Amount of Protein Required to Improve Muscle Mass in Older Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Data Collection
2.3. Muscle Mass Measurement
2.4. Dietary Intake Measurement
2.5. Statistical Analyses
3. Results
3.1. Characteristics of Participants
3.2. Muscle Mass and Gait Speed in Participants
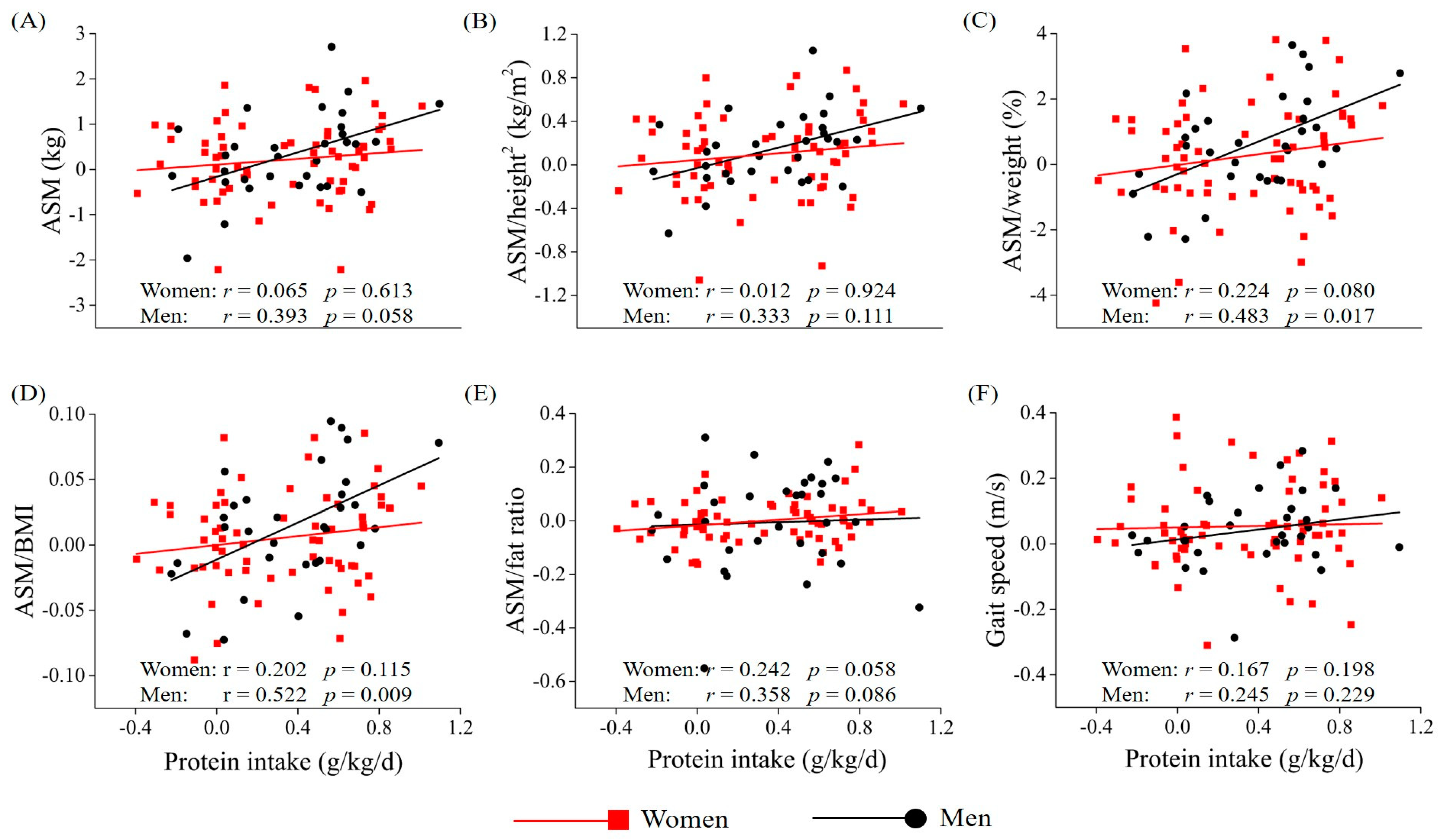
3.3. Association Between Protein Intake and Muscle Mass and Gait Speed
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- OECD iLibrary. Available online: http://dx.doi.org/10.1787/eco_surveys-kor-2018-en (accessed on 8 April 2020).
