Inflammation Adjustment by Two Methods Decreases the Estimated Prevalence of Zinc Deficiency in Malawi
Abstract
1. Introduction
2. Material and Methods
2.1. Adjustments of SZC
2.2. Statistical Analyses
2.3. Ethical Approval
3. Results
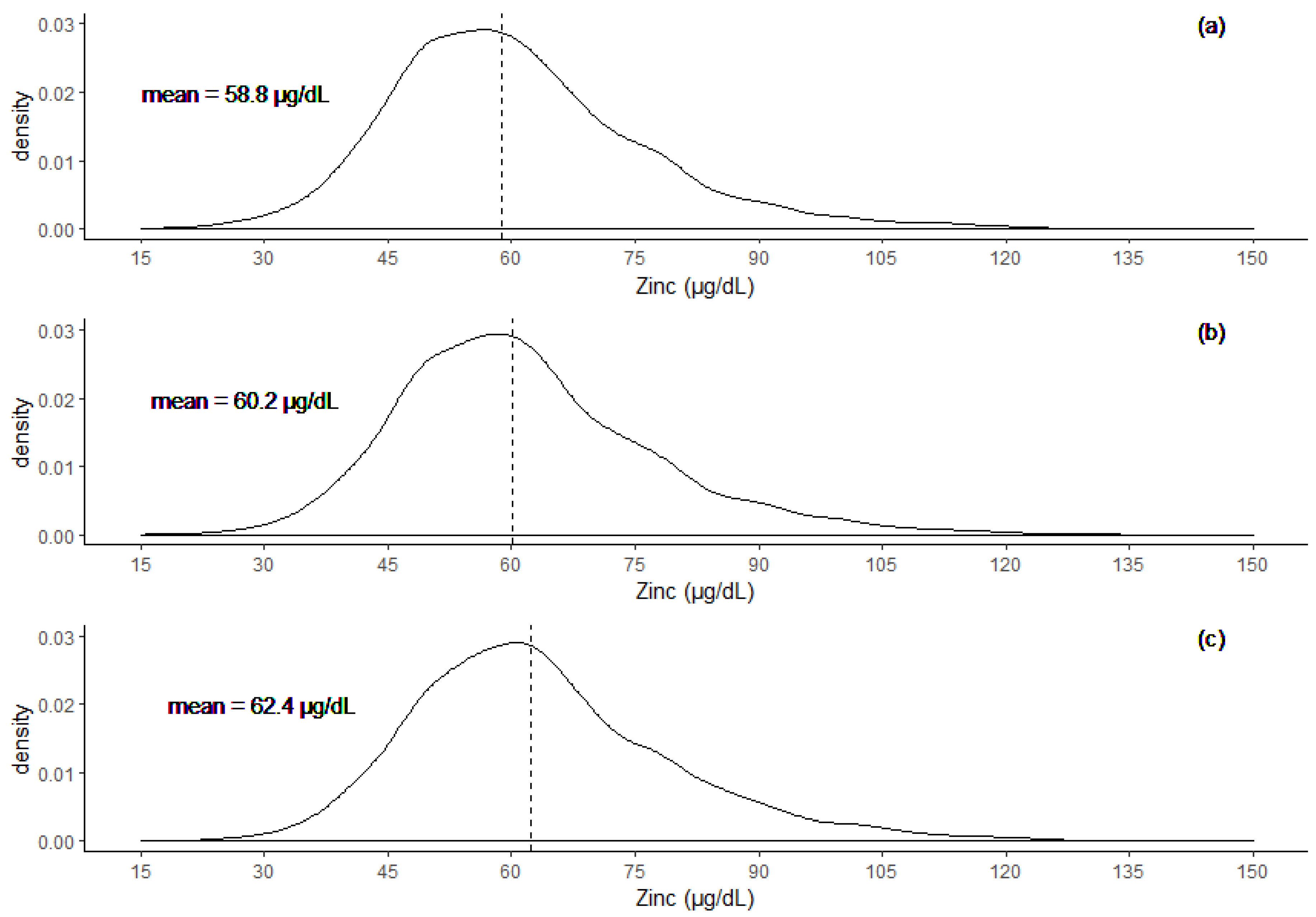
3.1. Adjustment of Serum Zinc Concentration (SZC)
3.2. Prevalence Point Estimates
3.3. Differences in SZC Across The Three Groups
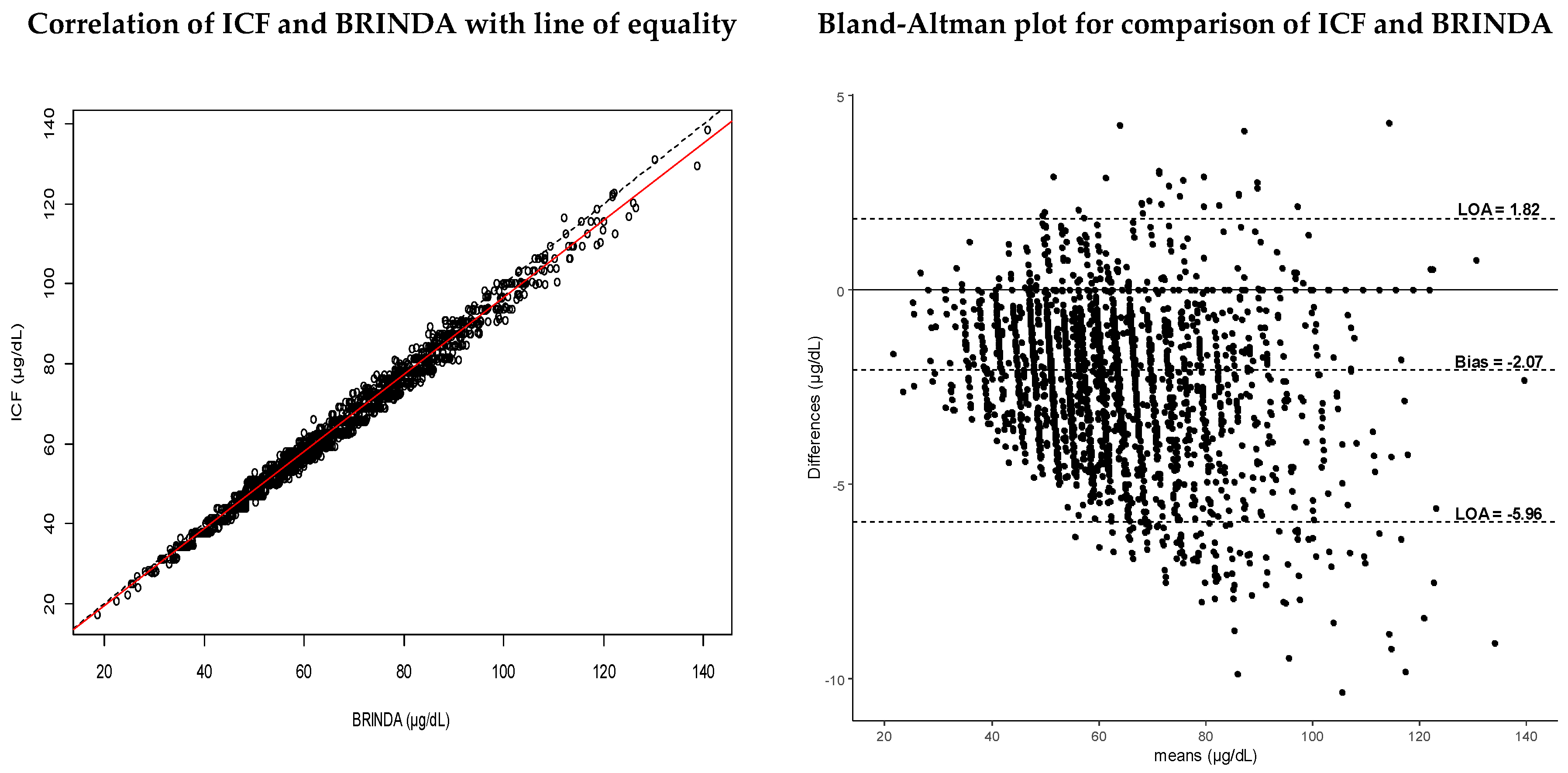
3.4. Assessment of Agreement Methods
3.5. Association Relationships between Serum Zinc Concentration (SZC) and Stunting
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- King, J.C.; Brown, K.H.; Gibson, R.S.; Krebs, N.F.; Lowe, N.M.; Siekmann, J.H.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND) - Zinc Review. J. Nutr. 2016, 146, 858S–885S. [Google Scholar] [CrossRef]
- Brown, K.H.; Rivera, J.A.; Bhutta, Z.; Gibson, R.S.; King, J.C.; Ruel, M.T.; Brittmarie, S.; Wasanwisut, E.; Hotz, C. International Zinc Nutrition Consultative Group (IZiNCG) Technical Document #1. Assessment of the Risk of Zinc Deficiency in Populations and Options for Its Control. Food Nutr. Bull. 2004, 25, s91–s203. [Google Scholar]
- Hess, S.Y. National risk of zinc deficiency as estimated by national surveys. Food Nutr. Bull. 2017, 38, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.L.F.; Black, R.E. Functional indicators for assessing zinc deficiency. Food Nutr. Bull. 2007, 28, S454–S479. [Google Scholar] [CrossRef] [PubMed]
- Lowe, N.M.; Fekete, K.; Desci, T. Methods of assessment of zinc status in humans: A systematic review. Am. J. Clin. Nutr. 2009, 89, 2040s–2051s. [Google Scholar] [CrossRef]
- King, J.C. Zinc: An essential but elusive nutrient. Am. J. Clin. Nutr. 2011, 94, 679S. [Google Scholar] [CrossRef]
- Wieringa, F.T.; Dijkhuizen, M.A.; Fiorentino, M.; Laillou, A.; Berger, J. Determination of zinc status in humans: Which indicator should we use? Nutrients 2015, 7, 3252–3263. [Google Scholar] [CrossRef]
- National Statistical Office(NSO); Community Health Sciences Unit(CHSU); Centers for Disease Control and Prevention (CDC); Emory University. Malawi Micronutrient Survey 2015-16; NSO: Atlanta, GA, USA; CHSU: Atlanta, GA, USA; CDC: Atlanta, GA, USA; Emory University: Atlanta, GA, USA, 2017. [Google Scholar]
