Hypoxia-Inducible Factor Inhibitors Derived from Marine Products Suppress a Murine Model of Neovascular Retinopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Marine Product Preparation
2.2. Luciferase Assay for Fish Screening
2.3. Real-Time PCR
2.4. Western Blotting
2.5. Animals
2.6. Oxygen-Induced Retinopathy Model and Administration of Fish Gradients
2.7. Statistical Analysis
3. Results
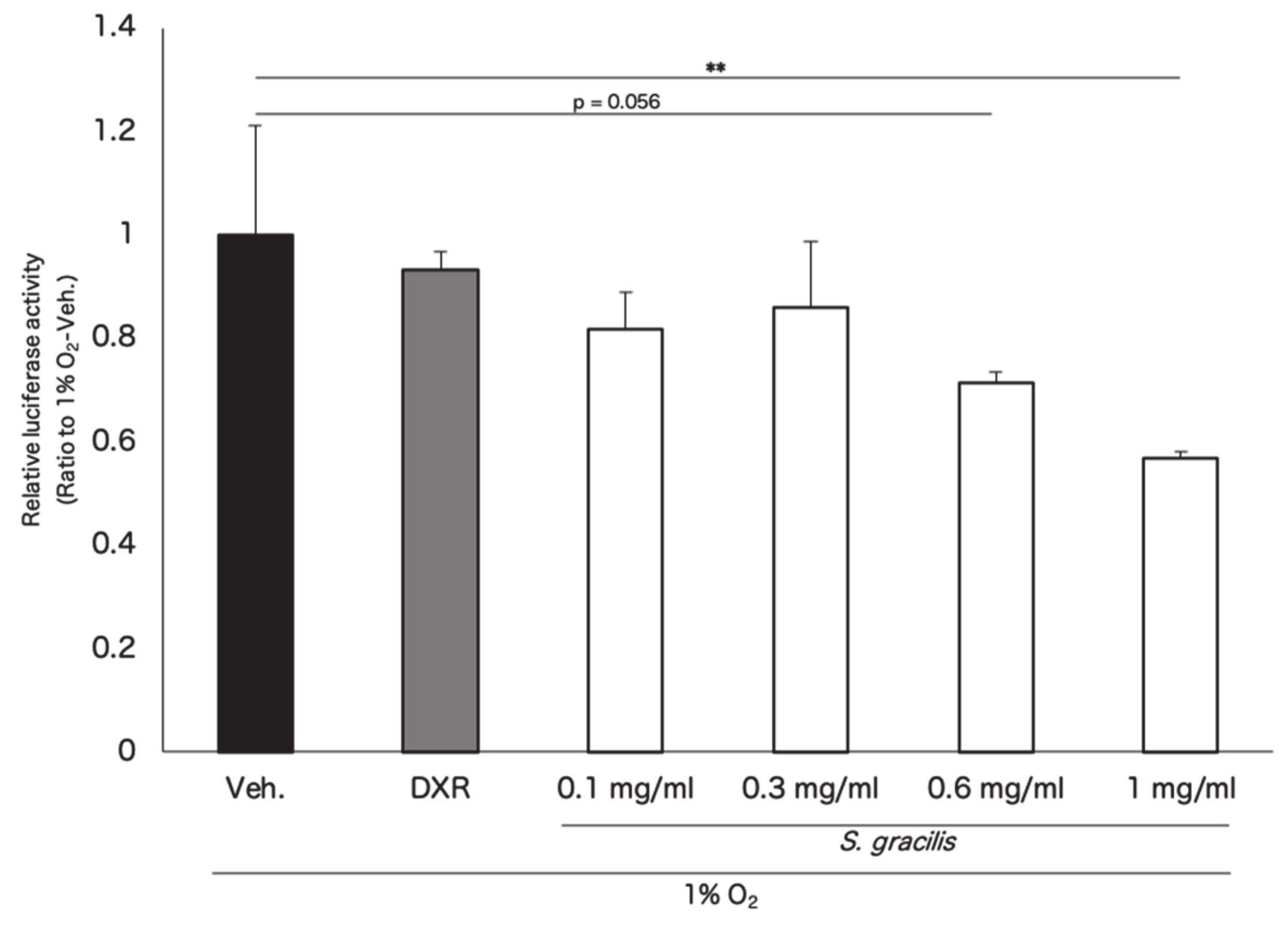
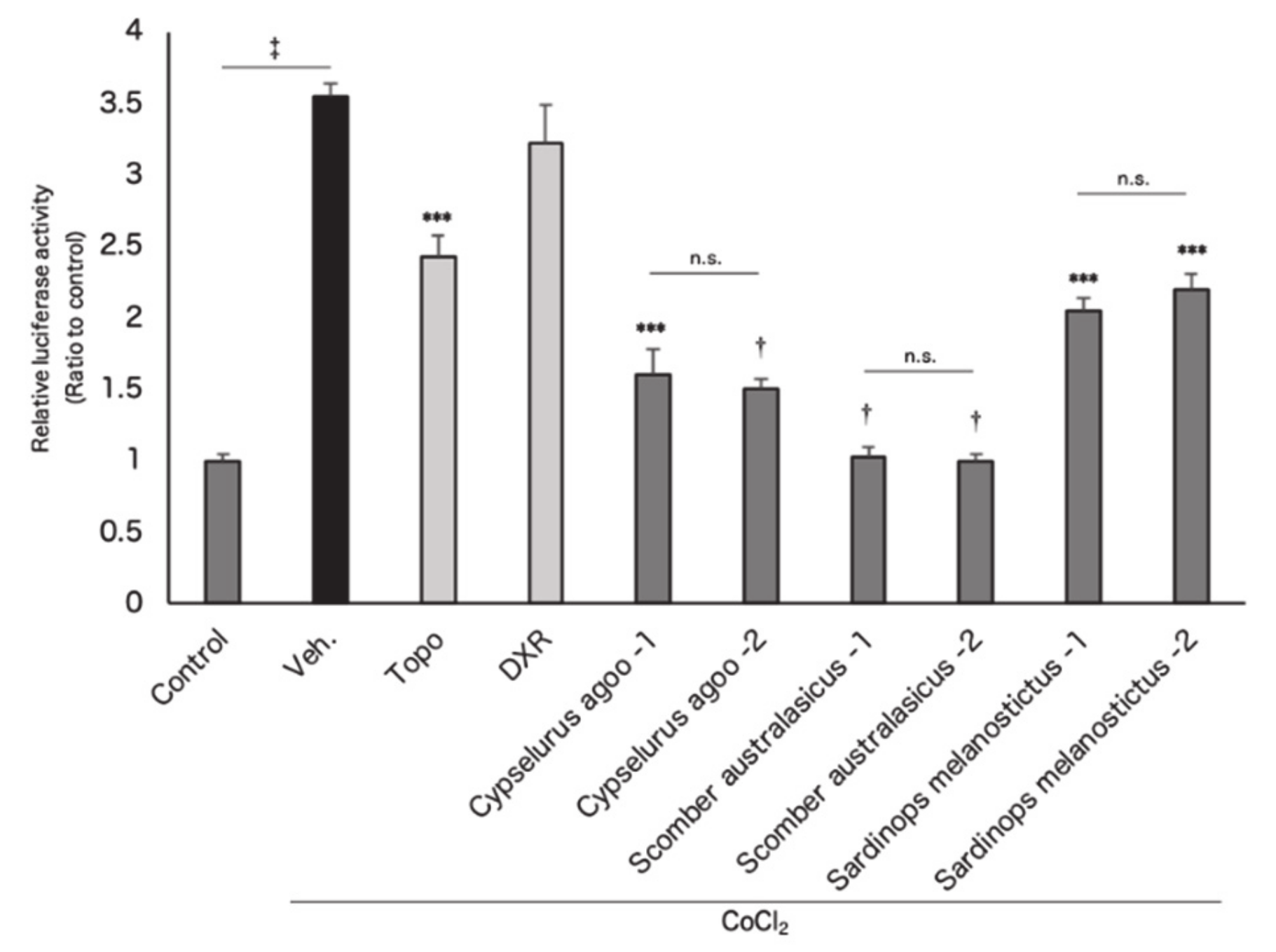
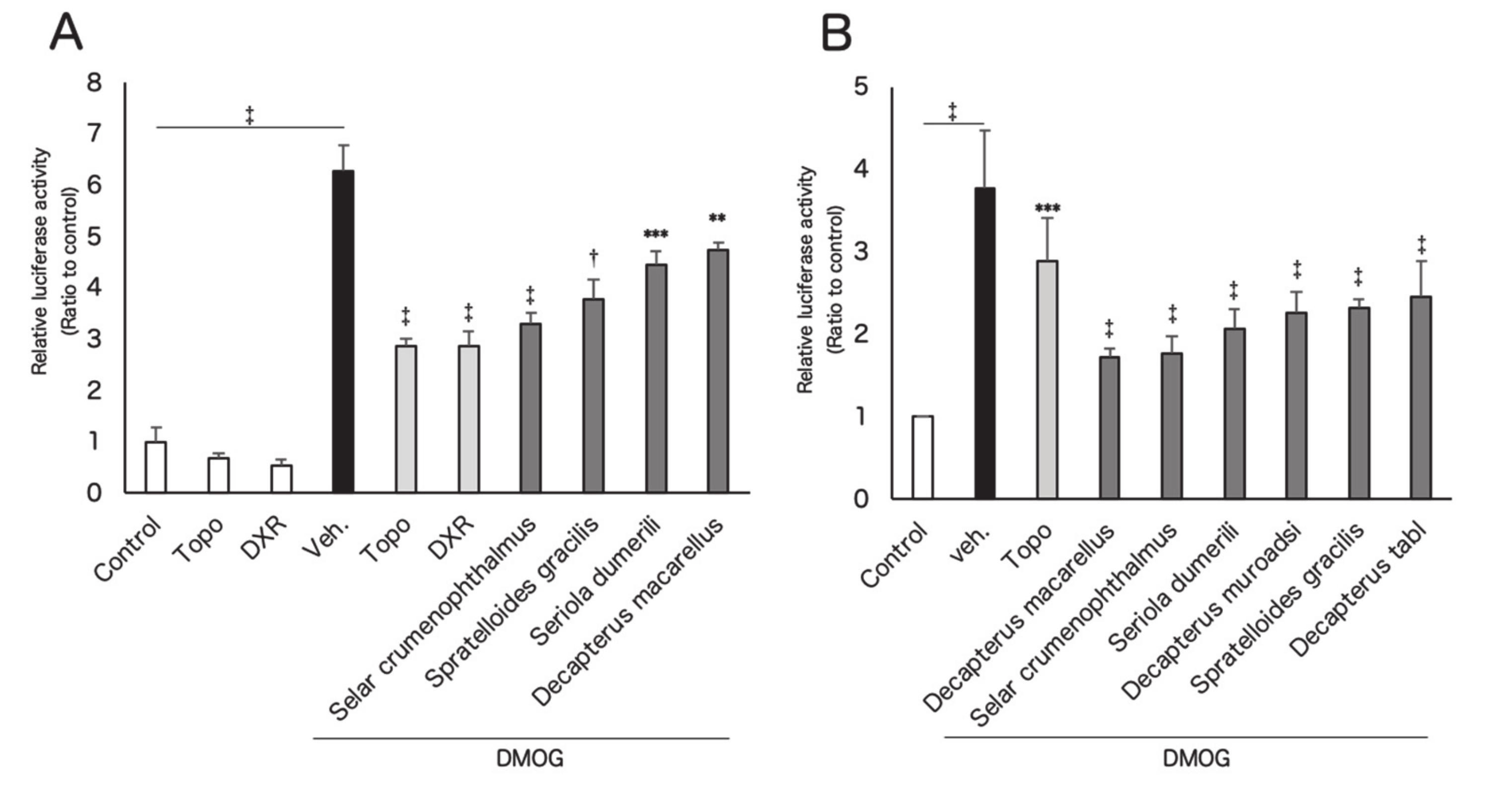
3.1. In Vitro Screening for Hypoxia-Inducible Factor (HIF) Inhibitors from Marine Products
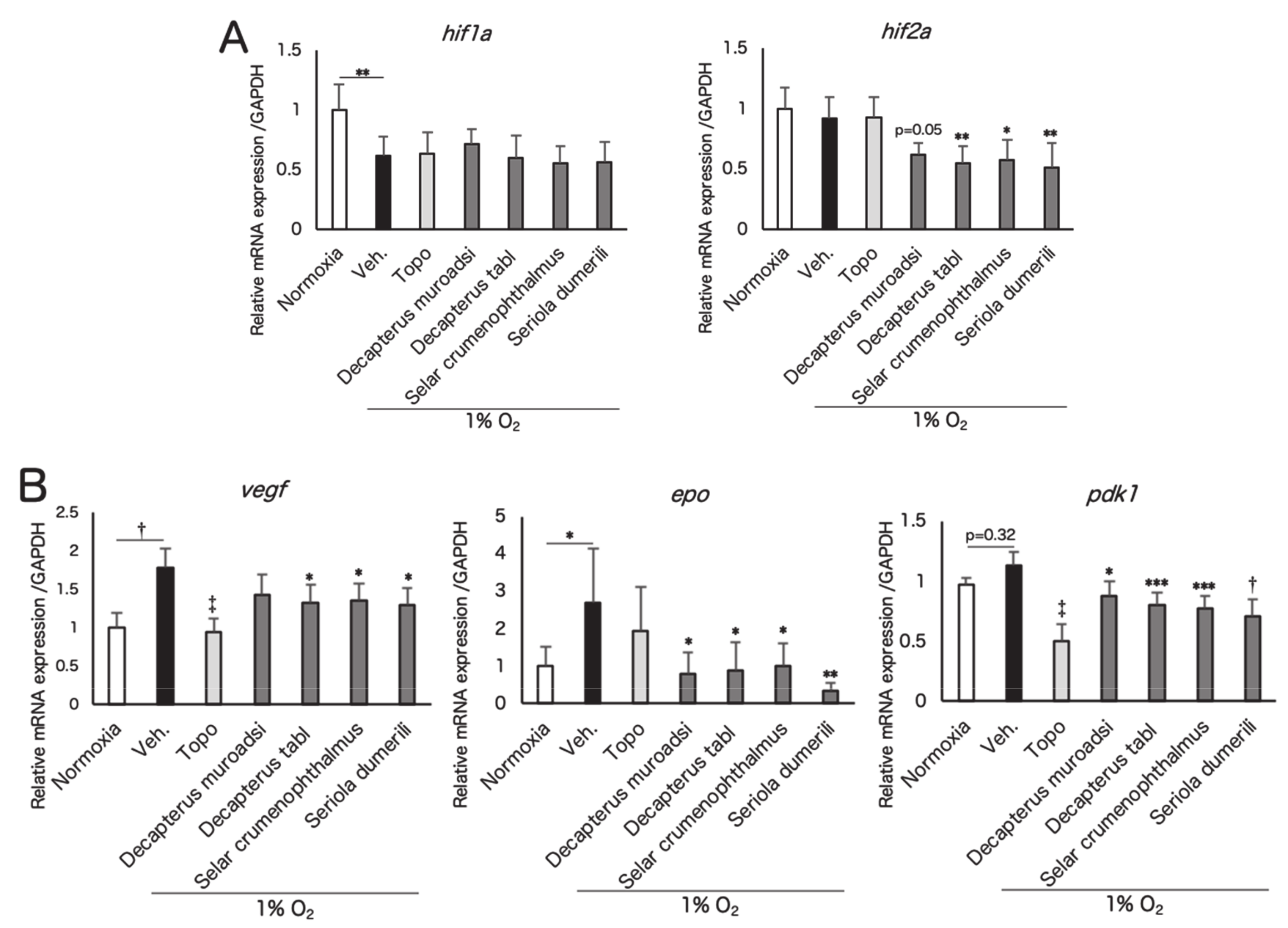
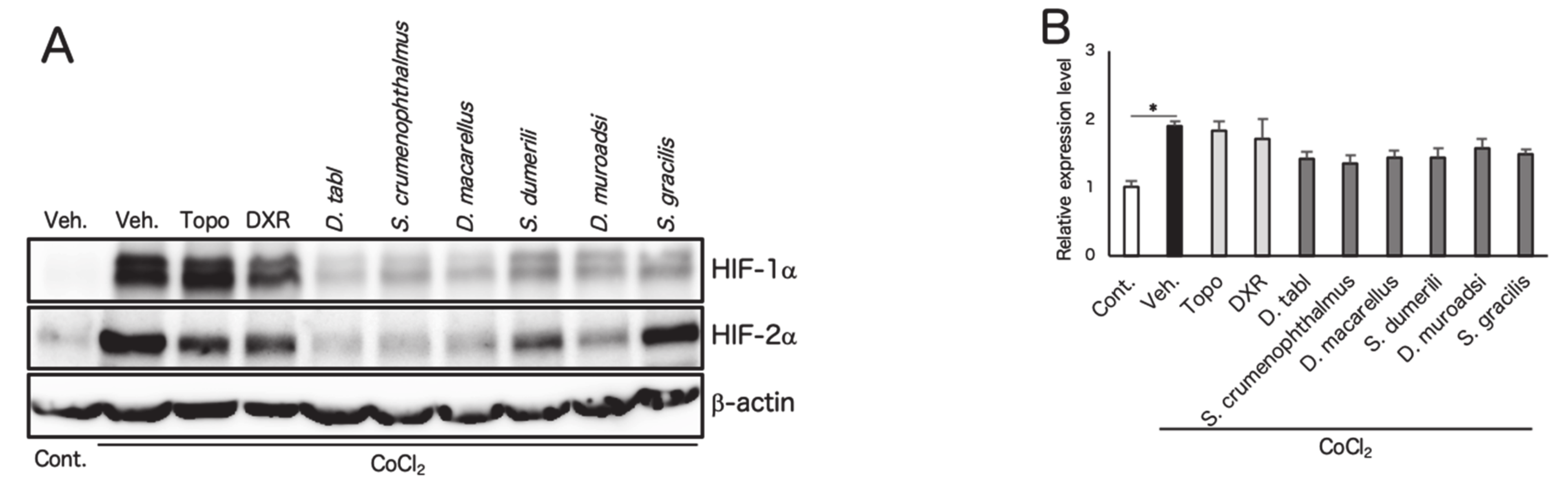
3.2. Screened Fish Ingredients Inhibit HIF and HIF Target Genes In Vitro
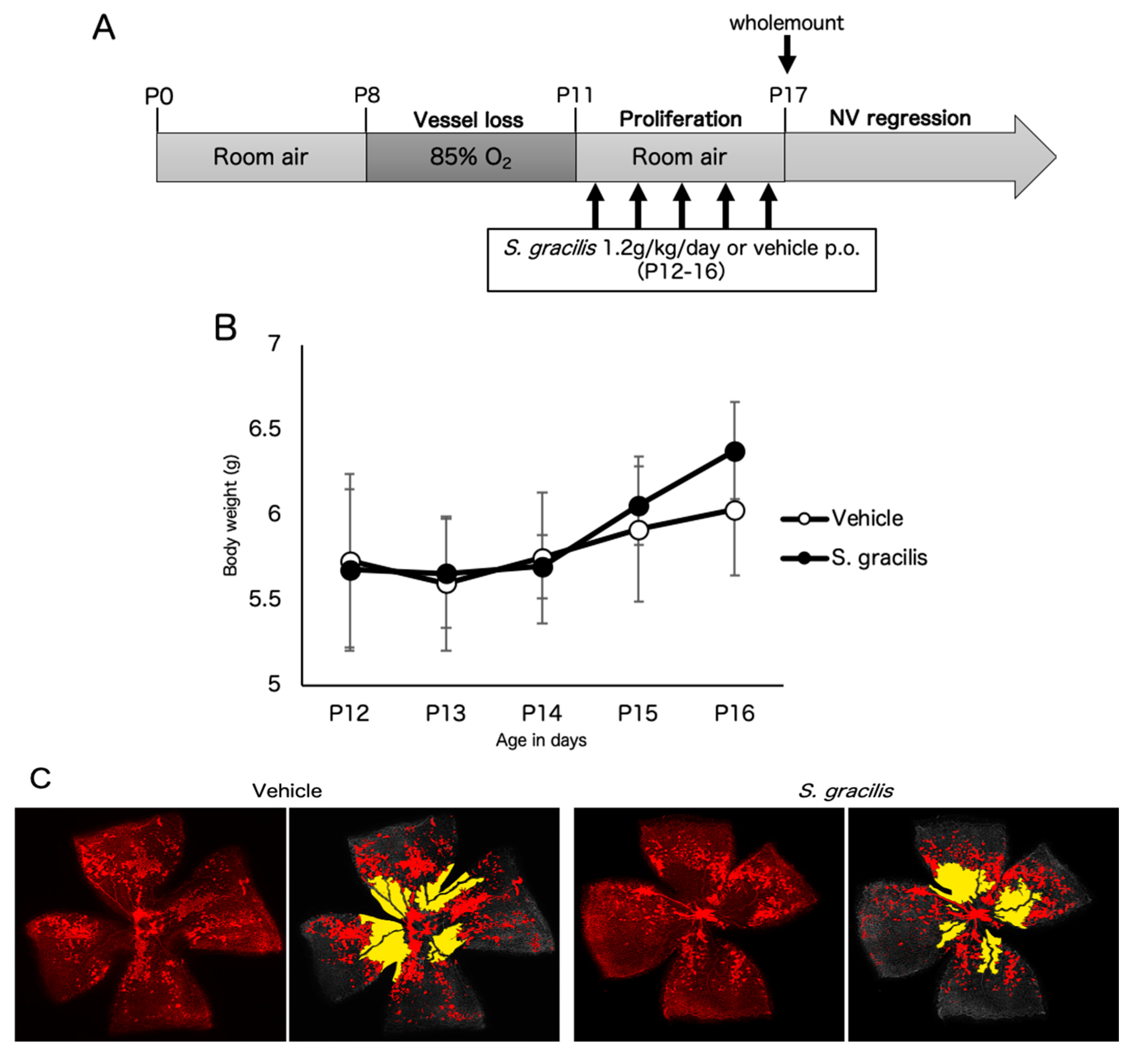
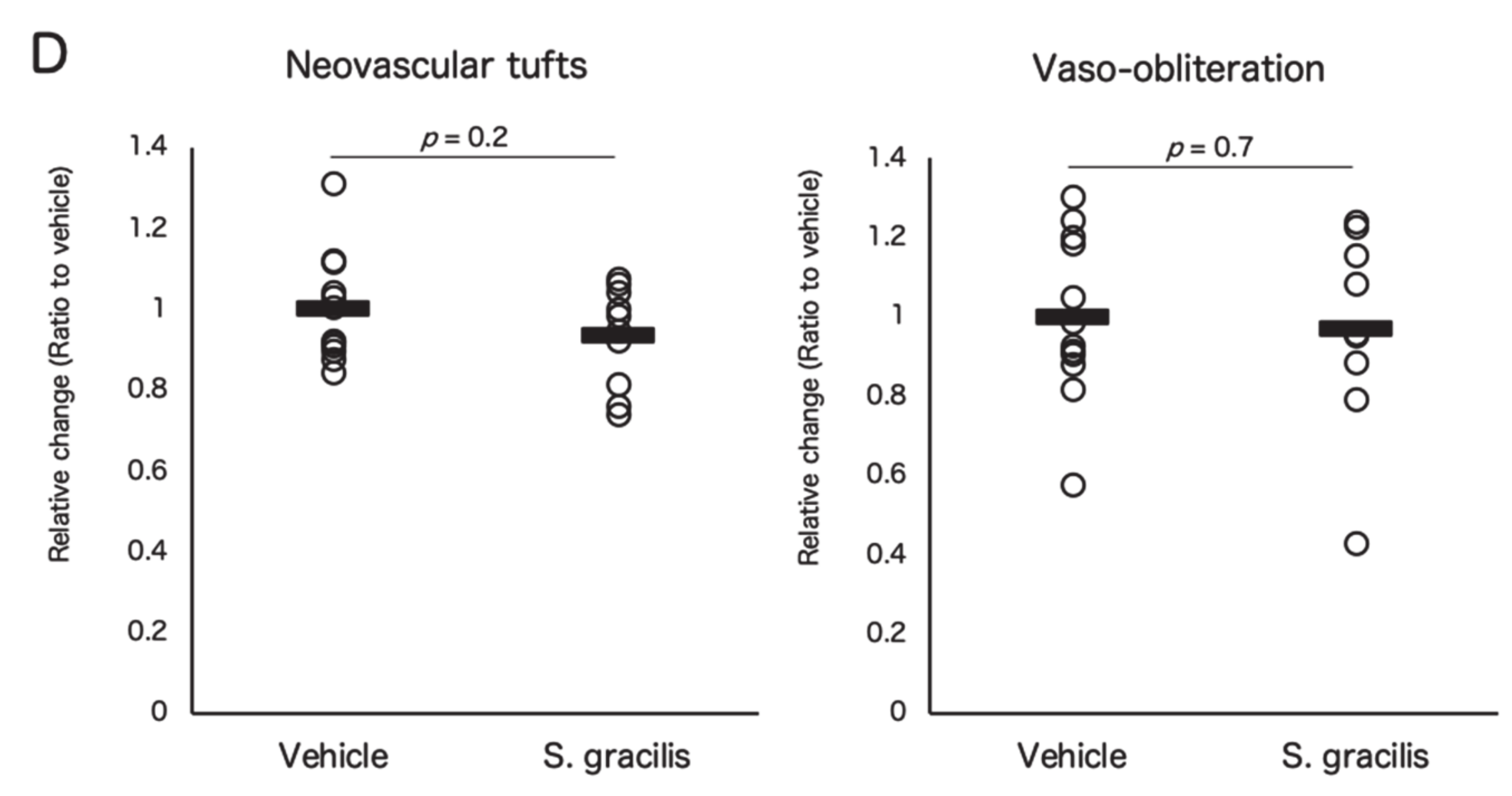
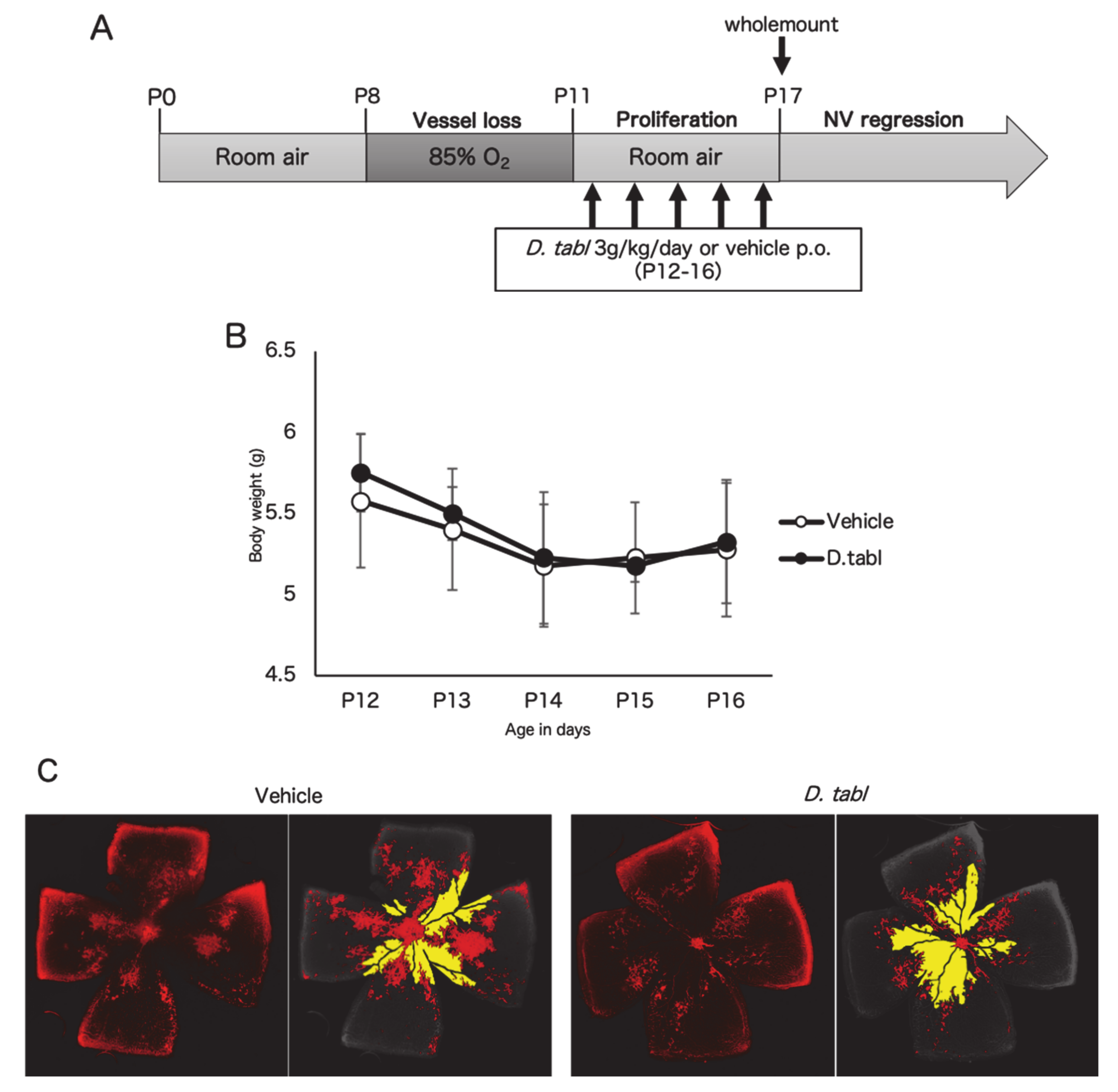
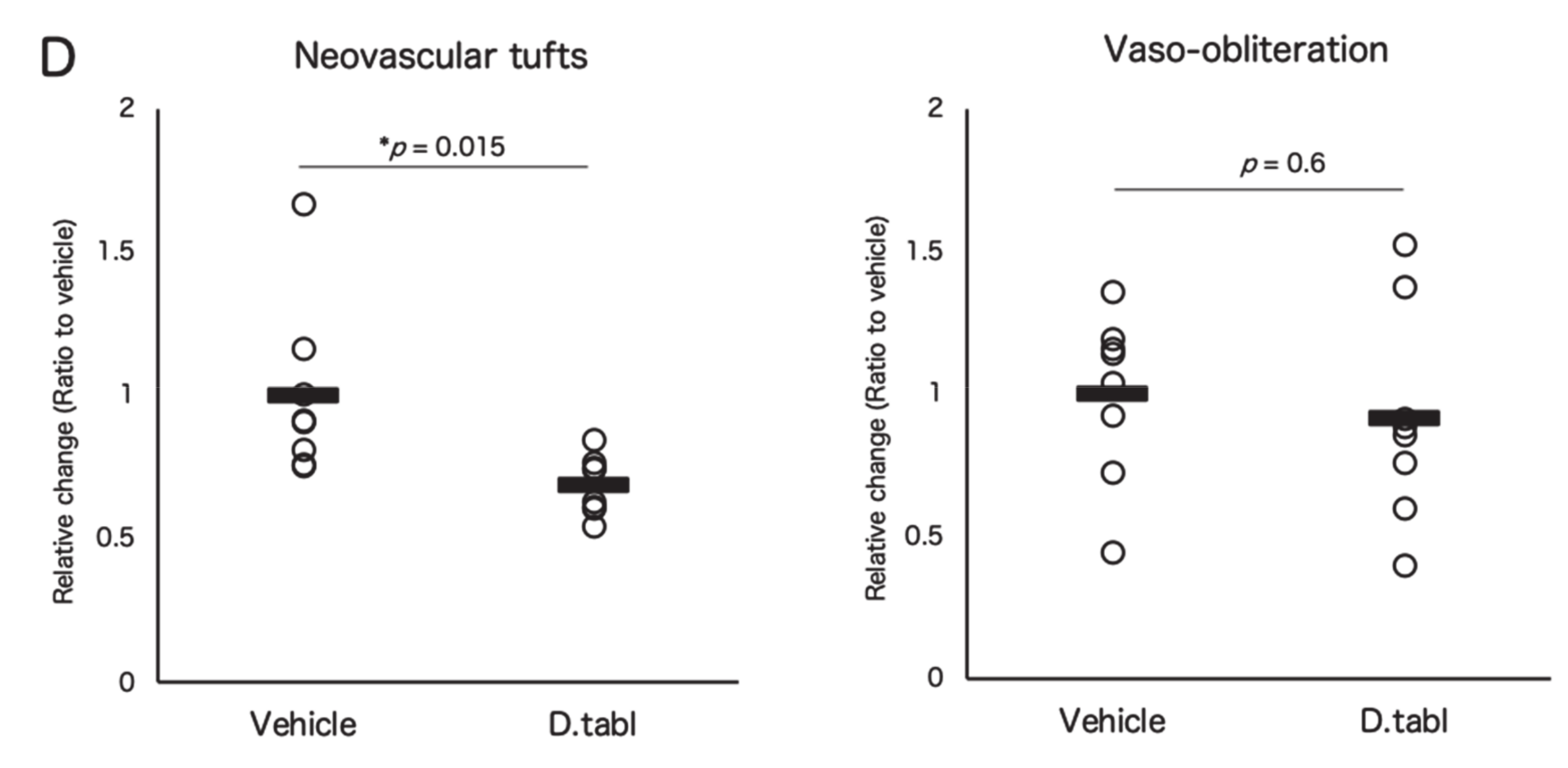
3.3. Fish Ingredients Suppressed Neovascularization in a Murine Oxygen-Induced Retinopathy (OIR) Model
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Species of Fish | Japanese Name | Sampling Parts | Rate of Change (%) |
|---|---|---|---|
| Scomber australasicus | Gomasaba | white muscle, dorsal | −75.0 |
| Fermented dried bonito | Honkare-bushi | - | −70.9 |
| Dried bonito | Ara-bushi | - | −70.9 |
| Katsuwonus pelamis | Katsuo | white muscle, dorsal | −68.0 |
| Scomber japonicus | Masaba | white muscle, dorsal | −68.0 |
| Thunnus obesus | Mebachi | white muscle, dorsal | −65.0 |
| Etrumeus teres | Urumeiwashi | skinless fillet | −56.5 |
| Cypselurus agoo | Tobiuo | white muscle, dorsal | −55.8 |
| Dried mackerel | Saba-bushi | - | −50.5 |
| Spratelloides gracilis | Kibinago | headless | −49.5 |
| Thunnus obesus | Mebachi | head muscle | −49.5 |
| Trachurus japonicus | Maaji | skinless fillet | −47.6 |
| Topotecan † | - | - | −40.0 |
| Thunnus obesus | Mebachi | Pectoral fin muscle | −39.8 |
| Scomberomorus niphonius | Sawara | white muscle, dorsal | −38.8 |
| Doxorubicin † | - | - | −37.5 |
| Selar crumenophthalmus | Meaji | skinless fillet | −35.5 |
