Interference on Iodine Uptake and Human Thyroid Function by Perchlorate-Contaminated Water and Food
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Overview on Iodine Metabolism in Healthy Humans
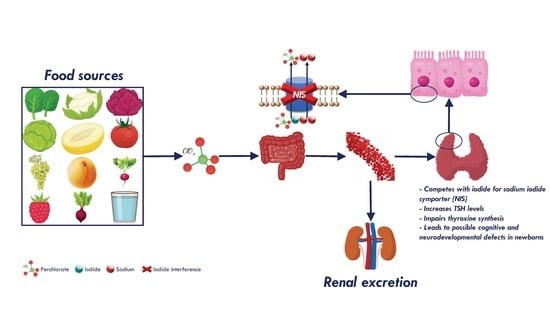
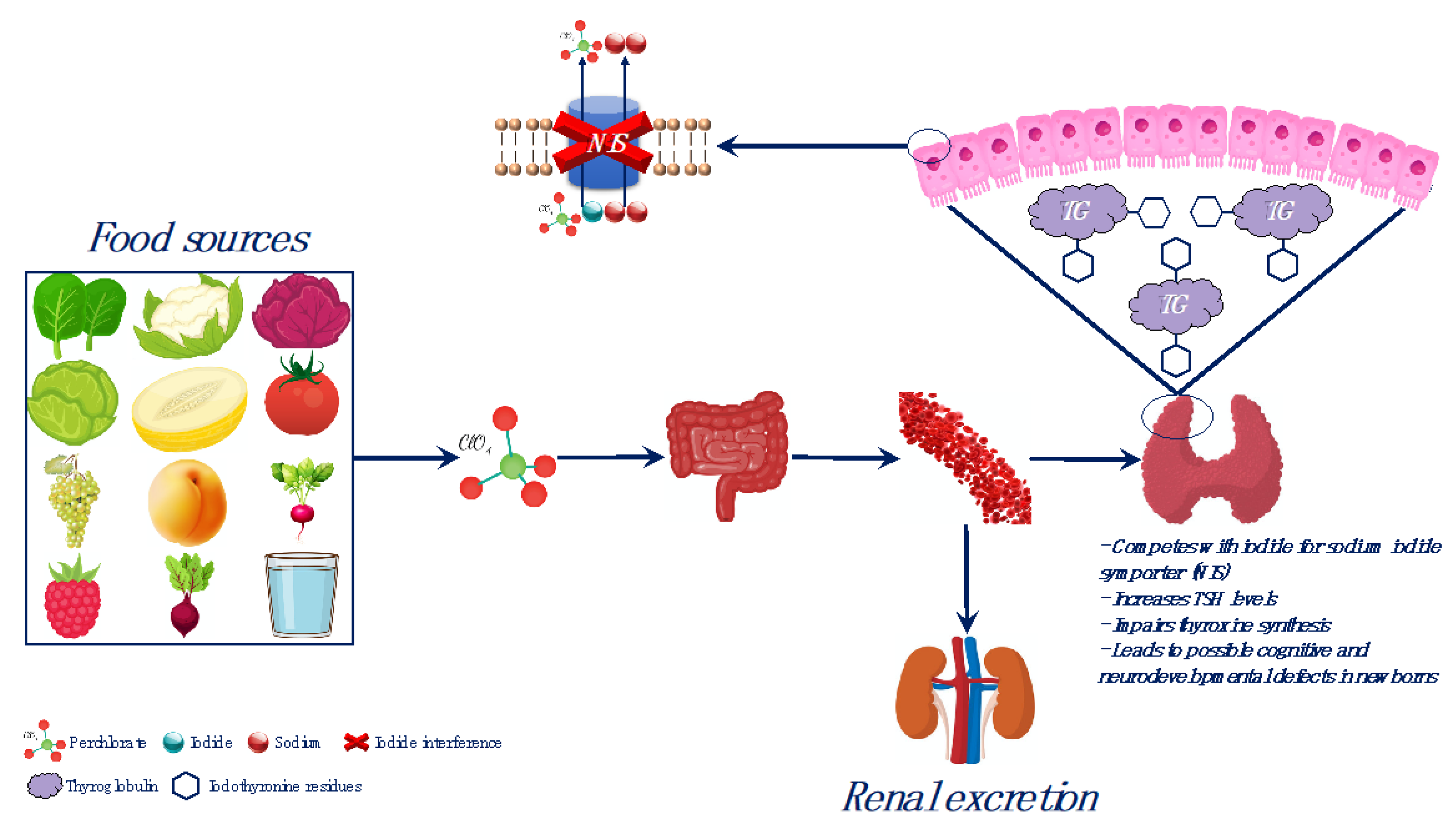
3.2. Perchlorate Compounds and Iodine Interference
3.3. Perchlorate Compounds in Food and Water
3.4. Chronic Esposure to Perchlorate Compounds by Food and Drinking Water
3.5. Perchlorate Compounds Toxicity
3.6. Overview on Other Halogenate Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Thomas Zoeller, R.; Brown, T.R.; Doan, L.L.; Gore, A.C.; Skakkebaek, N.E.; Soto, A.M.; Woodruff, T.J.; Vom Saal, F.S. Endocrine-disrupting chemicals and public health protection: A statement of principles from the Endocrine Society. Endocrinology 2012, 153, 4097–4110. [Google Scholar] [CrossRef]
- La Merrill, M.A.; Vandenberg, L.N.; Smith, M.T.; Goodson, W.; Browne, P.; Patisaul, H.B.; Guyton, K.Z.; Kortenkamp, A.; Cogliano, V.J.; Woodruff, T.J.; et al. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat. Rev. Endocrinol. 2020, 16, 45–57. [Google Scholar] [CrossRef]
- Langer, P.; Kočan, A.; Tajtáková, M.; Koška, J.; Rádiková, Ž.; Kšinantová, L.; Imrich, R.; Hučková, M.; Drobná, B.; Gašperíková, D.; et al. Increased thyroid volume, prevalence of thyroid antibodies and impaired fasting glucose in young adults from organochlorine cocktail polluted area: Outcome of transgenerational transmission? Chemosphere 2008, 73, 1145–1150. [Google Scholar] [CrossRef]
- Street, M.E.; Angelini, S.; Bernasconi, S.; Burgio, E.; Cassio, A.; Catellani, C.; Cirillo, F.; Deodati, A.; Fabbrizi, E.; Fanos, V.; et al. Current knowledge on endocrine disrupting chemicals (EDCs) from animal biology to humans, from pregnancy to adulthood: Highlights from a national italian meeting. Int. J. Mol. Sci. 2018, 19, 1647. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.M.; Fallahi, P.; Antonelli, A.; Benvenga, S. Environmental issues in thyroid diseases. Front. Endocrinol. 2017, 8, 50. [Google Scholar] [CrossRef]
- Yilmaz, B.; Terekeci, H.; Sandal, S.; Kelestimur, F. Endocrine disrupting chemicals: Exposure, effects on human health, mechanism of action, models for testing and strategies for prevention. Rev. Endocr. Metab. Disord. 2019, 21, 127–147. [Google Scholar] [CrossRef]
- Boas, M.; Feldt-Rasmussen, U.; Main, K.M. Thyroid effects of endocrine disrupting chemicals. Mol. Cell. Endocrinol. 2012, 355, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Calsolaro, V.; Pasqualetti, G.; Niccolai, F.; Caraccio, N.; Monzani, F. Thyroid disrupting chemicals. Int. J. Mol. Sci. 2017, 18, 2583. [Google Scholar] [CrossRef] [PubMed]
- Mughal, B.B.; Fini, J.B.; Demeneix, B.A. Thyroid-disrupting chemicals and brain development: An update. Endocr. Connect. 2018, 7, R160–R186. [Google Scholar] [CrossRef] [PubMed]
- Melzer, D.; Rice, N.; Depledge, M.H.; Henley, W.E.; Galloway, T.S. Association between serum perfluorooctanoic acid (PFOA) and thyroid disease in the U.S. National Health and Nutrition Examination Survey. Environ. Health Perspect. 2010, 118, 686–692. [Google Scholar] [CrossRef]
- MacKay, H.; Abizaid, A. A plurality of molecular targets: The receptor ecosystem for bisphenol-A (BPA). Horm. Behav. 2018, 101, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhou, Y.; Fu, C.; Wang, H.; Huang, P.; Wang, B.; Su, M.; Jiang, F.; Fang, H.; Zhao, Q.; et al. Influence of Bisphenol A on Thyroid Volume and Structure Independent of Iodine in School Children. PLoS ONE 2015, 10, e0141248. [Google Scholar] [CrossRef]