- Galloway, P.; McMillan, D.C.; Sattar, N. Effect of the inflammatory response on trace element and vitamin status. Ann. Clin. Biochem. 2000, 37, 289–297. [Google Scholar] [CrossRef]
- Namaste, S.M.; Aaron, G.J.; Varadhan, R.; Peerson, J.M.; Suchdev, P.S. Methodologic approach for the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am. J. Clin. Nutr. 2017, 106, 333S–347S. [Google Scholar]
- Thurnham, D.I.; Mccabe, L.D.; Haldar, S.; Wieringa, F.T.; Northrop-clewes, C.A.; Mccabe, G.P. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: A meta-analysis. Am. J. Clin. Nutr. 2010, 92, 546–555. [Google Scholar] [CrossRef]
- MacDonell, S.O.; Miller, J.C.; Harper, M.J.; Reid, M.R.; Haszard, J.J.; Gibson, R.S.; Houghton, L.A. A comparison of methods for adjusting biomarkers of iron, zinc, and selenium status for the effect of inflammation in an older population: A case for interleukin 6. Am. J. Clin. Nutr. 2018, 107, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Namaste, S.M.; Serdula, M.; Suchdev, P.S.; Rohner, F.; Flores-Ayala, R.; Addo, O.Y.; Raiten, D.J. Adjusting total body iron for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am. J. Clin. Nutr. 2017, 106, 383S–389S. [Google Scholar] [PubMed]
- Karakochuk, C.D.; Barr, S.I.; Boy, E.; Bahizire, E.; Tugirimana, P.L.; Akilimali, P.Z.; Houghton, L.A.; Green, T.J. The effect of inflammation on serum zinc concentrations and the prevalence estimates of population-level zinc status among Congolese children aged 6–59 months. Eur. J. Clin. Nutr. 2017, 71, 1467–1470. [Google Scholar] [CrossRef] [PubMed]
- Diana, A.; Haszard, J.J.; Purnamasari, D.M.; Nurulazmi, I.; Luftimas, D.E.; Rahmania, S.; Nugraha, G.I.; Erhardt, J.; Gibson, R.S.; Houghton, L.; et al. Iron, zinc, vitamin A and selenium status in a cohort of Indonesian infants after adjusting for inflammation using several different approaches. Br. J. Nutr. 2017, 118, 830–839. [Google Scholar] [CrossRef]
- Mburu, A.S.W.W.; Thurnham, D.I.; Mwaniki, D.L.; Muniu, E.M.; Alumasa, F.M. The influence of inflammation on plasma zinc concentration in apparently healthy, HIV Kenyan adults and zinc responses after a multi-micronutrient supplement. Eur. J. Clin. Nutr. 2010, 64, 510–517. [Google Scholar] [CrossRef]