| Sardinops melanostictus | Maiwasi | whole | −33.3 |
| Decapterus macarellus | Kusayamoro | skinless fillet | −31.0 |
| Seriola dumerili | Kanpachi | white muscle, dorsal | −26.5 |
| Coryphaena hippurus | Siira | white muscle, dorsal | −25.0 |
| Pagrus major | Madai | white muscle, dorsal | −24.3 |
| Dried sardine | Iwashi-bushi | - | −24.3 |
| Rhabdosargus sarba | Hedai | white muscle, dorsal | −23.7 |
| Panulirus japonicus | Ise-ebi | muscle, abdomen | −14.5 |
| Seriola quinqueradiata | Buri | white muscle, dorsal | −11.5 |
| Haliotis diversicolor aquatilis | Tokobushi | muscle, foot | −10.0 |
| Scomberoides lysan | Ikekatsuo | skinless fillet | −4.9 |
| Marsupenaeus japonicus | Kuruma-ebi | muscle, abdomen | −0.0 |
| CoCl2 | - | - | 0 |
| Species of Fish | Japanese Name | Sampling Parts | Rate of Change (%) |
|---|---|---|---|
| CoCl2 | - | - | 0 |
| Oncorhynchus mykiss | Nijimasu | white muscle, dorsal | 1.9 |
| Lateolabrax latus | Hirasuzuki | white muscle, skinless fillet | 4.4 |
| Crassostrea gigas | Magaki | edible part | 8.0 |
| Haliotis gigantea | Megai-awabi | muscle, foot | 9.5 |
| Gerres equulus | Kurosagi | white muscle, skinless fillet | 12.5 |
| Caranx sexfasciatus | Gingameaji | skinless fillet | 16.9 |
| Plecoglossus altivelis altivelis | Ayu | skinless fillet | 17.4 |
| Dasyatis akajei | Akaei | white muscle, dorsal | 24.5 |
| Caranx ignobilis | Rounin-aji | skinless fillet | 29.9 |
| Charonia lampas sauliae | Boushu-bora | muscle, foot | 30.5 |
| Oplegnathus punctatus | Ishigakidai | white muscle, dorsal | 36.5 |
| Plectorhinchus cinctus | Koshodai | white muscle, skinless fillet | 39.5 |
| Guinusia dentipes | Shojin-kani | muscle, cheliped and ambulatory leg | 39.5 |
| Girella punctata | Mejina | white muscle, dorsal | 44.0 |
| Stephanolepis cirrhifer | Kawahagi | white muscle, skinless fillet | 47.0 |
| Lateolabrax japonicus | Suzuki | white muscle, skinless fillet | 53.0 |
| Scombrops boops | Mutu | headless | 75.0 |
| Paralichthys olivaceus | Hirame | white muscle, skinless fillet | 77.0 |
