Breast Cancer Prevention-Is there a Future for Sulforaphane and Its Analogs?
Abstract
1. Introduction
2. Epidemiology and Characteristics of Breast Cancer
3. Breast Cancer Prevention
3.1. Chemoprevention
3.1.1. Registered Drugs and Clinical Trials in Breast Cancer Prevention
3.1.2. Natural Compounds in Breast Cancer Prevention: Preclinical Studies
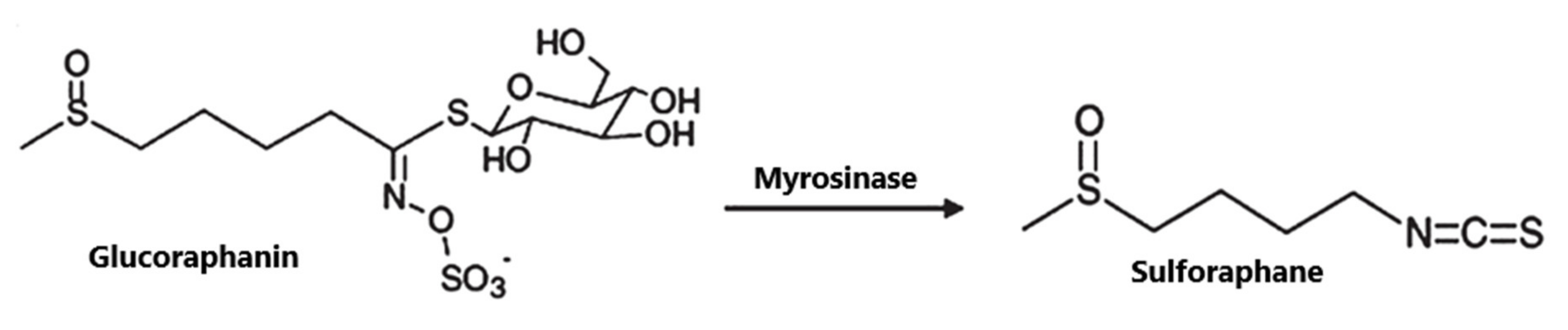
4. SFN: Structure, Occurrence and Metabolism
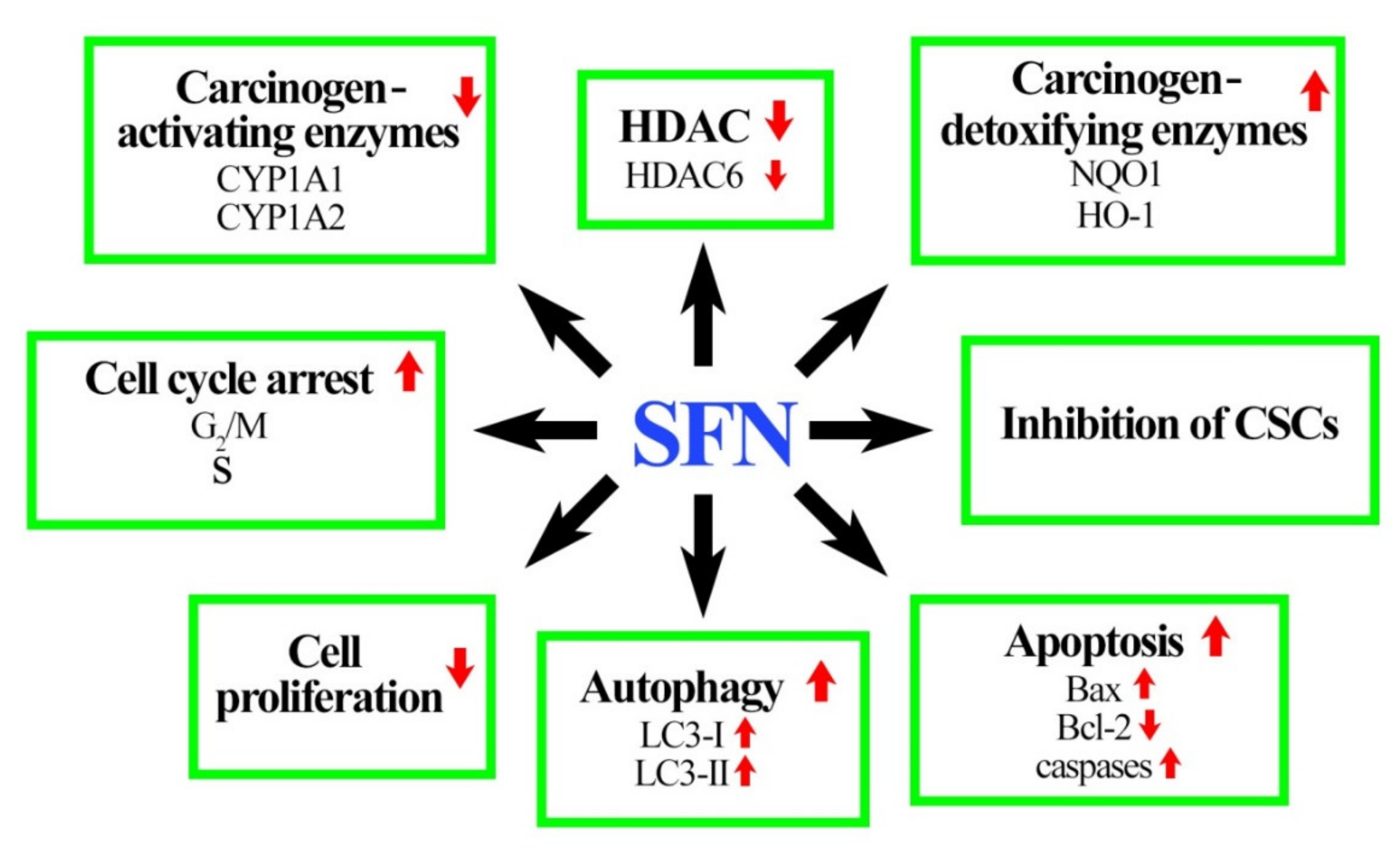
5. Role of SFN in Breast Cancer Prevention
5.1. In Vitro Studies Demonstate the Efficacy of SFN
5.1.1. Phase I of Xenobiotic Metabolism
5.1.2. Phase II and Phase III of Xenobiotic Metabolism
5.1.3. SFN Inhibits Growth of Breast Cancer Cells
5.1.4. SFN Induces Cancer Cell Cycle Arrest and Death
5.1.5. SFN Inhibits Breast CSC
5.2. In Vivo Studies Demonstate the Efficacy of SFN
6. SFN Analogs
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Adami, H.-O.; Signorello, L.B.; Trichopoulos, D. Towards an understanding of breast cancer etiology. Semin. Cancer Biol. 1998, 8, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Michels, K.B.; Mohllajee, A.P.; Roset-Bahmanyar, E.; Beehler, G.P.; Moysich, K.B. Diet and breast cancer: A review of the prospective observational studies. Cancer 2007, 109, 2712–2749. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.F.; Bhat, S.H.; Husain, E.; Abu-Duhier, F.; Hadi, S.M.; Sarkar, F.H.; Ahmad, A. Cancer chemopreventive pharmacology of phytochemicals derived from plants of dietary and non-dietary origin: Implication for alternative and complementary approaches. Phytochem. Rev. 2014, 13, 811–833. [Google Scholar] [CrossRef]