- Lim, K.; Booth, A.; Szymlek-Gay, E.A.; Gibson, R.S.; Bailey, K.B.; Irving, D.; Nowson, C.; Riddell, L. Associations between dietary iron and zinc intakes, and between biochemical iron and zinc status in women. Nutrients 2015, 7, 2983–2999. [Google Scholar] [CrossRef]
- Mwangi, M.N.; Echoka, E.; Knijff, M.; Kaduka, L.; Werema, B.G.; Kinya, F.M.; Mutisya, R.; Muniu, E.M.; Demir, A.Y.; Verhoef, H.; et al. Iron status of Kenyan pregnant women after adjusting for inflammation using BRINDA regression analysis and other correction methods. Nutrients 2019, 11, 420. [Google Scholar] [CrossRef]
- Suchdev, P.S.; Namaste, S.M.L.; Aaron, G.J.; Raiten, D.J.; Brown, K.H.; Flores-ayala, R. Overview of the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) Project. Adv. Nutr. 2016, 7, 349–356. [Google Scholar] [CrossRef]
- Grant, F.K.E.; Suchdev, P.S.; Flores-ayala, R.; Conrad, R.; Ramakrishnan, U.; Ruth, L.J.; Martorell, R.; Program, H.S. Correcting for inflammation changes estimates of iron deficiency among rural Kenyan pre-school children. J. Nutr. 2016, 142, 105–111. [Google Scholar] [CrossRef]
- Larson, L.M.; Namaste, S.M.; Williams, A.M.; Engle-Stone, R.; Addo, O.Y.; Suchdev, P.S.; Wirth, J.P.; Temple, V.; Serdula, M.; Northrop-Clewes, C.A. Adjusting retinol-binding protein concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am. J. Clin. Nutr. 2017, 106, 390S–401S. [Google Scholar]
- Rohner, F.; Namaste, S.M.; Larson, L.M.; Addo, O.Y.; Mei, Z.; Suchdev, P.S.; Williams, A.M.; Sakr Ashour, F.A.; Rawat, R.; Raiten, D.J.; et al. Adjusting soluble transferrin receptor concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am. J. Clin. Nutr. 2017, 106, 372S–382S. [Google Scholar]
- Namaste, S.M.; Rohner, F.; Huang, J.; Bhushan, N.L.; Flores-Ayala, R.; Kupka, R.; Mei, Z.; Rawat, R.; Williams, A.M.; Raiten, D.J.; et al. Adjusting ferritin concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am. J. Clin. Nutr. 2017, 106, 359S–371S. [Google Scholar]
- Cichon, B.; Ritz, C.; Fabiansen, C.; Christensen, V.B.; Filteau, S.; Friis, H.; Kaestel, P. Assessment of regression models for adjustment of iron status biomarkers for inflammation in children with moderate acute malnutrition in Burkina Faso. J. Nutr. 2016, 147, 125–132. [Google Scholar] [CrossRef]