| Turbo sazae | Sazae | muscle, foot | 80.0 |
| Meretrix lusoria | Hamaguri | edible part | 83.0 |
| Sphyraena japonica | Yamatokamasu | white muscle, dorsal | 87.5 |
| Oncorhynchus masou ishikawae | Amago | skinless fillet | 88.5 |
| Ruditapes philippinarum | Asari | edible part | 96.5 |
| Anguilla japonica | Nihon-unagi | skinless fillet | 98.1 |
| Sphyraena pinguis | Akakamasu | white muscle, dorsal | 104.2 |
| Siganus fuscescens | Aigo | white muscle, dorsal | 104.2 |
| Heteropriacanthus cruentatus | Gomahirekintoki | white muscle, skinless fillet | 105.5 |
| Trichiurus japonicus | Tachiuo | white muscle, skinless fillet | 108.5 |
| Prionurus scalprum | Nizadai | white muscle, dorsal | 117.5 |
| Diaphus suborbitalis | Senhadaka | headless | 127.7 |
| Stichopus japonica | Manamako | muscle | 129.5 |
| Beryx splendens | Kinmedai | white muscle, dorsal | 133.0 |
| Chelidonichthys spinosus | Houbou | white muscle, skinless fillet | 134.0 |
| Siganus fuscescens | Aigo | ovary | 142.5 |
| Parapristipoma trilineatum | Isaki | white muscle, skinless fillet | 147.5 |
| Goniistius zonatus | Takanohadai | white muscle, dorsal | 147.5 |
| Calotomus japonicus | Budai | white muscle, dorsal | 149.0 |
| Engraulis japonica | Katakuchi-iwashi | whole (larva) | 180.0 |
| Konosirus punctatus | Konoshiro | white muscle, skinless fillet | 182.0 |
| Lucensosergia lucens | Sakura-ebi | whole | 215.8 |
| Sphyraena pinguis | Akakamasu | testis | 230.8 |
| Species of Fish | Japanese Name | Sampling Parts | Rate of Change (%) | p Value |
|---|---|---|---|---|
| DMOG | - | - | 0 | - |
| Fermented dried bonito | Honkare-bushi | - | 1.0 | 0.999 |
| Seriola quinqueradiata | Buri | white muscle, dorsal | 3.8 | 0.871 |
| Coryphaena hippurus | Siira | white muscle, dorsal | 6.3 | 0.826 |
| Thunnus obesus | Mebachi | white muscle, dorsal | 7.7 | 0.919 |
| Cypselurus agoo | Tobiuo | white muscle, dorsal | 8.2 | 0.882 |
| Dried sardine | Iwashi-bushi | - | 10.9 | 0.039 |
| Scomber australasicus | Gomasaba | white muscle, dorsal | 11.1 | 0.529 |
| Sardinops melanostictus | Maiwasi | whole | 11.5 | 0.464 |