- Kim, M.; Won, C.W. Prevalence of sarcopenia in community-dwelling older adults using the definition of the European Working Group on Sarcopenia in Older People 2: Findings from the Korean Frailty and Aging Cohort Study. Age Ageing 2019, 48, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Shinkai, S.; Watanabe, S.; Kumagai, S.; Fujiwara, Y.; Amano, H.; Yoshida, H.; Ishizaki, T.; Yukawa, H.; Suzuki, T.; Shibata, H. Walking speed as a good predictor for the onset of functional dependence in a Japanese rural community population. Age Ageing 2000, 29, 441–446. [Google Scholar] [CrossRef]
- Landi, F.; Cruz-Jentoft, A.J.; Liperoti, R.; Russo, A.; Giovannini, S.; Tosato, M.; Capoluongo, E.; Bernabei, R.; Onder, G. Sarcopenia and mortality risk in frail older persons aged 80 years and older: Results from ilSIRENTE study. Age Ageing 2013, 42, 203–209. [Google Scholar] [CrossRef]
- Mijnarends, D.M.; Schols, J.M.; Meijers, J.M.; Tan, F.E.; Verlaan, S.; Luiking, Y.C.; Morley, J.E.; Halfens, R.J. Instruments to assess sarcopenia and physical frailty in older people living in a community (care) setting: Similarities and discrepancies. J. Am. Med. Dir. Assoc. 2015, 16, 301–308. [Google Scholar] [CrossRef]
- Dennison, E.M.; Sayer, A.A.; Cooper, C. Epidemiology of sarcopenia and insight into possible therapeutic targets. Nat. Rev. Rheumatol. 2017, 13, 340–347. [Google Scholar] [CrossRef]
- Rolland, Y.; van Kan, G.A.; Benetos, A.; Blain, H.; Bonnefoy, M.; Chassagne, P.; Jeandel, C.; Laroche, M.; Nourhashemi, F.; Orcel, P.; et al. Frailty, osteoporosis and hip fracture: Causes, consequences and therapeutic perspectives. J. Nutr. Health Aging 2008, 12, 335–346. [Google Scholar] [CrossRef]
- Coelho-Junior, H.J.; Rodrigues, B.; Uchida, M.; Marzetti, E. Low protein intake is associated with frailty in older adults: A systematic review and meta-analysis of observational studies. Nutrients 2018, 10, 1334. [Google Scholar] [CrossRef]
- Kerstetter, J.E.; Bihuniak, J.D.; Brindisi, J.; Sullivan, R.R.; Mangano, K.M.; Larocque, S.; Kotler, B.M.; Simpson, C.A.; Cusano, A.M.; Gaffney-Stomberg, E.; et al. The effect of a whey protein supplement on bone mass in older Caucasian adults. J. Clin. Endocrinol. Metab. 2015, 100, 2214–2222. [Google Scholar] [CrossRef]
- Deutz, N.E.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznaric, Z.; Nair, K.S.; et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Choi, J.E.; Hwang, H.S. Protein supplementation improves muscle mass and physical performance in undernourished prefrail and frail elderly subjects: A randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2018, 108, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.J.; Milan, A.M.; Mitchell, S.M.; Zeng, N.; Ramzan, F.; Sharma, P.; Knowles, S.O.; Roy, N.C.; Sjödin, A.; Wagner, K.H.; et