- Block, G.; Subar, A.; Patterson, B. Fruit, Vegetables, and Cancer Prevention: A Review of the Epidemiological Evidence. Nutr. Cancer 1992, 18, 1–29. [Google Scholar] [CrossRef]
- Abuajah, C.I.; Ogbonna, A.C.; Osuji, C.M. Functional components and medicinal properties of food: A review. J. Food Sci. Technol. 2015, 52, 2522–2529. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Higdon, J.; Delage, B.; Williams, D.; Dashwood, R. Cruciferous vegetables and human cancer risk: Epidemiologic evidence and mechanistic basis. Pharmacol. Res. 2007, 55, 224–236. [Google Scholar] [CrossRef]
- Thomson, C.A.; Newton, T.R.; Graver, E.J.; Jackson, K.A.; Reid, P.M.; Hartz, V.L.; Cussler, E.C.; Hakim, I.A. Cruciferous Vegetable Intake Questionnaire Improves Cruciferous Vegetable Intake Estimates. J. Am. Diet. Assoc. 2007, 107, 631–643. [Google Scholar] [CrossRef]
- Ambrosone, C.B.; McCann, S.E.; Freudenheim, J.L.; Marshall, J.R.; Zhang, Y.; Shields, P.G. Breast Cancer Risk in Premenopausal Women Is Inversely Associated with Consumption of Broccoli, a Source of Isothiocyanates, but Is Not Modified by GST Genotype. J. Nutr. 2004, 134, 1134–1138. [Google Scholar] [CrossRef]
- Fowke, J.H.; Chung, F.L.; Jin, F.; Qi, D.; Cai, Q.; Conaway, C.; Cheng, J.R.; Shu, X.O.; Gao, Y.T.; Zheng, W. Urinary isothiocyanate levels, Brassica, and human breast cancer. Cancer Res. 2003, 63, 3980–3986. [Google Scholar]
- Tomczyk, J.; Olejnik, A. Sulforafan—Potencjalny czynnik w prewencji i terapii chorób nowotworowych. Postepy Hig. Med. Dosw. 2010, 64, 590–603. [Google Scholar]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization: Cancer. Available online: http://www.who.int/mediacentre/factsheets/fs297/en/index.html (accessed on 26 March 2020).