| Katsuwonus pelamis | Katsuo | white muscle, dorsal | 13.9 | 0.206 |
| Pagrus major | Madai | white muscle, dorsal | 15.9 | 0.011 * |
| Scomber japonicus | Masaba | white muscle, dorsal | 21.6 | 0.004 ** |
| Dried mackel | Saba-bushi | - | 26.9 | 0.0002 *** |
| Scomberomorus niphonius | Sawara | white muscle, dorsal | 28.4 | 0.0001 *** |
| Etrumeus teres | Urumeiwashi | skinless fillet | 69.0 | 0.00004 † |
| Thunnus obesus | Mebachi | head muscle | 70.4 | 0.00002 † |
| Thunnus obesus | Mebachi | pectoral fin muscle | 79.5 | 0.000005 ‡ |
References
- Tarr, J.M.; Kaul, K.; Chopra, M.; Kohner, E.M.; Chibber, R. Pathophysiology of Diabetic Retinopathy. ISRN Ophthalmol. 2013, 2013, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cayabyab, R.; Ramanathan, R. Retinopathy of Prematurity: Therapeutic Strategies Based on Pathophysiology. Neonatology 2016, 109, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.I.; Evans, S.M.; Craft, J.R.; Capozzi, M.E.; McCollum, G.W.; Yang, R.; Marnett, L.J.; Uddin, M.J.; Jayagopal, A.; Penn, J.S. In Vivo Imaging of Retinal Hypoxia in a Model of Oxygen-Induced Retinopathy. Sci. Rep. 2016, 6, 31011. [Google Scholar] [CrossRef]
- Ding, J.; Wong, T.Y. Current epidemiology of diabetic retinopathy and diabetic macular edema. Curr. Diab. Rep. 2012, 12, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Resnikoff, S.; Pascolini, D.; Etya’ale, D.; Kocur, I.; Pararajasegaram, R.; Pokharel, G.P.; Mariotti, S.P. Global data on visual impairment in the year 2002. Bull. World Health Organ. 2004, 82, 844–851. [Google Scholar] [PubMed]
- Antonetti, D.A.; Klein, R.; Gardner, T.W. Diabetic retinopathy. N. Engl. J. Med. 2012, 13, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, D.; Suzuma, K.; Matsui, S.; Kurimoto, M.; Kiryu, J.; Kita, M.; Suzuma, I.; Ohashi, H.; Ojima, T.; Murakami, T.; et al. Erythropoietin as a retinal angiogenic factor in proliferative diabetic retinopathy. N. Engl. J. Med. 2005, 353, 782–792. [Google Scholar] [CrossRef]