al. The effects of dietary protein intake on appendicular lean mass and muscle function in elderly men: A 10-wk randomized controlled trial. Am. J. Clin. Nutr. 2017, 106, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Tieland, M.; van de Rest, O.; Dirks, M.L.; van der Zwaluw, N.; Mensink, M.; van Loon, L.J.; de Groot, L.C. Protein supplementation improves physical performance in frail elderly people: A randomized, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc. 2012, 13, 720–726. [Google Scholar] [CrossRef]
- Bosse, J.D.; Dixon, B.M. Dietary protein to maximize resistance training: A review and examination of protein spread and change theories. J. Int. Soc. Sports Nutr. 2012, 9, 42. [Google Scholar] [CrossRef]
- Morley, J.E.; Malmstrom, T.K.; Miller, D.K. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J. Nutr. Health Aging 2012, 16, 601–608. [Google Scholar] [CrossRef]
- Kang, Y.; Na, D.L.; Hahn, S. A validity study on the Korean Mini-Mental State Examination (K-MMSE) in dementia patients. J. Korean Neurol. Assoc. 1997, 15, 300–308. [Google Scholar]
- Won, C.W.; Rho, Y.G.; Kim, S.Y.; Cho, B.R.; Lee, Y.S. The validity and reliability of Korean Activities of Daily Living (K-ADL) scale. J. Korean Geriatr. Soc. 2002, 6, 98–106. [Google Scholar]
- Won, C.W.; Rho, Y.G.; Woo, D.S.; Lee, Y.S. The validity and reliability of Korean Instrumental Activities of Daily Living (K-IADL) scale. J. Korean Geriatr. Soc. 2002, 6, 273–280. [Google Scholar]
- Nestlé Nutrition Institute. Available online: https://www.mna-elderly.com (accessed on 8 April 2020).
- Kim, K.M.; Jang, H.C.; Lim, S. Differences among skeletal muscle mass indices derived from height-, weight-, and body mass index-adjusted models in assessing sarcopenia. Korean J. Intern. Med. 2016, 31, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Hong, S.; Kim, E.Y. Reference values of skeletal muscle mass for Korean children and adolescents using data from the Korean National Health and Nutrition Examination Survey 2009–2011. PLoS ONE 2016, 11, e0153383. [Google Scholar] [CrossRef] [PubMed]
- Greenland, S.; Pearce, N. Statistical foundations for model-based adjustments. Annu. Rev. Public Health 2015, 36, 89–108. [Google Scholar] [CrossRef] [PubMed]
- Aleman-Mateo, H.; Macias, L.; Esparza-Romero, J.; Astiazaran-Garcia, H.; Blancas, A.L. Physiological effects beyond the significant gain in muscle mass in sarcopenic elderly men: Evidence from a randomized clinical trial using a protein-rich food. Clin. Interv. Aging 2012, 7, 225–234. [Google Scholar] [CrossRef]
- Ten Haaf, D.S.M.; Eijsvogels, T.M.H.; Bongers, C.; Horstman, A.M.H.; Timmers, S.; de Groot, L.; Hopman, M.T.E. Protein supplementation improves lean body mass in physically active older adults: A randomized placebo-controlled trial. J. Cachexia Sarcopenia Muscle 2019, 10, 298–310. [Google Scholar] [CrossRef]