- Kopnin, B.P. Targets of Oncogenes and Tumor Suppressors: Key for Understanding Basic Mechanisms of Carcinogenesis. Biochemistry 2000, 65, 2–27. [Google Scholar] [PubMed]
- Misiewicz, I.; Kozar, A.; Skupinska, K.; Kowalska, E.; Lubinski, J.; Kasprzycka-Guttman, T. Inhibition of cell cycle and induction of apoptosis by sulforaphane in cell lines carrying various inherited BRCA1 mutations. Oncol. Rep. 2005, 13, 659–665. [Google Scholar] [CrossRef]
- Thangaraju, M.; Kaufmann, S.H.; Couch, F.J. BRCA1 facilitates stress-induced apoptosis in breast and ovarian cancer cell lines. J. Biol. Chem. 2000, 275, 33487–33496. [Google Scholar] [CrossRef]
- Ziegler, R.G.; Hoover, R.N.; Pike, M.C.; Hildesheim, A.; Nomura, A.M.Y.; West, D.W.; Wu-williams, A.H.; Kolonel, L.N.; Horn-ross, P.L.; Rosenthal, J.F.; et al. Migration patterns and breast cancer risk in Asian-American women. J. Natl. Cancer Inst. 1993, 85, 1819–1827. [Google Scholar] [CrossRef]
- Shao, W.; Brown, M. Advances in estrogen receptor biology: Prospects for improvements in targeted breast cancer therapy. Breast Cancer Res. 2004, 6, 39–52. [Google Scholar] [CrossRef]
- Ahmed, S.; Azad, K.A. Study of Receptor Status in Carcinoma Breast Patient. Chattagram Maa-O-Shishu Hosp. Med. Coll. J. 2017, 16, 48–50. [Google Scholar] [CrossRef]
- Parise, C.A.; Caggiano, V. Breast Cancer Survival Defined by the ER/PR/HER2 Subtypes and a Surrogate Classification according to Tumor Grade and Immunohistochemical Biomarkers. J. Cancer Epidemiol. 2014, 2014, 1–11. [Google Scholar] [CrossRef]
- Effi, A.B.; Aman, N.A.; Koui, B.S.; Koffi, K.D.; Traoré, Z.C.; Kouyate, M. Immunohistochemical determination of estrogen and progesterone receptors in breast cancer: Relationship with clinicopathologic factors in 302 patients in Ivory Coast. BMC Cancer 2017, 17, 115. [Google Scholar] [CrossRef]
- Hudis, C.A.; Gianni, L. Triple-Negative Breast Cancer: An Unmet Medical Need. Oncologist 2011, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Podo, F.; Buydens, L.M.C.; Degani, H.; Hilhorst, R.; Klipp, E.; Gribbestad, I.S.; Van Huffel, S.; van Laarhoven, W.H.M.; Luts, J.; Monleon, D.; et al. Triple-negative breast cancer: Present challenges and new perspectives. Mol. Oncol. 2010, 4, 209–229. [Google Scholar] [CrossRef] [PubMed]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Dontu, G.; Wicha, M.S. Mammary stem cells, self-renewal pathways, and carcinogenesis. Breast Cancer Res. 2005, 7, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.B.; Onder, T.T.; Jiang, G.; Tao, K.; Kuperwasser, C.; Weinberg, R.A.; Lander, E.S. Identification of Selective Inhibitors of Cancer Stem Cells by High-Throughput Screening. Cell 2009, 138, 645–659. [Google Scholar] [CrossRef]
- Saeg, F.; Anbalagan, M. Breast cancer stem cells and the challenges of eradication: A review of novel therapies. Stem Cell Investig. 2018, 5, 39. [Google Scholar] [CrossRef]
- Stasiołek, D.; Kwaśniewska, M.; Drygas, W. Rak sutka-wybrane czynniki ryzyka, prewencja pierwotna. Przegl. Lek. 2002, 59, 26–30. [Google Scholar]
- Zolfaroli, I.; Tarín, J.J.; Cano, A. The action of estrogens and progestogens in the young female breast. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 230, 204–207. [Google Scholar] [CrossRef]
- Struciński, P.; Ludwicki, J.K.; Góralczyk, K.; Czaja, K. Wybrane aspekty działania ksenoestrogenów z grupy persystentnych związków chloroorganicznych. Rocz. Państwowego Zakładu Hig. 2000, 51, 211–228. [Google Scholar]
- Maciążek-Jurczyk, M.; Maliszewska, M.; Szkudlarek-Haśnik, A. Chemoprewencja w raku piersi. Farm. Przegląd Nauk. 2010, 10, 34–40. [Google Scholar]
- International Agency for Research on Cancer. IARC Handbooks Breast Cancer Screening, Volume 15; International Agency for Research on Cancer: Lyon, France, 2016; pp. 281–469. [Google Scholar]