- Aiello, L.P.; Arrigg, P.G.; Shah, S.T.; Keyt, B.A.; Avery, R.L.; Jampel, H.D.; Pasquale, L.R.; Thieme, H.; King, G.L.; Iwamoto, M.A.; et al. Vascular Endothelial Growth Factor in Ocular Fluid of Patients with Diabetic Retinopathy and Other Retinal Disorders. N. Engl. J. Med. 1994, 331, 1480–1487. [Google Scholar] [CrossRef]
- Pieramici, D.J.; Rabena, M.D. Anti-VEGF therapy: Comparison of current and future agents. In Proceedings of the Eye 2008, 22, 1330–1336. [Google Scholar] [CrossRef]
- Maguire, M.G.; Martin, D.F.; Ying, G. shuang; Jaffe, G.J.; Daniel, E.; Grunwald, J.E.; Toth, C.A.; Ferris, F.L.; Fine, S.L. Five-Year Outcomes with Anti–Vascular Endothelial Growth Factor Treatment of Neovascular Age-Related Macular Degeneration: The Comparison of Age-Related Macular Degeneration Treatments Trials. Proc. Ophthalmol. 2016, 123, 1751–1761. [Google Scholar]
- Grunwald, J.E.; Daniel, E.; Huang, J.; Ying, G.S.; Maguire, M.G.; Toth, C.A.; Jaffe, G.J.; Fine, S.L.; Blodi, B.; Klein, M.L.; et al. Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology 2014, 121, 150–161. [Google Scholar] [CrossRef]
- Morin, J.; Luu, T.M.; Superstein, R.; Ospina, L.H.; Lefebvre, F.; Simard, M.N.; Shah, V.; Shah, P.S.; Kelly, E.N. Neurodevelopmental outcomes following bevacizumab injections for retinopathy of prematurity. Pediatrics 2016, 137, e20153218. [Google Scholar] [CrossRef] [PubMed]
- Laíns, I.; Figueira, J.; Santos, A.R.; Baltar, A.; Costa, M.; Nunes, S.; Farinha, C.; Pinto, R.; Henriques, J.; Silva, R. Choroidal thickness in diabetic retinopathy: The influence of antiangiogenic therapy. Retina 2014, 34, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Semenza, G.L. Purification and characterization of hypoxia-inducible factor. J. Biol. Chem. 1995, 270, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Kaelin, W.G.; Ratcliffe, P.J. Oxygen Sensing by Metazoans: The Central Role of the HIF Hydroxylase Pathway. Mol. Cell 2008, 30, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Mole, D.R.; Blancher, C.; Copley, R.R.; Pollard, P.J.; Gleadle, J.M.; Ragousis, J.; Ratcliffe, P.J. Genome-wide association of hypoxia-inducible factor (HIF)-1α and HIF-2α DNA binding with expression profiling of hypoxia-inducible transcripts. J. Biol. Chem. 2009, 284, 16767–16775. [Google Scholar] [CrossRef] [PubMed]