- Moore, D.R.; Tang, J.E.; Burd, N.A.; Rerecich, T.; Tarnopolsky, M.A.; Phillips, S.M. Differential stimulation of myofibrillar and sarcoplasmic protein synthesis with protein ingestion at rest and after resistance exercise. J. Physiol. 2009, 587, 897–904. [Google Scholar] [CrossRef]
- Kim, H.K.; Suzuki, T.; Saito, K.; Yoshida, H.; Kobayashi, H.; Kato, H.; Katayama, M. Effects of exercise and amino acid supplementation on body composition and physical function in community-dwelling elderly Japanese sarcopenic women: A randomized controlled trial. J. Am. Geriatr. Soc. 2012, 60, 12–23. [Google Scholar] [CrossRef]
- Moore, D.R.; Churchward-Venne, T.A.; Witard, O.; Breen, L.; Burd, N.A.; Tipton, K.D.; Phillips, S.M. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 57–62. [Google Scholar] [CrossRef]
- Yang, Y.; Breen, L.; Burd, N.A.; Hector, A.J.; Churchward-Venne, T.A.; Josse, A.R.; Tarnopolsky, M.A.; Phillips, S.M. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br. J. Nutr. 2012, 108, 1780–1788. [Google Scholar] [CrossRef]
- Devries, M.C.; McGlory, C.; Bolster, D.R.; Kamil, A.; Rahn, M.; Harkness, L.; Baker, S.K.; Phillips, S.M. Protein leucine content is a determinant of shorter- and longer-term muscle protein synthetic responses at rest and following resistance exercise in healthy older women: A randomized, controlled trial. Am. J. Clin. Nutr. 2018, 107, 217–226. [Google Scholar] [CrossRef]
- Smith, G.I.; Villareal, D.T.; Sinacore, D.R.; Shah, K.; Mittendorfer, B. Muscle protein synthesis response to exercise training in obese, older men and women. Med. Sci. Sports Exerc. 2012, 44, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, R.; Kato, Y.; Tange, C.; Nishita, Y.; Tomida, M.; Imai, T.; Ando, F.; Shimokata, H.; Arai, H. Protein intake per day and at each daily meal and skeletal muscle mass declines among older community dwellers in Japan. Public Health Nutr. 2020, 23, 1090–1097. [Google Scholar] [CrossRef] [PubMed]
- Ormsbee, M.J.; Willingham, B.D.; Marchant, T.; Binkley, T.L.; Specker, B.L.; Vukovich, M.D. Protein supplementation during a 6-month concurrent training program: Effect on body composition and muscular strength in sedentary individuals. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.M.; Verlaan, S.; Bautmans, I.; Brandt, K.; Donini, L.M.; Maggio, M.; McMurdo, M.E.T.; Mets, T.; Seal, C.; Wijers, S.L.; et al. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE Study: A randomized, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc. 2015, 16, 740–747. [Google Scholar] [CrossRef]