- Li, L.; Ying, Y.; Zhang, C.; Wang, W.; Li, Y.; Feng, Y.; Liang, J.; Song, H.; Wang, Y. Bisphenol A exposure and risk of thyroid nodules in Chinese women: A case-control study. Environ. Int. 2019, 126, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Leemans, M.; Couderq, S.; Demeneix, B.; Fini, J.B. Pesticides With Potential Thyroid Hormone-Disrupting Effects: A Review of Recent Data. Front. Endocrinol. 2019, 10, 743. [Google Scholar] [CrossRef] [PubMed]
- Campos, É.; Freire, C. Exposure to non-persistent pesticides and thyroid function: A systematic review of epidemiological evidence. Int. J. Hyg. Environ. Health 2016, 219, 481–497. [Google Scholar] [CrossRef] [PubMed]
- Witorsch, R.J. Critical analysis of endocrine disruptive activity of triclosan and its relevance to human exposure through the use of personal care products. Crit. Rev. Toxicol. 2014, 44, 535–555. [Google Scholar] [CrossRef] [PubMed]
- Demeneix, B.A. Evidence for Prenatal Exposure to Thyroid Disruptors and Adverse Effects on Brain Development. Eur. Thyroid J. 2019, 8, 283–292. [Google Scholar] [CrossRef]
- Pearce, E.N.; Braverman, L.E. Environmental pollutants and the thyroid. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 801–813. [Google Scholar] [CrossRef]
- Pironi, L.; Guidetti, M.; Agostini, F. Iodine status in intestinal failure in adults. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Nicola, J.P.; Carrasco, N.; Masini-Repiso, A.M. Dietary I− Absorption: Expression and Regulation of the Na+/I− Symporter in the Intestine. Vitam. Horm. 2015, 98, 1–31. [Google Scholar]
- Cavalieri, R.R. Iodine metabolism and thyroid physiology: Current concepts. Thyroid 1997, 7, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Ravera, S.; Reyna-Neyra, A.; Ferrandino, G.; Amzel, L.M.; Carrasco, N. The Sodium/Iodide Symporter (NIS): Molecular Physiology and Preclinical and Clinical Applications. Annu. Rev. Physiol. 2017, 79, 261–289. [Google Scholar] [CrossRef] [PubMed]
- Dai, G.; Levy, O.; Carrasco, N. Cloning and characterization of the thyroid iodide transporter. Nature 1996, 379, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, N. Iodide transport in the thyroid gland. BBA Rev. Biomembr. 1993, 1154, 65–82. [Google Scholar] [CrossRef]
- Milanesi, A.; Brent, G.A. Iodine and Thyroid Hormone Synthesis, Metabolism, and Action. Mol. Genet. Nutr. Asp. Major Trace Miner. 2017, 143–150. [Google Scholar]
- Lee, S.Y.; Chang, D.L.F.; He, X.; Pearce, E.N.; Braverman, L.E.; Leung, A.M. Urinary iodine excretion and serum thyroid function in adults after iodinated contrast administration. Thyroid 2015, 25, 471–477. [Google Scholar] [CrossRef]
- Dunn, J.T.; Dunn, A.D. Update on intrathyroidal iodine metabolism. Thyroid 2001, 11, 407–414. [Google Scholar] [CrossRef]
- Rohner, F.; Zimmermann, M.; Jooste, P.; Pandav, C.; Caldwell, K.; Raghavan, R.; Raiten, D.J. Biomarkers of Nutrition for Development—Iodine Review. J. Nutr. 2014, 144, 1322S–1342S. [Google Scholar] [CrossRef]
- Glinoer, D. The regulation of thyroid function in pregnancy: Pathways of endocrine adaptation from physiology to pathology. Endocr. Rev. 1997, 18, 404–433. [Google Scholar] [CrossRef]
- Osei, J.; Andersson, M.; van der Reijden, O.; Dold, S.; Smuts, C.M.; Baumgartner, J. Breast-milk iodine concentrations, iodine status, and thyroid function of breastfed infants aged 2–4 months and their mothers residing in a south african township. JCRPE J. Clin. Res. Pediatr. Endocrinol. 2016, 8, 381–391. [Google Scholar] [CrossRef]
- Guideline: Fortification of Food-Grade Salt with Iodine for the Prevention and Control of Iodine Deficiency Disorders. Available online: https://www.who.int/publications-detail/9789241507929 (accessed on 3 June 2020).