- Fiorentino, M.; Perignon, M.; Kuong, K.; Chamnan, C.; Berger, J.; Wieringa, F.T. Subclinical inflammation affects iron and Vitamin A but not zinc status assessment in Senegalese children and Cambodian children and women. Public Health Nutr. 2018, 21, 1266–1277. [Google Scholar] [CrossRef]
- Bayse, D.D.; Gunter, E.W.; Neese, J.W.; Turner, W.E. Laboratory Procedures used by the Clinical Division, Centers for Disease Control for the Second Health and Nutrition Examination Survey (NHANES II) 1976–1980; U.S Department of Health and Human Services: Atlanta, GA, USA, 1985. [Google Scholar]
- Hotz, C.; Peerson, J.M.; Brown, K.H. Suggested lower cutoffs of serum zinc concentrations for assessing zinc status: Reanalysis of the second National Health and Nutrition Examination Survey data (1976–1980). Am. J. Clin. Nutr. 2003, 78, 756–764. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef]
- World Health Organization. Physical Status: The Use and Interpretation of Anthropometry; World Health Organization: Geneva, Switzerland, 1995; Volume 1995. [Google Scholar]
- Ghashut, R.A.; McMillan, D.C.; Kinsella, J.; Vasilaki, A.T.; Talwar, D.; Duncan, A. The effect of the systemic inflammatory response on plasma zinc and selenium adjusted for albumin. Clin. Nutr. 2016, 35, 381–387. [Google Scholar] [CrossRef]
- Siyame, E.W.P.; Hurst, R.; Wawer, A.A.; Young, S.D.; Broadley, M.R.; Chilimba, A.D.C.; Ander, L.E.; Watts, M.J.; Chilima, B.; Gondwe, J.; et al. A high prevalence of zinc-but not iron-deficiency among women in Rural Malawi: A cross-sectional study. Int. J. Vitam. Nutr. Res. 2013, 83, 176–187. [Google Scholar] [CrossRef]
- Huddle, J.-M.; Gibson, R.S.; Cullinan, T.R. Is zinc a limiting nutrient in the diets of rural pregnant Malawian women? Br. J. Nutr. 1998, 79, 257–265. [Google Scholar] [CrossRef]
- de Benoist, B.; Darnton-Hill, I.; Davidsson, L.; Fontaine, O.; Hotz, C. Conclusions of the joint WHO/UNICEF/IAEA/IZiNCG interagency meeting on zinc status indicators. Food Nutr. Bull. 2007, 28, S480–S484. [Google Scholar] [CrossRef]
