefe’s Arch. Clin. Exp. Ophthalmol. 2013, 251, 2285. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Wilkins, M.; Kim, T.; Malyugin, B.; Mehta, J.S. Cataracts. Lancet 2017, 390, 600–612. [Google Scholar] [CrossRef]
- Thompson, J.; Lakhani, N. Cataracts. Prim. Care 2015, 42, 409–423. [Google Scholar] [CrossRef]
- Trevor-Roper, P.D. Cataracts. Br. Med. J. 1970, 3, 33–35. [Google Scholar] [CrossRef][Green Version]
- Gupta, V.B.; Rajagopala, M.; Ravishankar, B. Etiopathogenesis of cataract: An appraisal. Indian J. Ophthalmol. 2014, 62, 103–110. [Google Scholar] [CrossRef]
- Asbell, P.A.; Dualan, I.; Mindel, J.; Brocks, D.; Ahmad, M.; Epstein, S. Age-related cataract. Lancet 2005, 365, 599–609. [Google Scholar] [CrossRef]
- Reddy, V.N. Glutathione and its function in the lens—an overview. Exp. Eye Res. 1990, 50, 771–778. [Google Scholar] [CrossRef]
- Lim, J.C.; Grey, A.C.; Zahraei, A.; Donaldson, P.J. Age-dependent changes in glutathione metabolism pathways in the lens: New insights into therapeutic strategies to prevent cataract formation—A Review. Clin. Exp. Ophthalmol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lyle, B.J.; Mares-Perlman, J.A.; Klein, B.E.; Klein, R.; Greger, J.L. Antioxidant intake and risk of incident age-related nuclear cataracts in the Beaver Dam Eye Study. Am. J. Epidemiol. 1999, 149, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Karppi, J.; Laukkanen, J.A.; Kurl, S. Plasma lutein and zeaxanthin and the risk of age-related nuclear cataract among the elderly Finnish population. Br. J. Nutr. 2012, 108, 148–154. [Google Scholar] [CrossRef]
- Yeum, K.J.; Taylor, A.; Tang, G.; Russell, R.M. Measurement of carotenoids, retinoids, and tocopherols in human lenses. Investig. Ophthalmol. Vis. Sci. 1995, 36, 2756–2761. [Google Scholar]
- Gao, S.; Qin, T.; Liu, Z.; Caceres, M.A.; Ronchi, C.F.; Chen, C.Y.; Yeum, K.J.; Taylor, A.; Blumberg, J.B.; Liu, Y.; et al. Lutein and zeaxanthin supplementation reduces H2O2-induced oxidative damage in human lens epithelial cells. Mol. Vis. 2011, 17, 3180–3190. [Google Scholar]
- Chew, E.Y.; SanGiovanni, J.P.; Ferris, F.L.; Wong, W.T.; Agron, E.; Clemons, T.E.; Sperduto, R.; Danis, R.; Chandra, S.R.; Blodi, B.A.; et al. Lutein/zeaxanthin for the treatment of age-related cataract: AREDS2 randomized trial report no. 4. JAMA Ophthalmol. 2013, 131, 843–850. [Google Scholar] [CrossRef]
| Food Items | Lutein (μg/g Fresh Weight) | Zeaxanthin (μg/g Fresh Weight) |
|---|---|---|
| Vegetables | ||
| Basil | 70.5 | |
| Kale | 48.0–114.7 | |
| Leek | 36.8 | |
| Parsley | 64.0–106.5 | |
| Red pepper | 2.5–85.1 | 5.9–13.5 |
| Egg | ||
| Egg yolk | 3.8–13.2 | |
| Nuts | ||
| Pistachio | 7.7–49.0 | |
| Grains | ||
| Corn | 21.9 | 10.3 |
| Einkorn wheat | 7.4 | 0.9 |
| Khorasan wheat | 5.5 | 0.7 |
| Durum wheat | 5.4 | 0.5 |
| Basic Clinical Classification [59,60] | |||
|---|---|---|---|
| Stage | Drusen | Pigment Abnormalities | Additional Features |
| No aging changes | Absent | Absent | Nil |
| Normal aging changes | Small (≤63 μm) | Absent | Nil |
| Early AMD | Medium (>63 μm but ≤125 μm) | Absent | Nil |
| Intermediate AMD | Large (>125 μm) | Present | Nil |
| Late AMD | Large (>125 μm) | Present | Neovascular AMD/geographic atrophy |
| Age-Related Eye Disease Study (AREDS) Classification [61] | |
|---|---|
| Category | |
| 1 | No drusen/Small, non-extensive drusen in both eyes |
| 2 | Small, extensive drusen/Intermediate, non-extensive drusen/Pigment abnormalities in at least one eye |