- De Groot, L.; Abalovich, M.; Alexander, E.K.; Amino, N.; Barbour, L.; Cobin, R.H.; Eastman, C.J.; Lazarus, J.H.; Luton, D.; Mandel, S.J.; et al. Management of thyroid dysfunction during pregnancy and postpartum: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2012, 97, 2543–2565. [Google Scholar] [CrossRef] [PubMed]
- Triggiani, V.; Tafaro, E.; Giagulli, V.; Sabba, C.; Resta, F.; Licchelli, B.; Guastamacchia, E. Role of Iodine, Selenium and Other Micronutrients in Thyroid Function and Disorders. Endocr. Metab. Immune Disord. Drug Targets 2009, 9, 277–294. [Google Scholar] [CrossRef] [PubMed]
- Guastamacchia, E.; Giagulli, V.; Licchelli, B.; Triggiani, V. Selenium and Iodine in Autoimmune Thyroiditis. Endocr. Metab. Immune Disord. Targets 2015, 15, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Bizhanova, A.; Kopp, P. Minireview: The sodium-iodide symporter NIS and pendrin in iodide homeostasis of the thyroid. Endocrinology 2009, 150, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Wémeau, J.L.; Kopp, P. Pendred syndrome. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 213–224. [Google Scholar] [CrossRef]
- Leung, A.M.; Braverman, L.E. Consequences of excess iodine. Nat. Rev. Endocrinol. 2014, 10, 136–142. [Google Scholar] [CrossRef]
- Sundick, R.S.; Bagchi, N.; Brown, T.R. The role of iodine in thyroid autoimmunity: From chickens to humans: A review. Autoimmunity 1992, 13, 61–68. [Google Scholar] [CrossRef]
- Katagiri, R.; Yuan, X.; Kobayashi, S.; Sasaki, S. Effect of excess iodine intake on thyroid diseases in different populations: A systematic review and meta-analyses including observational studies. PLoS ONE 2017, 12, e0173722. [Google Scholar] [CrossRef]
- Stanbury, J.B.; Wyngaarden, J.B. Effect of perchlorate on the human thyroid gland. Metabolism 1952, 1, 533–539. [Google Scholar]
- Leung, A.M.; Pearce, E.N.; Braverman, L.E. Perchlorate, iodine and the thyroid. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 133–141. [Google Scholar] [CrossRef]
- Tonacchera, M.; Pinchera, A.; Dimida, A.; Ferrarini, E.; Agretti, P.; Vitti, P.; Santini, F.; Crump, K.; Gibbs, J. Relative potencies and additivity of perchlorate, thiocyanate, nitrate, and iodide on the inhibition of radioactive iodide uptake by the human sodium iodide symporter. Thyroid 2004, 14, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Concilio, S.C.; Zhekova, H.R.; Noskov, S.Y.; Russell, S.J. Inter-species variation in monovalent anion substrate selectivity and inhibitor sensitivity in the sodium iodide symporter (NIS). PLoS ONE 2020, 15, e0229085. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.X.; Ding, M.H.; Li, Y.G.; Liu, Q.; Peng, K.L. Dose-Response Relationship between Orally Administered Ammonium Perchlorate and Urine Perchlorate Concentrations in Rats: Possible Biomarker to Quantify Environmental Ammonium Perchlorate Exposure on Thyroid Homeostasis. Arch. Environ. Occup. Health 2015, 70, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Hershman, J.M. Perchlorate and thyroid function: What are the environmental issues? Thyroid 2005, 15, 427–431. [Google Scholar] [CrossRef]
- Suh, M.; Abraham, L.; Hixon, J.G.; Proctor, D.M. The effects of perchlorate, nitrate, and thiocyanate on free thyroxine for potentially sensitive subpopulations o. The 2001–2002 and 2007–2008 National Health and Nutrition Examination Surveys. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 579–587. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the risks to public health related to the presence of perchlorate in food, in particular fruits and vegetables. EFSA J. 2014, 12, 3869. [Google Scholar] [CrossRef]
- Kounaves, S.P.; Stroble, S.T.; Anderson, R.M.; Moore, Q.; Catling, D.C.; Douglas, S.; Mckay, C.P.; Ming, D.W.; Smith, P.H.; Tamppari, L.K.; et al. Discovery of natural Perchlorate in the Antarctic Dry Valleys and its global implications. Environ. Sci. Technol. 2010, 44, 2360–2364. [Google Scholar] [CrossRef]
- Calderón, R.; Palma, P.; Parker, D.; Molina, M.; Godoy, F.A.; Escudey, M. Perchlorate levels in soil and waters from the Atacama Desert. Arch. Environ. Contam. Toxicol. 2014, 66, 155–161. [Google Scholar] [CrossRef]