- Joy, E.J.M.; Ander, E.L.; Young, S.D.; Black, C.R.; Watts, M.J.; Chilimba, A.D.C.; Chilima, B.; Siyame, E.W.P.; Kalimbira, A.A.; Hurst, R.; et al. Dietary mineral supplies in Africa. Physiol. Plant. 2014, 151, 208–229. [Google Scholar] [CrossRef]
- Joy, E.J.M.; Kumssa, D.B.; Broadley, M.R.; Watts, M.J.; Young, S.D.; Chilimba, A.D.C.; Ander, E.L. Dietary mineral supplies in Malawi: Spatial and socioeconomic assessment. BMC Nutr. 2015, 1, 42. [Google Scholar] [CrossRef]
- Manary, M.J.; Hotz, C.; Krebs, N.F.; Gibson, R.S.; Westcott, J.E.; Broadhead, R.L.; Hambidge, K.M. Zinc homeostasis in Malawian children consuming a high-phytate, maize-based diet. Am. J. Clin. Nutr. 2002, 75, 1057–1061. [Google Scholar] [CrossRef]
- Hurst, R.; Siyame, E.W.P.; Young, S.D.; Chilimba, A.D.C.; Joy, E.J.M.; Black, C.R.; Ander, E.L.; Watts, M.J.; Chilima, B.; Gondwe, J.; et al. Soil-type influences human selenium status and underlies widespread selenium deficiency risks in Malawi. Sci. Rep. 2013, 3, 1425. [Google Scholar] [CrossRef]
- Joy, E.J.M.; Broadley, M.R.; Young, S.D.; Black, C.R.; Chilimba, A.D.C.; Ander, E.L.; Barlow, T.S.; Watts, M.J. Soil type influences crop mineral composition in Malawi. Sci. Total Environ. 2015, 505, 587–595. [Google Scholar] [CrossRef]
- Manary, M.J.; Abrams, S.A.; Griffin, I.J.; Quimper, M.M.; Shulman, R.J.; Hamzo, M.G.; Chen, Z.; Maleta, K.; Manary, M.J. Perturbed zinc homeostasis in rural 3-5-y-old malawian children is associated with abnormalities in intestinal permeability attributed to tropical enteropathy. Pediatr. Res. 2010, 67, 671–675. [Google Scholar] [CrossRef]
- Bhutta, Z.A. Effect of infections and environmental factors on growth and nutritional status in developing countries. J. Pediatr. Gastroenterol. Nutr. 2006, 43, S13–S21. [Google Scholar] [CrossRef]
- Quihui, L.; Morales, G.G.; Méndez, R.O.; Leyva, J.G.; Esparza, J.; Valencia, M.E. Could giardiasis be a risk factor for low zinc status in schoolchildren from northwestern Mexico? A cross-sectional study with longitudinal follow-up. BMC Public Health 2010, 10, 85. [Google Scholar] [CrossRef]
- de Gier, B.; Mpabanzi, L.; Vereecken, K.; van der Werff, S.D.; D’Haese, P.C.; Fiorentino, M.; Khov, K.; Perignon, M.; Chamnan, C.; Berger, J.; et al. Height, zinc and soil-transmitted helminth infections in schoolchildren: A study in Cuba and Cambodia. Nutrients 2015, 7, 3000–3010. [Google Scholar] [CrossRef]