- Miwa, Y.; Hoshino, Y.; Shoda, C.; Jiang, X.; Tsubota, K.; Kurihara, T. Pharmacological HIF inhibition prevents retinal neovascularization with improved visual function in a murine oxygen-induced retinopathy model. Neurochem. Int. 2019, 128, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, T.; Westenskow, P.D.; Bravo, S.; Aguilar, E.; Friedlander, M. Targeted deletion of Vegfa in adult mice induces vision loss. J. Clin. Invest. 2012, 122, 4213–4217. [Google Scholar] [CrossRef]
- Yu, T.; Tang, B.; Sun, X. Development of inhibitors targeting hypoxia-inducible factor 1 and 2 for cancer therapy. Yonsei Med. J. 2017, 58, 489–496. [Google Scholar] [CrossRef]
- Kunimi, H.; Miwa, Y.; Inoue, H.; Tsubota, K.; Kurihara, T. A novel HIF inhibitor halofuginone prevents neurodegeneration in a murine model of retinal ischemia-reperfusion. Int. J. Mol. Sci. 2019, 20, 3171. [Google Scholar] [CrossRef]
- Skulas-Ray, A.C.; Wilson, P.W.F.; Harris, W.S.; Brinton, E.A.; Kris-Etherton, P.M.; Richter, C.K.; Jacobson, T.A.; Engler, M.B.; Miller, M.; Robinson, J.G.; et al. Omega-3 Fatty Acids for the Management of Hypertriglyceridemia: A Science Advisory From the American Heart Association. Circulation 2019, 140, e673–e691. [Google Scholar] [CrossRef]
- Sala-Vila, A.; Díaz-López, A.; Valls-Pedret, C.; Cofán, M.; García-Layana, A.; Lamuela-Raventós, R.M.; Castañer, O.; Zanon-Moreno, V.; Martinez-Gonzalez, M.A.; Toledo, E.; et al. Dietary marine ω-3 fatty acids and incident sight-threatening retinopathy in middle-aged and older individuals with type 2 diabetes: Prospective investigation from the PREDIMED trial. JAMA Ophthalmol. 2016, 134, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Henmi, H.; Nishioka, F. Extractive components in the skeletal muscle from ten different species of scombroid fishes. Fisheries Science 1994, 60, 473–478. [Google Scholar] [CrossRef][Green Version]