| 3 | Intermediate extensive drusen/Large drusen/Noncentral geographic atrophy in at least one eye |
| 4 | Advanced age-related macular degeneration defined by geographic atrophy, retinal pigment epithelial detachment in one eye, choroidal neovascularization or scars of confluent photocoagulation/Visual acuity less than 20/32 induced by lesions like large drusen in the fovea in only one eye due to nonadvanced age-related macular degeneration |
| Name | Study Design | Subject | Results | |
|---|---|---|---|---|
| Arch Ophthalmol 1993 [76] | Eye Disease Case-Control Study (EDCCS) | Case control study | 421 AMD patients, 615 controls | High serum lutein level reduces neovascular AMD risk |
| Richer 2004 [84] | Lutein Antioxidant Supplementation Trial (LAST) | Randomized control trial, 12-month follow up | 90 Atrophic AMD patients in USA | Lutein supplements improve visual function |
| Richer 2007 [78] | Lutein Antioxidant Supplementation Trial II (LASTII) | Randomized control trial, 12-month follow up | 90 Atrophic AMD patients in USA | Lutein increases macular pigment optical density (MPOD) |
| Tan 2008 [75] | The Blue Mountains Eye Study (BMES) | Population based cohort study, follow up after 5 and 10 years | 2454 Australians aged ≥49 | High lutein intake reduces long-term AMD risk |
| Neelam 2008 [85] | Carotenoids and co-antioxidants in age-related maculopathy (CARMA) study | Randomized control trial, 12-month follow up | 433 Caucasian AMD patients aged ≥55 | Lutein increases both macular pigment level and visual acuity |
| Ho 2011 [86] | The Rotterdam Study | Nested case-control study, mean 8.6 year follow up | 2167 individuals aged ≥55 with genetic variants CFH Y402H and LOC387715 A69S | High lutein intake reduces early AMD risk in those at high genetic risk |
| Weigert 2011 [79] | Lutein Intervention Study Austria (LISA) | Randomized control trial, 6-month follow up | 126 AMD patients | Lutein increases MPOD |
| Age-Related Eye Disease Study 2 Research Group 2013 [50] | Age-Related Eye Disease Study 2 (AREDS2) | Randomized control trial | 4203 intermediate or advanced AMD patients aged 50 to 85 | AREDS2 formula containing lutein reduces progression to advanced AMD |
| Murray 2013 [80] | Combination of Lutein Effects in the Aging Retina (CLEAR) study | Randomized control trial, 12- month duration | 72 patients, mean age of 70.5 | Lutein increases MPOD and slows down visual acuity reduction |
| Name | Study Design | Subject | Results | |
|---|---|---|---|---|
| VandenLangenberg 1998 [82] | Beaver Dam Study | Population based cohort study, 5-year incidence | 1709 adults in USA | Too few incidence, unable to show association of lutein with age-related maculopathy |
| Moeller 2006 [83] | Carotenoids in Age-related Eye Disease Study (CAREDS) | Population based ancillary study, 6 years prevalence | 1787 women, aged 50–79 | Lowered odds ratio of intermediate AMD only in age group >75 |
| Cho 2008 [87] | Nurses’ Health Study, Health Professionals Follow-up Study | Prospective follow-up study | 77562 women 40,866 men, aged ≥50 | Lutein intake not strongly related to age-related maculopathy |
| Cho 2008 [81] | Nurses’ Health Study, Health Professionals Follow-up Study | Prospective follow-up study | 71494 women and 41,564 men, aged ≥50 | Lutein has no protective role against early AMD |
| DR Severity Scale | Characteristics |
|---|---|
| No apparent retinopathy | No recognizable diabetic fundus changes |