- Kumarathilaka, P.; Oze, C.; Indraratne, S.P.; Vithanage, M. Perchlorate as an emerging contaminant in soil, water and food. Chemosphere 2016, 150, 667–677. [Google Scholar] [CrossRef]
- Furdui, V.I.; Zheng, J.; Furdui, A. Anthropogenic Perchlorate Increases since 1980 in the Canadian High Arctic. Environ. Sci. Technol. 2018, 52, 972–981. [Google Scholar] [CrossRef]
- Furdui, V.I.; Tomassini, F. Trends and sources of perchlorate in Arctic snow. Environ. Sci. Technol. 2010, 44, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, P.K.; Martinelango, P.K.; Jackson, W.A.; Anderson, T.A.; Tian, K.; Tock, R.W.; Rajagopalan, S. The origin of naturally occurring perchlorate: The role of atmospheric processes. Environ. Sci. Technol. 2005, 39, 1569–1575. [Google Scholar] [CrossRef]
- Kannan, K.; Praamsma, M.L.; Oldi, J.F.; Kunisue, T.; Sinha, R.K. Occurrence of perchlorate in drinking water, groundwater, surface water and human saliva from India. Chemosphere 2009, 76, 22–26. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, T.; Gan, Z.; Sun, H. Spatial distribution of perchlorate, iodide and thiocyanate in the aquatic environment of Tianjin, China: Environmental source analysis. Chemosphere 2014, 111, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Munster, J.; Hanson, G.N.; Jackson, W.A.; Rajagopalan, S. The fallout from fireworks: Perchlorate in total deposition. Water Air Soil Pollut. 2009, 198, 149–153. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Anderson, T.; Cox, S.; Harvey, G.; Cheng, Q.; Jackson, W.A. Perchlorate in Wet Deposition Across North America. Environ. Sci. Technol. 2009, 43, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Urbansky, E.T.; Brown, S.K. Perchlorate retention and mobility in soils. J. Environ. Monit. 2003, 5, 455–462. [Google Scholar] [CrossRef]
- Dugan, N.R.; Williams, D.J.; Meyer, M.; Schneider, R.R.; Speth, T.F.; Metz, D.H. The impact of temperature on the performance of anaerobic biological treatment of perchlorate in drinking water. Water Res. 2009, 43, 1867–1878. [Google Scholar] [CrossRef]
- Calderón, R.; Palma, P.; Eltit, K.; Arancibia-Miranda, N.; Silva-Moreno, E.; Yu, W. Field study on the uptake, accumulation and risk assessment of perchlorate in a soil-chard/spinach system: Impact of agronomic practices and fertilization. Sci. Total Environ. 2020, 719, 137411. [Google Scholar] [CrossRef]
- Calderón, R.; Godoy, F.; Escudey, M.; Palma, P. A review of perchlorate (ClO4−) occurrence in fruits and vegetables. Environ. Monit. Assess. 2017, 189, 82. [Google Scholar] [CrossRef]
- Wang, Z.; Forsyth, D.; Lau, B.P.Y.; Pelletier, L.; Bronson, R.; Gaertner, D. Estimated dietary exposure of canadians to perchlorate through the consumption of fruits and vegetables available in Ottawa markets. J. Agric. Food Chem. 2009, 57, 9250–9255. [Google Scholar] [CrossRef] [PubMed]
- Vega, M.; Nerenberg, R.; Vargas, I.T. Perchlorate contamination in Chile: Legacy, challenges, and potential solutions. Environ. Res. 2018, 164, 316–326. [Google Scholar] [CrossRef]
- Steinmaus, C.M. Perchlorate in Water Supplies: Sources, Exposures, and Health Effects. Curr. Environ. Health Rep. 2016, 3, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Iannece, P.; Motta, O.; Tedesco, R.; Carotenuto, M.; Proto, A. Determination of Perchlorate in Bottled Water from Italy. Water 2013, 5, 767–779. [Google Scholar] [CrossRef]
- Agrarmarkt Informations-Gesellschaft MbH. European Statistics Handbook—FRUIT LOGISTICA 2019. Available online: www.AMI-informiert.de (accessed on 2 March 2020).
- OEC. Chile (CHL) Exports, Imports, and Trade Partners. Available online: https://oec.world/en/profile/country/chl/ (accessed on 11 May 2020).
- Chilean Table Grape Exports to Europe Increase by 36%. Available online: https://www.freshplaza.com/article/9196058/chilean-table-grape-exports-to-europe-increase-by-36/ (accessed on 11 May 2020).
- Chile—Trade—European Commission. Available online: https://ec.europa.eu/trade/policy/countries-and-regions/countries/chile/ (accessed on 11 May 2020).