- Alemán-Mateo, H.; Carreón, V.R.; Macias, L.; Astiazaran-García, H.; Gallegos-Aguilar, A.C.; Enriquez, J.R. Nutrient-rich dairy proteins improve appendicular skeletal muscle mass and physical performance, and attenuate the loss of muscle strength in older men and women subjects: A single-blind randomized clinical trial. Clin. Interv. Aging 2014, 9, 1517–1525. [Google Scholar] [CrossRef]
- ten Haaf, D.S.M.; Nuijten, M.A.H.; Maessen, M.F.H.; Horstman, A.M.H.; Eijsvogels, T.M.H.; Hopman, M.T.E. Effects of protein supplementation on lean body mass, muscle strength, and physical performance in nonfrail community-dwelling older adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2018, 108, 1043–1059. [Google Scholar] [CrossRef]
- Kim, C.O.; Lee, K.R. Preventive effect of protein-energy supplementation on the functional decline of frail older adults with low socioeconomic status: A community-based randomized controlled study. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 309–316. [Google Scholar] [CrossRef]
- Miki, A.; Hashimoto, Y.; Matsumoto, S.; Ushigome, E.; Fukuda, T.; Sennmaru, T.; Tanaka, M.; Yamazaki, M.; Fukui, M. Protein intake, especially vegetable protein intake, is associated with higher skeletal muscle mass in elderly patients with type 2 diabetes. J. Diabetes Res. 2017, 2017, 7985728. [Google Scholar] [CrossRef]
- Silva, T.R.; Spritzer, P.M. Skeletal muscle mass is associated with higher dietary protein intake and lower body fat in postmenopausal women: A cross-sectional study. Menopause 2017, 24, 502–509. [Google Scholar] [CrossRef]
- Lemieux, F.C.; Filion, M.E.; Barbat-Artigas, S.; Karelis, A.D.; Aubertin-Leheudre, M. Relationship between different protein intake recommendations with muscle mass and muscle strength. Climacteric 2014, 17, 294–300. [Google Scholar] [CrossRef]
- McLean, R.R.; Shardell, M.D.; Alley, D.E.; Cawthon, P.M.; Fragala, M.S.; Harris, T.B.; Kenny, A.M.; Peters, K.W.; Ferrucci, L.; Guralnik, J.M.; et al. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: The foundation for the National Institutes of Health (FNIH) sarcopenia project. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.H.; Kim, K.M.; Kim, J.H.; Moon, J.H.; Choi, S.H.; Lim, S.; Lim, J.Y.; Kim, K.W.; Park, K.S.; Jang, H.C. Predictive values of the new sarcopenia index by the foundation for the national institutes of health sarcopenia project for mortality among older Korean adults. PLoS ONE 2016, 11, e016634. [Google Scholar] [CrossRef] [PubMed]
| Change in Protein Intake (g/kg/day) | Total (n = 96) | Women (n = 65) | Men (n = 31) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tertile 1 (Mean, −0.06) | Tertile 2 (Mean, 0.36) | Tertile 3 (Mean, 0.72) | p | Tertile 1 (≤0.04) | Tertile 2 (0.05–0.57) | Tertile 3 (>0.57) | p | Tertile 1 (≤0.15) | Tertile 2 (0.16–0.54) | Tertile 3 (>0.54) | p | |