- Swe, K.M.M.; Abas, A.B.L.; Bhardwaj, A.; Barua, A.; Nair, N.S. Zinc supplements for treating thalassaemia and sickle cell disease. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef] [PubMed]
- Sales, M.C.; de Queiroz, E.O.; Paiva, A.d.A. Association between anemia and subclinical infection in children in Paraíba state, Brazil. Rev. Bras. Hematol. Hemoter. 2011, 33, 96–99. [Google Scholar] [CrossRef]
- Thurnham, D.I.; Mburu, A.S.W.; Mwaniki, D.L.; De Wagt, A. Micronutrients in childhood and the influence of subclinical inflammation. Proc. Nutr. Soc. 2005, 64, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.H. Effect of infections on plasma zinc concentration and implications for zinc status assessment in low-income countries. Am. J. Clin. Nutr. 1998, 68, 425S–429S. [Google Scholar] [CrossRef] [PubMed]
- Besecker, B.Y.; Exline, M.C.; Hollyfield, J.; Phillips, G.; DiSilvestro, R.A.; Wewers, M.D.; Knoell, D.L. A comparison of zinc metabolism, inflammation, and disease severity in critically ill infected and noninfected adults early after intensive care unit admission. Am. J. Clin. Nutr. 2011, 93, 1356–1364. [Google Scholar] [CrossRef]
- Thurnham, D.I.; Mccabe, G.P. Influence of infection and inflammation on biomarkers of nutritional status with an emphasis on vitamin A and iron. In Report: Priorities in the Assessment of Vitamin A and Iron Status in Populations, Panama City, Panama, 15-17 September 2010; World Health Organization: Geneva, Switzerland, 2012; pp. 63–80. [Google Scholar]
- Laillou, A.; Pham, T. Van; Tran, N.T.; Le, H.T.; Wieringa, F.; Rohner, F.; Fortin, S.; Le, M.B.; Tran, D.T.; Moench-Pfanner, R.; et al. Micronutrient deficits are still public health issues among women and young children in Vietnam. PLoS ONE 2012, 7, e34906. [Google Scholar] [CrossRef]
- Tessema, M.; De Groote, H.; Brouwer, I.D.; Feskens, E.J.M.; Belachew, T.; Zerfu, D.; Belay, A.; Demelash, Y.; Gunaratna, N.S. Soil zinc is associated with serum zinc but not with linear growth of children in Ethiopia. Nutrients 2019, 11, 221. [Google Scholar] [CrossRef]