- Arcella, D.; Binaglia, M.; Vernazza, F. Dietary exposure assessment to perchlorate in the European population. EFSA J. 2017, 15, e05043. [Google Scholar]
- Dong, H.; Xiao, K.; Xian, Y.; Wu, Y.; Zhu, L. A novel approach for simultaneous analysis of perchlorate (ClO4−) and bromate (BrO3−) in fruits and vegetables using modified QuEChERS combined with ultrahigh performance liquid chromatography-tandem mass spectrometry. Food Chem. 2019, 270, 196–203. [Google Scholar] [CrossRef]
- Vejdovszky, K.; Grossgut, R.; Unterluggauer, H.; Inreiter, N.; Steinwider, J. Risk assessment of dietary exposure to perchlorate for the Austrian population. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2018, 35, 623–631. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, H.; Zhou, L.; Luo, F.; Zhang, X.; Chen, Z. Quantitative determination and contamination pattern of perchlorate in tea by ultra performance liquid chromatography and tandem mass spectrometry. Food Chem. 2019, 274, 180–186. [Google Scholar] [CrossRef]
- Huber, D.R.; Blount, B.C.; Mage, D.T.; Letkiewicz, F.J.; Kumar, A.; Allen, R.H. Estimating perchlorate exposure from food and tap water based on US biomonitoring and occurrence data. J. Expo. Sci. Environ. Epidemiol. 2011, 21, 395–407. [Google Scholar] [CrossRef]
- Blount, B.C.; Valentin-Blasini, L.; Osterloh, J.D.; Mauldin, J.P.; Pirkle, J.L. Perchlorate exposure of the US population, 2001–2002. J. Expo. Sci. Environ. Epidemiol. 2007, 17, 400–407. [Google Scholar] [CrossRef]
- Zoeller, T.R. Environmental chemicals targeting thyroid. Hormones 2010, 9, 28–40. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R.; Lee, D.H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V.; et al. Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; McCarthy, A.M.; Stohl, H.; Ibrahim, S.; Jeong, C.; Braverman, L.E.; Ma, W.; He, X.; Mestman, J.H.; Schuller, K.E.; et al. Urinary Iodine, Perchlorate, and Thiocyanate Concentrations in U.S. Lactating Women. Thyroid 2017, 27, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.M.; LaMar, A.; He, X.; Braverman, L.E.; Pearce, E.N. Iodine status and thyroid function of Boston-area vegetarians and vegans. J. Clin. Endocrinol. Metab. 2011, 96, E1303–E1307. [Google Scholar] [CrossRef] [PubMed]
- De Groef, B.; Decallonne, B.R.; Van der Geyten, S.; Darras, V.M.; Bouillon, R. Perchlorate versus other environmental sodium/iodide symporter inhibitors: Potential thyroid-related health effects. Eur. J. Endocrinol. 2006, 155, 17–25. [Google Scholar] [CrossRef]
- Téllez, R.T.; Chacón, P.M.; Abarca, C.R.; Blount, B.C.; Van Landingham, C.B.; Crump, K.S.; Gibbs, J.P. Long-term environmental exposure to perchlorate through drinking water and thyroid function during pregnancy and the neonatal period. Thyroid 2005, 15, 963–975. [Google Scholar] [CrossRef]
- Maffini, M.V.; Trasande, L.; Neltner, T.G. Perchlorate and Diet: Human Exposures, Risks, and Mitigation Strategies. Curr. Environ. Health Rep. 2016, 3, 107–117. [Google Scholar] [CrossRef]
- Borjan, M.; Marcella, S.; Blount, B.; Greenberg, M.; Zhang, J.; Murphy, E.; Valentin-Blasini, L.; Robson, M. Perchlorate exposure in lactating women in an urban community in New Jersey. Sci. Total Environ. 2011, 409, 460–464. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, Q.; Sun, H.W.; Rao, J.; Kannan, K. Perchlorate and iodide in whole blood samples from infants, children, and adults in Nanchang, China. Environ. Sci. Technol. 2010, 44, 6947–6953. [Google Scholar] [CrossRef]
- Valentín-Blasini, L.; Blount, B.C.; Otero-Santos, S.; Cao, Y.; Bernbaum, J.C.; Rogan, W.J. Perchlorate exposure and dose estimates in infants. Environ. Sci. Technol. 2011, 45, 4127–4132. [Google Scholar] [CrossRef]
- Lau, F.K.; Decastro, B.R.; Mills-Herring, L.; Tao, L.; Valentin-Blasini, L.; Alwis, K.U.; Blount, B.C. Urinary perchlorate as a measure of dietary and drinking water exposure in a representative sample of the United States population 2001–2008. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 207–214. [Google Scholar] [CrossRef]
- Crump, C.; Michaud, P.; Téllez, R.; Reyes, C.; Gonzalez, G.; Montgomery, E.L.; Crump, K.S.; Lobo, G.; Becerra, C.; Gibbs, J.P. Does perchlorate in drinking water affect thyroid function in newborns or school-age children? J. Occup. Environ. Med. 2000, 42, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Levie, D.; Korevaar, T.I.M.; Bath, S.C.; Murcia, M.; Dineva, M.; Llop, S.; Espada, M.; van Herwaarden, A.E.; de Rijke, Y.B.; Ibarluzea, J.M.; et al. Association of Maternal Iodine Status With Child IQ: A Meta-Analysis of Individual Participant Data. J. Clin. Endocrinol. Metab. 2019, 104, 5957–5967. [Google Scholar] [CrossRef] [PubMed]
- Knight, B.A.; Shields, B.M.; He, X.; Pearce, E.N.; Braverman, L.E.; Sturley, R.; Vaidya, B. Effect of perchlorate and thiocyanate exposure on thyroid function of pregnant women from South-West England: A cohort study. Thyroid Res. 2018, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.M.; Manley, S.W.; Morris, J.C.; Powell, K.A.; Bergert, E.R.; Mortimer, R.H. Sodium iodide symporter (NIS) gene expression in human placenta. Placenta 2001, 22, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Blount, B.C.; Rich, D.Q.; Valentin-Blasini, L.; Lashley, S.; Ananth, C.V.; Murphy, E.; Smulian, J.C.; Spain, B.J.; Barr, D.; Ledoux, T.; et al. Perinatal exposure to perchlorate, thiocyanate, and nitrate in New Jersey mothers and newborns. Environ. Sci. Technol. 2009, 43, 7543–7549. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, Y.; Wang, D.; Li, R.; Chen, X.; Mo, W.; Qin, X.; Sun, H.; Kannan, K. Placental transfer of and infantile exposure to perchlorate. Chemosphere 2016, 144, 948–954. [Google Scholar] [CrossRef]
- Pearce, E.N.; Alexiou, M.; Koukkou, E.; Braverman, L.E.; He, X.; Ilias, I.; Alevizaki, M.; Markou, K.B. Perchlorate and thiocyanate exposure and thyroid function in first-trimester pregnant women from Greece. Clin. Endocrinol. 2012, 77, 471–474. [Google Scholar] [CrossRef]