- Okabe, K.; Kobayashi, S.; Yamada, T.; Kurihara, T.; Tai-Nagara, I.; Miyamoto, T.; Mukouyama, Y.S.; Sato, T.N.; Suda, T.; Ema, M.; et al. Neurons limit angiogenesis by titrating VEGF in retina. Cell 2014, 159, 584–596. [Google Scholar] [CrossRef]
- Connor, K.M.; Krah, N.M.; Dennison, R.J.; Aderman, C.M.; Chen, J.; Guerin, K.I.; Sapieha, P.; Stahl, A.; Willett, K.L.; Smith, L.E.H. Quantification of oxygen-induced retinopathy in the mouse: A model of vessel loss, vessel regrowth and pathological angiogenesis. Nat. Protoc. 2009, 4, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Torii, S.; Kurihara, A.; Li, X.Y.; Yasumoto, K.I.; Sogawa, K. Inhibitory effect of extracellular histidine on cobalt-induced HIF-1α expression. J. Biochem. 2011, 149, 171–176. [Google Scholar] [CrossRef]
- Berlow, R.B.; Dyson, H.J.; Wright, P.E. Hypersensitive termination of the hypoxic response by a disordered protein switch. Nature 2017, 543, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Wolfensohn, S.; Lloyd, M. Conduct of Minor Procedures. In Handbook of Laboratory Animal Management and Welfare, 3rd ed.; Wolfensohn, S., Lloyd, M., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2003; pp. 150–181. [Google Scholar]
- Zhang, J.L.; Wang, Z.; Hu, W.; Chen, S.S.; Lou, X.E.; Zhou, H.J. DHA regulates angiogenesis and improves the efficiency of CDDP for the treatment of lung carcinoma. Microvasc. Res. 2013, 87, 14–24. [Google Scholar] [CrossRef]
- Zendedel, A.; Habib, P.; Dang, J.; Lammerding, L.; Hoffmann, S.; Beyer, C.; Slowik, A. Omega-3 polyunsaturated fatty acids ameliorate neuroinflammation and mitigate ischemic stroke damage through interactions with astrocytes and microglia. J. Neuroimmunol. 2015, 278, 200–211. [Google Scholar] [CrossRef]
- Kurihara, T.; Westenskow, P.D.; Gantner, M.L.; Usui, Y.; Schultz, A.; Bravo, S.; Aguilar, E.; Wittgrove, C.; Friedlander, M.S.H.; Paris, L.P.; et al. Hypoxia-induced metabolic stress in retinal pigment epithelial cells is sufficient to induce photoreceptor degeneration. Elife 2016, 5, 14319. [Google Scholar] [CrossRef]