| Age (years) | 76.97 ± 3.78 | 76.79 ± 3.42 | 77.06 ± 3.80 | 0.954 a | 77.24 ± 3.51 | 76.68 ± 3.30 | 77.91 ± 3.80 | 0.520 a | 76.40 ± 4.45 | 77.00 ± 3.80 | 75.20 ± 3.23 | 0.563 a |
| BMI (kg/m2) | 24.37 ± 4.48 | 24.29 ± 2.67 | 23.76 ± 2.46 | 0.656 a | 25.47 ± 4.67 | 24.23 ± 2.73 | 23.97 ± 2.46 | 0.441 a | 22.05 ± 3.10 | 24.43 ± 2.69 | 23.30 ± 2.52 | 0.165 a |
| ASM (kg) | 14.86 ± 3.27 | 15.32 ± 3.59 | 14.78 ± 2.93 | 0.932 b | 13.41 ± 2.24 | 13.29 ± 1.63 | 13.41 ± 2.12 | 0.972 a | 17.91 ± 3.02 | 19.39 ± 2.91 | 17.80 ± 2.09 | 0.483 b |
| ASM/height2 (kg/m2) | 6.26 ± 0.89 | 6.18 ± 0.88 | 6.10 ± 0.76 | 0.856 b | 6.04 ± 0.85 | 5.79 ± 0.51 | 5.85 ± 0.65 | 0.500 a | 6.71 ± 0.83 | 6.97 ± 0.95 | 6.66 ± 0.71 | 0.657 a |
| ASM/weight (%) | 26.23 ± 4.52 | 25.57 ± 3.39 | 25.81 ± 3.28 | 0.873 b | 24.15 ± 3.53 | 24.08 ± 2.66 | 24.47 ± 2.36 | 0.891 a | 30.59 ± 3.01 | 28.56 ± 2.68 | 28.74 ± 3.20 | 0.247 a |
| ASM/BMI | 0.63 ± 0.17 | 0.63 ± 0.14 | 0.63 ± 0.13 | 0.905 b | 0.54 ± 0.09 | 0.55 ± 0.07 | 0.56 ± 0.07 | 0.555 a | 0.82 ± 0.12 | 0.79 ± 0.09 | 0.77 ± 0.10 | 0.610 a |
| ASM:fat ratio | 1.09 ± 0.51 | 0.98 ± 0.40 | 1.11 ± 0.66 | 0.735 b | 0.81 ± 0.27 | 0.80 ± 0.26 | 0.82 ± 0.16 | 0.979 a | 1.66 ± 0.39 | 1.35 ± 0.39 | 1.74 ± 0.89 | 0.169 a |
| Gait speed (m/s) | 0.97 ± 0.32 | 0.98 ± 0.32 | 1.00 ± 0.34 | 0.978 a | 0.91 ± 0.30 | 0.96 ± 0.27 | 0.96 ± 0.34 | 0.853 a | 1.09 ± 0.34 | 1.02 ± 0.41 | 1.05 ± 0.35 | 0.913 a |
| Living alone, n (%) | 17 (54.8) | 19 (57.6) | 18 (56.3) | 0.976 c | 13 (61.9) | 14 (63.6) | 16 (72.7) | 0.720 c | 4 (40.0) | 5 (45.5) | 2 (20.0) | 0.446 c |
| Smoking, n (%) | 0.197 c | 0.380 c | 0.105 c | |||||||||
| Never | 22 (71.0) | 20 (60.6) | 24 (75.0) | 19 (90.5) | 20 (90.9) | 22 (100.0) | 3 (30.0) | 0 (0.0) | 2 (20.0) | |||
| Former | 8 (25.8) | 9 (27.3) | 3 (9.4) | 2 (9.5) | 1 (4.5) | 0 (0.0) | 6 (60.0) | 8 (72.7) | 3 (30.0) | |||
| Current | 1 (3.2) | 4 (12.1) | 5 (15.6) | 0 (0.0) | 1 (4.5) | 0 (0.0) | 1 (10.0) | 3 (27.3) | 5 (50.0) | |||
| Alcohol drinking, n (%) | 23 (74.2) | 21 (63.6) | 22 (68.8) | 0.661 c | 14 (66.7) | 11 (50.0) | 14 (63.6) | 0.490 c | 9 (90.0) | 10 (90.9) | 8 (80.0) | 0.717 c |
| Comorbidity, n (%) d | 0.756 c | 0.992 c | 0.476 c | |||||||||
| 0 | 10 (32.3) | 9 (27.3) | 6 (18.8) | 4 (19.0) | 4 (18.2) | 4 (18.2) | 6 (60.0) | 5 (45.5) | 2 (20.0) | |||
| 1 | 12 (38.7) | 12 (36.4) | 15 (46.9) | 9 (42.9) | 8 (36.4) | 9 (40.9) | 3 (30.0) | 4 (36.4) | 6 (60.0) | |||
| ≥2 | 9 (29.0) | 12 (36.4) | 11 (34.4) | 8 (38.1) | 10 (45.5) | 9 (40.9) | 1 (10.0) | 2 (18.2) | 2 (20.0) | |||
| Cognitive impairment, n (%) e | 6 (19.4) | 10 (30.3) | 11 (34.4) | 0.392 c | 5 (23.8) | 9 (40.9) | 6 (27.3) | 0.435 c | 1 (10.0) | 1 (9.1) | 5 (50.0) | 0.042 c |
| ADL disability, n (%) | 7 (22.6) | 11 (33.3) | 6 (18.8) | 0.371 c | 4 (19.0) | 7 (31.8) | 5 (22.7) | 0.604 c | 3 (30.0) | 4 (36.4) | 1 (10.0) | 0.361 c |