- Beinner, M.A.; Amorim, F.R.D.; Silva, J.B.B.D.; Menezes, M.Â.D.B.C.; Jansen, A.K.; Lamounier, J.A. Plasma zinc and hair zinc levels, anthropometric status and food intake of children in a rural area of Brazil. Rev. Nutr. 2011, 23, 75–83. [Google Scholar] [CrossRef][Green Version]
- Huey, S.L.; Mehta, S. Stunting: The need for application of advances in technology to understand a complex health problem. Ebiomedicine 2016, 6, 26–27. [Google Scholar] [CrossRef]
- Golden, M.H.N. Malnutrition. In Textbook of Pediatric Gastroenterology and Nutrition; Guandalini, S., Ed.; Taylor & Francis: Hampshire, UK, 2004; pp. 424–489. ISBN 9781841843155. [Google Scholar]
- Knez, M.; Stangoulis, J.C.R.; Zec, M.; Debeljak-Martacic, J.; Pavlovic, Z.; Gurinovic, M.; Glibetic, M. An initial evaluation of newly proposed biomarker of zinc status in humans - linoleic acid: Dihomo-γ-linolenic acid (LA:DGLA) ratio. Clin. Nutr. ESPEN 2016, 15, 85–92. [Google Scholar] [CrossRef]
| Serum Zinc Concentration Values µmol/L (µg/dL) a | ||||
|---|---|---|---|---|
| Age Group | < 10 years | > 10 years | ||
| Children | Females | Males | ||
| Non-Pregnant | Pregnant b | |||
| Morning Fasting | N/A | 10.7 (70) | 8.6 (56) 1st trimester:7.6 (50) 2nd/3rd trimester: | 11.3 (74) |
| Morning non-fasting | 9.9 (65) | 10.1 (66) | 10.7 (70) | |
| Afternoon/evening | 8.7 (57) | 9.0 (59) | 9.3 (61) | |
| Group | Mean SZC ± SD (µg/dL) | Mean CRP ± SD (mg/L) | Mean AGP ± SD (g/L) |
|---|---|---|---|
| Men | 62.3 ± 14.1 | 2.7 ± 6.1 | 0.7 ± 0.4 |
| WRA | 60.1 ± 14.4 | 2.5 ± 8.5 | 0.7 ± 0.4 |
| SAC | 62.4 ± 15.8 | 4.3 ± 11.6 | 1.0 ± 0.6 |
| PSC | 58.4 ± 14.9 | 6.3 ± 14.5 | 1.4 ± 0.8 |
| Total | 58.8 ± 15.0 | 5.9 ± 13.9 | 1.3 ± 0.8 |
| Group | Inflammation Category | Correction Factors (ICF) | Internal Reference Values (BRINDA) | |
|---|---|---|---|---|
| CRP | AGP | |||
| Men | 0.13 | 0.37 | ||
| Normal | - | |||
| Incubation | 1.060 | |||
| Early | 1.049 | |||
| Late | 1.058 | |||
| WRA | 0.15 | 0.39 | ||
| Normal | - | |||
| Incubation | 0.966 | |||
| Early | 1.008 | |||
| Late | 1.076 | |||
| SAC | 0.12 | 0.46 | ||
| Normal | - | |||
| Incubation | 1.070 | |||
| Early | 1.164 | |||
| Late | 1.037 | |||
| PSC | 0.19 | 0.56 | ||
| Normal | - | |||