| Mild Non-proliferative diabetic retinopathy (NPDR) | Presence of at least one microaneurysm |
| Moderate NPDR | Presence of microaneurysms, intraretinal hemorrhages or venous beading |
| Severe NPDR | Presence of hemorrhages in all 4 fundus quadrants, venous beading in at least 2 quadrants, or intraretinal microvascular abnormalities (IRMA) |
| PDR | Presence of neovascularization of the disc, the retina, the iris, or the angle, or presence of vitreous hemorrhage or tractional retinal detachment |
| Study Design | Subject | Results | |
|---|---|---|---|
| Davies 2002 [104] | Case control study | 30 non-diabetic subjects 26 diabetic subjects | MPOD is lower in diabetic subjects compared to non-diabetic group. MPOD is also lower in diabetic patients with DR compared to diabetic patients without |
| Lima 2010 [105] | Case control study | 14 non-diabetic subjects 17 diabetic subjects without DR 12 diabetic subjects with NPDR | MPOD is lower in type 2 diabetic subjects (with or without DR) compared to non-diabetic group |
| Study Design | Subject | Results | |
|---|---|---|---|
| Brazionis 2009 [106] | Cross-sectional study | 78 diabetic subjects without DR 33 diabetic subjects with NPDR | Plasma level of combined lutein and zeaxanthin is lower in patients with NPDR than those without NPDR |
| Hu 2011 [107] | Interventional study | 30 non-diabetic subjects 30 NPDR subjects with lutein supplement 30 NPDR subjects without lutein supplement | Administration of lutein and zeaxanthin increase their plasma levels and improve visual acuity and contrast sensitivity in NPDR |
| Zhang 2017 [42] | Randomized control trial | 31 NPDR subjects randomized into lutein and placebo group | Administration of lutein improves contrast sensitivity in NPDR patients |
| Subjects | Treatment | Results | |
|---|---|---|---|
| Romagnoli 2011 [128] | Preterm infants <33 week gestational age 31 treatment group 32 control group | lutein (0.5 mg/kg), zeaxanthin (0.02 mg/kg) | Lutein did not lead to significance difference in ROP incidence |
| Rubin 2012 [129] | Preterm infants <33 week gestational age 92 treatment group 91 control group | lutein/zeaxanthin, lycopene and β-carotene (24 kcal/oz in hospital, 22 kcal/oz post-discharge) | Supplementation raised plasma lutein level and rod photoreceptor sensitivity. No significance difference in ROP incidence |
| Dani 2012 [127] | Preterm infants <33 week gestational age 58 treatment group 56 control group | lutein (0.14 mg), zeaxanthin (0.006 mg) | Lutein did not affect outcome of ROP |
| Manzoni 2013 [130] | 229 preterm infants <32 week gestational age | lutein (0.14 mg), zeaxanthin (0.0006 mg) | Lutein did not lead to significance difference in ROP outcome |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.H.; Lee, J.C.-Y.; Leung, H.H.; Lam, W.C.; Fu, Z.; Lo, A.C.Y. Lutein Supplementation for Eye Diseases. Nutrients 2020, 12, 1721. https://doi.org/10.3390/nu12061721
Li LH, Lee JC-Y, Leung HH, Lam WC, Fu Z, Lo ACY. Lutein Supplementation for Eye Diseases. Nutrients. 2020; 12(6):1721. https://doi.org/10.3390/nu12061721
Chicago/Turabian StyleLi, Long Hin, Jetty Chung-Yung Lee, Ho Hang Leung, Wai Ching Lam, Zhongjie Fu, and Amy Cheuk Yin Lo. 2020. "Lutein Supplementation for Eye Diseases" Nutrients 12, no. 6: 1721. https://doi.org/10.3390/nu12061721
APA StyleLi, L. H., Lee, J. C.-Y., Leung, H. H., Lam, W. C., Fu, Z., & Lo, A. C. Y. (2020). Lutein Supplementation for Eye Diseases. Nutrients, 12(6), 1721. https://doi.org/10.3390/nu12061721