| IADL disability, n (%) | 11 (35.5) | 14 (42.4) | 16 (50.0) | 0.507 c | 5 (23.8) | 8 (36.4) | 8 (34.6) | 0.599 c | 6 (60.0) | 6 (54.5) | 8 (80.0) | 0.446 c |
| Frailty, n (%) | 2 (6.5) | 10 (30.3) | 6 (18.8) | 0.051 c | 2 (9.5) | 6 (27.3) | 5 (22.7) | 0.321 c | 0 (0.0) | 4 (36.4) | 1 (10.0) | 0.063 c |
| MNA score | 20.32 ± 2.15 | 20.35 ± 2.47 | 21.11 ± 1.92 | 0.287 b | 20.05 ± 2.09 | 20.34 ± 2.33 | 20.98 ± 1.81 | 0.356 b | 20.90 ± 2.26 | 20.36 ± 2.85 | 21.40 ± 2.23 | 0.228 a |
| Protein intake (g/kg/day) | 0.93 ± 0.29 f | 0.79 ± 0.21 f | 0.72 ± 0.20 g | 0.008 a | 0.83 ± 0.29 f | 0.80 ± 0.20 g | 0.67 ± 0.13 g | 0.018 a | 1.12 ± 0.21 f | 0.79 ± 0.24 g | 0.82 ± 0.28 g | 0.008 a |
| Change in Protein Intake (g/kg/day) | Total (n = 96) | Women (n = 65) | Men (n = 31) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tertile 1 (Mean, −0.06) | Tertile 2 (Mean, 0.36) | Tertile 3 (Mean, 0.72) | p | Tertile 1 (≤0.04) | Tertile 2 (0.05–0.57) | Tertile 3 (>0.57) | p | Tertile 1 (≤0.15) | Tertile 2 (0.16–0.54) | Tertile 3 (>0.54) | p | |
| ASM (kg) | 0.07 ± 0.88 | 0.18 ± 0.73 | 0.51 ± 0.95 | 0.075 a | 0.14 ± 0.86 | 0.22 ± 0.81 | 0.29 ± 0.91 | 0.976 a | −0.08 ± 0.96 c | 0.10 ± 0.56 c | 1.01 ± 0.85 d | 0.012 a |
| ASM/height2 (kg/m2) | 0.04 ± 0.37 | 0.09 ± 0.31 | 0.21 ± 0.39 | 0.174 a | 0.06 ± 0.39 | 0.09 ± 0.35 | 0.14 ± 0.40 | 0.904 a | −0.01 ± 0.34 c | 0.07 ± 0.21 c | 0.38 ± 0.33 d | 0.019 a |
| ASM/weight (%) | −0.06 ± 1.67 c | 0.32 ± 1.27 c | 0.86 ± 1.72 d | 0.026 a | −0.03 ± 1.76 | 0.40 ± 1.47 | 0.39 ± 1.72 | 0.393 a | −0.13 ± 1.57 c | 0.18 ± 0.78 d | 1.88 ± 1.26 d | 0.002 a |
| ASM/BMI | −0.00 ± 0.04 c | 0.01 ± 0.03 c | 0.02 ± 0.04 d | 0.028 a | −0.00 ± 0.04 | 0.01 ± 0.03 | 0.01 ± 0.04 | 0.427 a | −0.01 ± 0.04 c | 0.00 ± 0.03 d | 0.05 ± 0.04 d | 0.003 a |
| ASM:fat ratio | −0.04 ± 0.14 c | 0.02 ± 0.10 c | 0.01 ± 0.12 d | 0.033 a | −0.03 ± 0.08 | 0.01 ± 0.07 | 0.01 ± 0.10 | 0.200 a | −0.06 ± 0.23 | 0.02 ± 0.14 | 0.02 ± 0.17 | 0.147 a |
| Gait speed (m/s) | 0.04 ± 0.12 | 0.04 ± 0.14 | 0.07 ± 0.13 | 0.066 b | 0.06 ± 0.13 | 0.04 ± 0.14 | 0.07 ± 0.14 | 0.398 b | 0.00 ± 0.07 | 0.04 ± 0.14 | 0.07 ± 0.11 | 0.070 a |
| Change in Protein Intake (g/kg/day) | n | ASM (kg) | ASM/Height2 (kg/m2) | ASM/Weight (%) | ASM/BMI | ASM:Fat Ratio | Gait Speed (m/s) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | pa | β | pa | β | pa | β | pa | β | pa | β | pb | ||
| Total | |||||||||||||
| Tertile 1 (mean, −0.06) | 31 | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | ||||||
| Tertile 2 (mean, 0.36) | 33 | 0.074 | 0.509 | 0.016 | 0.888 | 0.119 | 0.301 | 0.148 | 0.201 | 0.207 | 0.066 | 0.006 | 0.947 |
| Tertile 3 (mean, 0.72) | 32 | 0.175 | 0.135 | 0.120 | 0.311 | 0.304 | 0.012 | 0.314 | 0.010 | 0.318 | 0.008 | 0.170 | 0.079 |