- Charatcharoenwitthaya, N.; Ongphiphadhanakul, B.; Pearce, E.N.; Somprasit, C.; Chanthasenanont, A.; He, X.; Chailurkit, L.; Braverman, L.E. The association between perchlorate and thiocyanate exposure and thyroid function in first-trimester pregnant Thai women. J. Clin. Endocrinol. Metab. 2014, 99, 2365–2371. [Google Scholar] [CrossRef]
- Steinmaus, C.; Pearl, M.; Kharrazi, M.; Blount, B.C.; Miller, M.D.; Pearce, E.N.; Valentin-Blasini, L.; DeLorenze, G.; Hoofnagle, A.N.; Liaw, J. Thyroid hormones and moderate exposure to perchlorate during pregnancy in women in southern California. Environ. Health Perspect. 2016, 124, 861–867. [Google Scholar] [CrossRef]
- Pearce, E.N.; Lazarus, J.H.; Smyth, P.P.A.; He, X.; Dall’Amico, D.; Parkes, A.B.; Burns, R.; Smith, D.F.; Maina, A.; Bestwick, J.P.; et al. Perchlorate and thiocyanate exposure and thyroid function in first-trimester pregnant women. J. Clin. Endocrinol. Metab. 2010, 95, 3207–3215. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.N.; Spencer, C.A.; Mestman, J.H.; Lee, R.H.; Bergoglio, L.M.; Mereshian, P.; He, X.; Leung, A.M.; Braverman, L.E. Effect of environmental perchlorate on thyroid function in pregnant women from Córdoba, Argentina, and Los Angeles, California. Endocr. Pract. 2011, 17, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Horton, M.K.; Blount, B.C.; Valentin-Blasini, L.; Wapner, R.; Whyatt, R.; Gennings, C.; Factor-Litvak, P. CO-occurring exposure to perchlorate, nitrate and thiocyanate alters thyroid function in healthy pregnant women. Environ. Res. 2015, 143, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.N.; Okosieme, O.E.; Murphy, R.; Hales, C.; Chiusano, E.; Maina, A.; Joomun, M.; Bestwick, J.P.; Smyth, P.; Paradice, R.; et al. Maternal perchlorate levels in women with borderline thyroid function during pregnancy and the cognitive development of their offspring: Data from the controlled antenatal thyroid study. J. Clin. Endocrinol. Metab. 2014, 99, 4291–4298. [Google Scholar] [CrossRef]
- Brent, G.A. Perchlorate exposure in pregnancy and cognitive outcomes in children: It’s not your mother’s thyroid. J. Clin. Endocrinol. Metab. 2014, 99, 4066–4068. [Google Scholar] [CrossRef][Green Version]
- Rubin, R.; Pearl, M.; Kharrazi, M.; Blount, B.C.; Miller, M.D.; Pearce, E.N.; Valentin-Blasini, L.; DeLorenze, G.; Liaw, J.; Hoofnagle, A.N.; et al. Maternal perchlorate exposure in pregnancy and altered birth outcomes. Environ. Res. 2017, 158, 72–81. [Google Scholar] [CrossRef]
- Buffler, P.A.; Kelsh, M.A.; Lau, E.C.; Edinboro, C.H.; Barnard, J.C.; Rutherford, G.W.; Daaboul, J.J.; Palmer, L.; Lorey, F.W. Thyroid function and perchlorate in drinking water: An evaluation among California newborns, 1998. Environ. Health Perspect. 2006, 114, 798–804. [Google Scholar] [CrossRef]
- Amitai, Y.; Winston, G.; Sack, J.; Wasser, J.; Lewis, M.; Blount, B.C.; Valentin-Blasini, L.; Fisher, N.; Israeli, A.; Leventhal, A. Gestational exposure to high perchlorate concentrations in drinking water and neonatal thyroxine levels. Thyroid 2007, 17, 843–850. [Google Scholar] [CrossRef]
- Steinmaus, C.; Miller, M.D.; Smith, A.H. Perchlorate in drinking water during pregnancy and neonatal thyroid hormone levels in California. J. Occup. Environ. Med. 2010, 52, 1217–1224. [Google Scholar] [CrossRef]
- Mervish, N.A.; Pajak, A.; Teitelbaum, S.L.; Pinney, S.M.; Windham, G.C.; Kushi, L.H.; Biro, F.M.; Valentin-Blasini, L.; Blount, B.C.; Wolff, M.S. Thyroid antagonists (perchlorate, thiocyanate, and nitrate) and childhood growth in a longitudinal study of U.S. girls. Environ. Health Perspect. 2016, 124, 542–549. [Google Scholar] [CrossRef]
- McMullen, J.; Ghassabian, A.; Kohn, B.; Trasande, L. Identifying subpopulations vulnerable to the thyroid-blocking effectsof perchlorateandthiocyanate. J. Clin. Endocrinol. Metab. 2017, 102, 2637–2645. [Google Scholar] [CrossRef] [PubMed]
- Van Sande, J.; Massart, C.; Beauwens, R.; Schoutens, A.; Costagliola, S.; Dumont, J.E.; Wolff, J. Anion selectivity by the sodium iodide symporter. Endocrinology 2003, 144, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Jianjie, C.; Wenjuan, X.; Jinling, C.; Jie, S.; Ruhui, J.; Meiyan, L. Fluoride caused thyroid endocrine disruption in male zebrafish (Danio rerio). Aquat. Toxicol. 2016, 171, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Yoffe, D.; Frim, R.; Ukeles, S.D.; Dagani, M.J.; Barda, H.J.; Benya, T.J.; Sanders, D.C. Bromine Compounds. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; pp. 1–31. [Google Scholar]
- Bromine as a Drinking-Water Disinfectant Alternative Drinking-Water Disinfectants: Bromine. 2018. Available online: http://apps.who.int/bookorders (accessed on 27 May 2020).
- Thewlis, B.H. The fate of potassium bromate when used as a breadmaking improver. J. Sci. Food Agric. 1974, 25, 1471–1475. [Google Scholar] [CrossRef]
- Shanmugavel, V.; Komala Santhi, K.; Kurup, A.H.; Kalakandan, S.; Anandharaj, A.; Rawson, A. Potassium bromate: Effects on bread components, health, environment and method of analysis: A review. Food Chem. 2020, 311, 125964. [Google Scholar] [CrossRef] [PubMed]
- Mitsumori, K.; Maita, K.; Kosaka, T.; Miyaoka, T.; Shirasu, Y. Two-year oral chronic toxicity and carcinogenicity study in rats of diets fumigated with methyl bromide. Food Chem. Toxicol. 1990, 28, 109–119. [Google Scholar] [CrossRef]
- Kurokawa, Y.; Maekawa, A.; Takahashi, M.; Hayashi, Y. Toxicity and carcinogenicity of potassium bromate—A new renal carcinogen. Environ. Health Perspect. 1990, 87, 309–335. [Google Scholar]
- Umemura, T.; Sai, K.; Takagi, A.; Hasegawa, R.; Kurokawa, Y. A possible role for cell proliferation in potassium bromate (KBrO3) carcinogenesis. J. Cancer Res. Clin. Oncol. 1993, 119, 463–469. [Google Scholar] [CrossRef]
- Last eval.: Potassium Bromate (IARC Summary & Evaluation, Volume 73, 1999). Available online: http://www.inchem.org/documents/iarc/vol73/73-17.html (accessed on 31 May 2020).