| Incubation | 1.069 | |||
| Early | 1.105 | |||
| Late | 1.004 | |||
| Group | Log β-Coefficient | Intercept (95% CI) | R2 | p-Value | |
|---|---|---|---|---|---|
| CRP (95% CI) | AGP (95% CI) | ||||
| Men | −0.033 (−0.056, −0.010) | 0.022 (−0.061, 0.105) | 61.83 (58.79, 65.01) | 0.03 | 0.013 |
| WRA | −0.007 (−0.020, 0.006) | −0.030 (−0.076, 0.015) | 57.70 (56.23, 59.21) | 0.01 | 0.047 |
| SAC | −0.021 (−0.032, −0.009) | −0.037 (−0.081, 0.006) | 59.40 (58.29, 60.53) | 0.05 | <0.001 |
| PSC | −0.026 (−0.035, −0.015) | −0.013 (−0.046, 0.020) | 58.12 (57.21, 59.04) | 0.04 | <0.001 |
| Inflammation a | Serum Zinc b | |||
|---|---|---|---|---|
| Unadjusted | ICF | BRINDA | ||
| Prev. (95% CI) | Prev. (95% CI) | Prev. (95% CI) | Prev. (95% CI) | |
| Men | 14 (9, 22) | 71 (60, 79) | 69 (60, 78) | 61 (48, 72) |
| WRA | 14 (10, 17) | 66 (59, 72) | 65 (58, 71) | 63 (56, 70) |
| SAC | 34 (29, 39) | 56 (48, 63) | 51 (44, 59) | 45 (38, 52) |
| PSC | 57 (51, 62) | 61 (55, 67) | 58 (52, 64) | 52 (46, 58) |
| Total | 52 (47, 57) | 62 (56, 67) | 59 (53, 64) | 52 (46, 58) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Likoswe, B.H.; Phiri, F.P.; Broadley, M.R.; Joy, E.J.M.; Patson, N.; Maleta, K.M.; Phuka, J.C. Inflammation Adjustment by Two Methods Decreases the Estimated Prevalence of Zinc Deficiency in Malawi. Nutrients 2020, 12, 1563. https://doi.org/10.3390/nu12061563
Likoswe BH, Phiri FP, Broadley MR, Joy EJM, Patson N, Maleta KM, Phuka JC. Inflammation Adjustment by Two Methods Decreases the Estimated Prevalence of Zinc Deficiency in Malawi. Nutrients. 2020; 12(6):1563. https://doi.org/10.3390/nu12061563
Chicago/Turabian StyleLikoswe, Blessings H., Felix P. Phiri, Martin R. Broadley, Edward J. M. Joy, Noel Patson, Kenneth M. Maleta, and John C. Phuka. 2020. "Inflammation Adjustment by Two Methods Decreases the Estimated Prevalence of Zinc Deficiency in Malawi" Nutrients 12, no. 6: 1563. https://doi.org/10.3390/nu12061563
APA StyleLikoswe, B. H., Phiri, F. P., Broadley, M. R., Joy, E. J. M., Patson, N., Maleta, K. M., & Phuka, J. C. (2020). Inflammation Adjustment by Two Methods Decreases the Estimated Prevalence of Zinc Deficiency in Malawi. Nutrients, 12(6), 1563. https://doi.org/10.3390/nu12061563