| Women | |||||||||||||
| Tertile 1 (≤0.04) | 21 | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | ||||||
| Tertile 2 (0.04 < to ≤ 0.57) | 22 | −0.007 | 0.960 | −0.088 | 0.512 | 0.126 | 0.376 | 0.157 | 0.284 | 0.221 | 0.143 | 0.000 | 0.998 |
| Tertile 3 (>0.57) | 22 | 0.029 | 0.842 | −0.026 | 0.850 | 0.196 | 0.191 | 0.172 | 0.262 | 0.256 | 0.107 | 0.151 | 0.182 |
| Men | |||||||||||||
| Tertile 1 (≤0.15) | 10 | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | ||||||
| Tertile 2 (0.15 < to ≤ 0.54) | 11 | 0.361 | 0.102 | 0.275 | 0.258 | 0.144 | 0.526 | 0.199 | 0.385 | 0.301 | 0.161 | 0.188 | 0.282 |
| Tertile 3 (>0.54) | 10 | 0.357 | 0.144 | 0.393 | 0.148 | 0.591 | 0.026 | 0.615 | 0.023 | 0.509 | 0.030 | 0.286 | 0.130 |
| Change in Protein Intake (g/kg/day) | n | ASM (kg) | ASM/Height2 (kg/m2) | ASM/Weight (%) | ASM/BMI | ASM:Fat Ratio | Gait Speed (m/s) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | pa | β | pa | β | pa | β | pa | β | pa | β | pb | ||
| Total | |||||||||||||
| Tertile 1 (≤0.11) | 32 | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | ||||||
| Tertile 2 (0.11–0.56) | 32 | 0.067 | 0.545 | −0.002 | 0.984 | 0.104 | 0.359 | 0.143 | 0.210 | 0.113 | 0.315 | 0.033 | 0.732 |
| Tertile 3 (>0.56) | 32 | 0.171 | 0.142 | 0.109 | 0.355 | 0.293 | 0.014 | 0.307 | 0.010 | 0.262 | 0.026 | 0.183 | 0.059 |
| Women | |||||||||||||
| Tertile 1 (≤0.11) | 24 | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | ||||||
| Tertile 2 (0.11–0.56) | 19 | −0.027 | 0.839 | −0.103 | 0.433 | 0.100 | 0.464 | 0.124 | 0.374 | 0.128 | 0.379 | −0.003 | 0.975 |
| Tertile 3 (>0.56) | 22 | 0.019 | 0.894 | −0.032 | 0.817 | 0.178 | 0.222 | 0.149 | 0.316 | 0.202 | 0.195 | 0.149 | 0.172 |
| Men | |||||||||||||
| Tertile 1 (≤0.11) | 8 | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | ||||||
| Tertile 2 (0.11–0.56) | 13 | 0.395 | 0.074 | 0.352 | 0.159 | 0.257 | 0.277 | 0.318 | 0.172 | 0.263 | 0.232 | 0.319 | 0.080 |
| Tertile 3 (>0.56) | 10 | 0.475 | 0.082 | 0.520 | 0.090 | 0.704 | 0.017 | 0.739 | 0.012 | 0.525 | 0.039 | 0.378 | 0.051 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Park, Y. Amount of Protein Required to Improve Muscle Mass in Older Adults. Nutrients 2020, 12, 1700. https://doi.org/10.3390/nu12061700
Kim D, Park Y. Amount of Protein Required to Improve Muscle Mass in Older Adults. Nutrients. 2020; 12(6):1700. https://doi.org/10.3390/nu12061700
Chicago/Turabian StyleKim, Doyeon, and Yongsoon Park. 2020. "Amount of Protein Required to Improve Muscle Mass in Older Adults" Nutrients 12, no. 6: 1700. https://doi.org/10.3390/nu12061700
APA StyleKim, D., & Park, Y. (2020). Amount of Protein Required to Improve Muscle Mass in Older Adults. Nutrients, 12(6), 1700. https://doi.org/10.3390/nu12061700