- FDA CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=172.730 (accessed on 31 May 2020).
- Wu, Z.; He, C.; Han, W.; Song, J.; Li, H.; Zhang, Y.; Jing, X.; Wu, W. Exposure pathways, levels and toxicity of polybrominated diphenyl ethers in humans: A review. Environ. Res. 2020, 187, 109531. [Google Scholar] [CrossRef]
- Pavelka, S. Metabolism of Bromide and Its Interference With the Metabolism of Iodine. Physiol. Res. 2004, 53 (Suppl. 1), S81–S90. [Google Scholar]
- Block, J. Nineteenth-Century Homeopathic Materia Medica Texts Predict Source Materials Whose Physiological Actions Influence Thyroid Activity. Homeopathy 2019, 108, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.K.; Gupta, N.; Kumar, V.; Khan, S.A.; Kumar, A. A review of emerging adsorbents and current demand for defluoridation of water: Bright future in water sustainability. Environ. Int. 2018, 111, 80–108. [Google Scholar] [CrossRef] [PubMed]
- Kurwadkar, S. Occurrence and distribution of organic and inorganic pollutants in groundwater. Water Environ. Res. 2019, 91, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- La Fluorazione Delle Acque in Italia. Available online: https://www.epicentro.iss.it/cavo_orale/nota (accessed on 27 May 2020).
- WHO. Water-Related Diseases. Available online: https://www.who.int/water_sanitation_health/diseases-risks/diseases/fluorosis/en/ (accessed on 31 May 2020).
- Srivastava, S.; Flora, S.J.S. Fluoride in Drinking Water and Skeletal Fluorosis: A Review of the Global Impact. Curr. Environ. Health Rep. 2020, 7. [Google Scholar] [CrossRef]
- Waugh, D.T. Fluoride exposure induces inhibition of sodium/iodide symporter (NIS) contributing to impaired iodine absorption and iodine deficiency: Molecular mechanisms of inhibition and implications for public health. Int. J. Environ. Res. Public Health 2019, 16, 1086. [Google Scholar] [CrossRef]
- Nakamoto, T.; Ralph Rawls, H. Fluoride exposure in early life as the possible root cause of disease in later life. J. Clin. Pediatr. Dent. 2018, 42, 325–330. [Google Scholar] [CrossRef]
- Johnston, N.R.; Strobel, S.A. Principles of fluoride toxicity and the cellular response: A review. Arch. Toxicol. 2020, 94, 1051–1069. [Google Scholar] [CrossRef]
- Hegde, R.M.; Rego, R.M.; Potla, K.M.; Kurkuri, M.D.; Kigga, M. Bio-inspired materials for defluoridation of water: A review. Chemosphere 2020, 253. [Google Scholar] [CrossRef]
- Leung, A.K.C.; Leung, A.A.C. Evaluation and management of the child with hypothyroidism. World J. Pediatr. 2019, 15, 124–134. [Google Scholar] [CrossRef]
- Hay, I.; Hynes, K.L.; Burgess, J.R. Mild-to-moderate gestational iodine deficiency processing disorder. Nutrients 2019, 11, 19674. [Google Scholar] [CrossRef]
- Urinary Iodine Concentrations for Determining Iodine Status in Populations. Available online: https://apps.who.int/iris/bitstream/handle/10665/85972/WHO_NMH_NHD_EPG_13.1_eng.pdf?ua=1 (accessed on 3 June 2020).
- Zimmermann, M.B. Iodine deficiency. Endocr. Rev. 2009, 30, 376–408. [Google Scholar] [CrossRef] [PubMed]
- Baldini, E.; Virili, C.; D’Armiento, E.; Centanni, M.; Ulisse, S. Iodine status in schoolchildren and pregnant women of lazio, a central region of Italy. Nutrients 2019, 11, 1674. [Google Scholar] [CrossRef] [PubMed]
- Giordano, C.; Barone, I.; Marsico, S.; Bruno, R.; Bonofiglio, D.; Catalano, S.; Andò, S. Endemic goiter and iodine prophylaxis in calabria, a region of southern Italy: Past and present. Nutrients 2019, 11, 2428. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Andersson, M. Assessment of iodine nutrition in populations: Past, present, and future. Nutr. Rev. 2012. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Eastman, C.J. NATURE REVIEWS | ENDOCRINOLOGY The changing epidemiology of iodine deficiency. Nat. Publ. Gr. 2012. [Google Scholar] [CrossRef]
- Soldin, O.P.; Braverman, L.E.; Lamm, S.H. Perchlorate Clinical Pharmacology and Human Health: A Review. Ther. Drug Monit. 2001, 23, 316. [Google Scholar] [CrossRef]
- Murray, C.W.; Egan, S.K.; Kim, H.; Beru, N.; Bolger, P.M. US food and drug administration’s total diet study: Dietary intake of perchlorate and iodine. J. Expo. Sci. Environ. Epidemiol. 2008, 18, 571–580. [Google Scholar] [CrossRef]
- Mantovani, A. Endocrine disrupters and the safety of food chains. Horm. Res. Paediatr. 2016, 86, 279–288. [Google Scholar] [CrossRef]
- Council, N.R. Health Implications of Perchlorate Ingestion. 2005. Available online: https://books.google.com/books?hl=it&lr=&id=05F0iOqvwgAC&oi=fnd&pg=PR1&ots=1byRT87Mff&sig=Kf3cQjhexKXGQ24WspdAKos7iNM (accessed on 10 May 2020).