| Species of Fish | Japanese Name | Sampling Parts | Rate of Change (%) | p value |
|---|---|---|---|---|
| Topotecan † | - | - | −54.4 | 0.0000000 ‡ |
| Doxorubicin † | - | - | −54.4 | 0.0000000 ‡ |
| Selar crumenophthalmus | Meaji | skinless fillet | −47.2 | 0.0000001 ‡ |
| Seriola dumerili | Kanpachi | white muscle, dorsal | −29.0 | 0.00003 † |
| Spratelloides gracilis | Kibinago | headless | −27.9 | 0.0003 *** |
| Decapterus macarellus | Kusayamoro | skinless fillet | −24.4 | 0.0001 *** |
| Panulirus japonicus | Ise-ebi | muscle, abdomen | −17.2 | 0.069 |
| Sulculus diversicolo supertexta | Tokobushi | Muscle, foot | −12.2 | 0.283 |
| Trachurus japonicus | Maaji | skinless fillet | −8.8 | 0.127 |
| Dried bonito | Ara-bushi | - | −3.8 | 0.999 |
| Scomberoides lysan | Ikekatsuo | skinless fillet | −2.9 | 0.999 |
| Rhabdosargus sarba | Hedai | white muscle, dorsal | −1.4 | 0.999 |
| DMOG | - | - | 0 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shoda, C.; Miwa, Y.; Nimura, K.; Okamoto, K.; Yamagami, S.; Tsubota, K.; Kurihara, T. Hypoxia-Inducible Factor Inhibitors Derived from Marine Products Suppress a Murine Model of Neovascular Retinopathy. Nutrients 2020, 12, 1055. https://doi.org/10.3390/nu12041055
Shoda C, Miwa Y, Nimura K, Okamoto K, Yamagami S, Tsubota K, Kurihara T. Hypoxia-Inducible Factor Inhibitors Derived from Marine Products Suppress a Murine Model of Neovascular Retinopathy. Nutrients. 2020; 12(4):1055. https://doi.org/10.3390/nu12041055
Chicago/Turabian StyleShoda, Chiho, Yukihiro Miwa, Kazumi Nimura, Kazutoshi Okamoto, Satoru Yamagami, Kazuo Tsubota, and Toshihide Kurihara. 2020. "Hypoxia-Inducible Factor Inhibitors Derived from Marine Products Suppress a Murine Model of Neovascular Retinopathy" Nutrients 12, no. 4: 1055. https://doi.org/10.3390/nu12041055
APA StyleShoda, C., Miwa, Y., Nimura, K., Okamoto, K., Yamagami, S., Tsubota, K., & Kurihara, T. (2020). Hypoxia-Inducible Factor Inhibitors Derived from Marine Products Suppress a Murine Model of Neovascular Retinopathy. Nutrients, 12(4), 1055. https://doi.org/10.3390/nu12041055