- Lawrence, J.; Lamm, S.; Braverman, L.E. Low dose perchlorate (3 mg daily) and thyroid function. Thyroid 2001, 11, 295. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.E.; Lamm, S.H.; Pino, S.; Richman, K.; Braverman, L.E. The effect of short-term low-dose perchlorate on various aspects of thyroid function. Thyroid 2000, 10, 659–663. [Google Scholar] [CrossRef]
- Greer, M.A.; Goodman, G.; Pleus, R.C.; Greer, S.E. Health effects perchlorate contamination: The dose response for inhibition of thyroidal radioiodine uptake in humans. Environ. Health Perspect. 2002, 110, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.P.; Ahmad, R.; Crump, K.S.; Houck, D.P.; Leveille, T.S.; Findley, J.E.; Francis, M. Evaluation of a population with occupational exposure to airborne ammonium perchlorate for possible acute or chronic effects on thyroid function. J. Occup. Environ. Med. 1998, 40, 1072–1082. [Google Scholar] [CrossRef] [PubMed]
- Lamm, S.H.; Braverman, L.E.; Li, F.X.; Richman, K.; Pino, S.; Howearth, G. Thyroid health status of ammonium perchlorate workers: A cross-sectional occupational health study. J. Occup. Environ. Med. 1999, 41, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Braverman, L.E.; He, X.; Pino, S.; Cross, M.; Magnani, B.; Lamm, S.H.; Kruse, M.B.; Engel, A.; Crump, K.S.; Gibbs, J.P. The Effect of Perchlorate, Thiocyanate, and Nitrate on Thyroid Function in Workers Exposed to Perchlorate Long-Term. J. Clin. Endocrinol. Metab. 2005, 90, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Alexeeff, G.V.; Rodriquez, M.; Brown Governor, E.G., Jr. Office of Environmental Health Hazard Assessment OEHHA Adopts Updated Public Health Goal for Perchlorate. 2015. Available online: www.oehha.ca.gov (accessed on 10 May 2020).
- WHO|JECFA. Evaluations of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/chemical.aspx?chemID=5885 (accessed on 11 May 2020).
- Srinivasan, A.; Viraraghavan, T. Perchlorate: Health effects and technologies for its removal from water resources. Int. J. Environ. Res. Public Health 2009, 6, 1418–1442. [Google Scholar] [CrossRef]
- Batista, J.R.; McGarvey, F.X.; Vieira, A.R. The Removal of Perchlorate from Waters Using Ion-Exchange Resins. In Perchlorate in the Environment; Springer: Boston, MA, USA, 2000; pp. 135–145. [Google Scholar]
- Xu, J.; Song, Y.; Min, B.; Steinberg, L.; Logan, B.E. Microbial degradation of perchlorate: Principles and applications. Environ. Eng. Sci. 2003, 20, 405–422. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, G.; Zhang, Z. TiO2-based catalysts for photocatalytic reduction of aqueous oxyanions: State-of-the-art and future prospects. Environ. Int. 2020, 136. [Google Scholar] [CrossRef]
- Han, J.; Kong, C.; Heo, J.; Yoon, Y.; Lee, H.; Her, N. Removal of perchlorate using reverse osmosis and nanofiltration membranes. Environ. Eng. Res. 2012, 17, 185–190. [Google Scholar] [CrossRef]
- Roquebert, V.; Booth, S.; Cushing, R.S.; Crozes, G.; Hansen, E. Electrodialysis reversal (EDR) and ion exchange as polishing treatment for perchlorate treatment. Desalination 2000, 131, 285–291. [Google Scholar] [CrossRef]
- Kumar, E.; Bhatnagar, A.; Hogland, W.; Marques, M.; Sillanpää, M. Interaction of inorganic anions with iron-mineral adsorbents in aqueous media—A review. Adv. Colloid Interface Sci. 2014, 203, 11–21. [Google Scholar] [CrossRef]
- Kumar, E.; Bhatnagar, A.; Ji, M.; Jung, W.; Lee, S.H.; Kim, S.J.; Lee, G.; Song, H.; Choi, J.Y.; Yang, J.S.; et al. Defluoridation from aqueous solutions by granular ferric hydroxide (GFH). Water Res. 2009, 43, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Susarla, S.; Collette, T.W.; Garrison, A.W.; Wolfe, N.L.; Mccutcheon, S.C. Perchlorate identification in fertilizers. Environ. Sci. Technol. 1999, 33, 3469–3472. [Google Scholar] [CrossRef]
- Calderon, R.; Rajendiran, K.; Kim, U.J.; Palma, P.; Arancibia-Miranda, N.; Silva-Moreno, E.; Corradini, F. Sources and fates of perchlorate in soils in Chile: A case study of perchlorate dynamics in soil-crop systems using lettuce (Lactuca sativa) fields. Environ. Pollut. 2020, 264. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisco, G.; De Tullio, A.; Giagulli, V.A.; De Pergola, G.; Triggiani, V. Interference on Iodine Uptake and Human Thyroid Function by Perchlorate-Contaminated Water and Food. Nutrients 2020, 12, 1669. https://doi.org/10.3390/nu12061669
Lisco G, De Tullio A, Giagulli VA, De Pergola G, Triggiani V. Interference on Iodine Uptake and Human Thyroid Function by Perchlorate-Contaminated Water and Food. Nutrients. 2020; 12(6):1669. https://doi.org/10.3390/nu12061669
Chicago/Turabian StyleLisco, Giuseppe, Anna De Tullio, Vito Angelo Giagulli, Giovanni De Pergola, and Vincenzo Triggiani. 2020. "Interference on Iodine Uptake and Human Thyroid Function by Perchlorate-Contaminated Water and Food" Nutrients 12, no. 6: 1669. https://doi.org/10.3390/nu12061669
APA StyleLisco, G., De Tullio, A., Giagulli, V. A., De Pergola, G., & Triggiani, V. (2020). Interference on Iodine Uptake and Human Thyroid Function by Perchlorate-Contaminated Water and Food. Nutrients, 12(6), 1669. https://doi.org/10.3390/nu12061669