- Baxter, N.; Canadian Task Force on Preventive Health Care. Preventive health care, 2001 update: Should women be routinely taught breast self-examination to screen for breast cancer? Can. Med. Assoc. J. 2001, 164, 1837–1846. [Google Scholar]
- Geisel, J.; Raghu, M.; Hooley, R. The Role of Ultrasound in Breast Cancer Screening: The Case for and Against Ultrasound. Semin. Ultrasound CT MRI 2018, 39, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Rocco, N.; Catanuto, G.; Nava, M.B. What is the evidence behind conservative mastectomies? Gland Surg. 2015, 4, 506–518. [Google Scholar] [CrossRef] [PubMed]
- van Verschuer, V.M.T.; Maijers, M.C.; van Deurzen, C.H.M.; Koppert, L.B. Oncological safety of prophylactic breast surgery: Skin-sparing and nipple-sparing versus total mastectomy. Gland Surg. 2015, 4, 467–475. [Google Scholar] [CrossRef]
- Fahey, J.W. Dietary Phytochemical Delivery: Glucosinolates/Isothiocyanates. Nutr. Today 2002, 37, 214–217. [Google Scholar] [CrossRef]
- Wattenberg, L.W. Chemoprophylaxis of Carcinogenesis: A Review. Cancer Res. 1966, 26, 1520–1526. [Google Scholar]
- Sporn, M.; Dunlop, N.; Newton, D.; Smith, J. Prevention of chemical carcinogenesis by vitamin A and its synthetic analogs (retinoids). Fed. Proc. 1976, 35, 1332–1338. [Google Scholar]
- Bonovas, S.; Tsantes, A.; Drosos, T.; Sitaras, N.M. Cancer chemoprevention: A summary of the current evidence. Anticancer Res. 2008, 28, 1857–1866. [Google Scholar]
- Wattenberg, L.W. Chemoprevention of cancer. Cancer Res. 1985, 45, 1–8. [Google Scholar] [CrossRef]
- Joseph, M.A.; Moysich, K.B.; Freudenheim, J.L.; Shields, P.G.; Bowman, E.D.; Zhang, Y.; Marshall, J.M.; Ambrosone, C.B. Cruciferous vegetables, genetic polymorphisms in glutathione S-transferases M1 and T1, and prostate cancer risk. Nutr. Cancer 2004, 50, 206–213. [Google Scholar] [CrossRef]
- Cummings, S.R.; Eckert, S.; Krueger, K.A.; Grady, D.; Powles, T.J.; Cauley, J.A.; Norton, L.; Nickelsen, T.; Bjarnason, N.H.; Morrow, M.; et al. The Effect of Raloxifene on Risk of Breast Cancer in Postmenopausal Women. J. Am. Med. Assoc. 1999, 281, 2189–2197. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Available online: https://www.ema.europa.eu (accessed on 26 March 2020).
- Pinsky, P.F.; Miller, E.; Heckman-stoddard, B.; Minasian, L. Use of Raloxifene and Tamoxifen by Breast Cancer Risk Level in a Medicare-1 eligible Cohort. Am. J. Obstet. Gynecol. 2018, 218, 606.e1–606.e9. [Google Scholar] [CrossRef]
- Deyati, A.; Sanam, R.D.; Guggilla, S.R.; Pidugu, V.R.; Novac, N. Molecular biomarkers in clinical development: What could we learn from the clinical trial registry? Per. Med. 2014, 11, 381–393. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov: Anastrozole in Preventing Breast Cancer in Postmenopausal Women at Increased Risk of Breast Cancer (IBIS II). Available online: https://clinicaltrials.gov/ct2/show/NCT00078832 (accessed on 26 March 2020).
- ClinicalTrials.gov: Alternative Dosing of Exemestane Before Surgery in Treating Postmenopausal Patients with Stage 0-II Estrogen Positive Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT02598557?term=chemoprevention&recrs=abdf&cond=Breast+Cancer&draw=2 (accessed on 26 March 2020).
- LaCroix, A.Z.; Powles, T.; Osborne, C.K.; Wolter, K.; Thompson, J.R.; Thompson, D.D.; Allred, D.C.; Armstrong, R.; Cummings, S.R.; Eastell, R.; et al. Breast cancer incidence in the randomized PEARL trial of lasofoxifene in postmenopausal osteoporotic women. J. Natl. Cancer Inst. 2010, 102, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Smith, J.A.; Axelrod, D.M.; Ameri, P.; Levitt, H.; Danoff, A.; Lesser, M.; de Angelis, C.; Illa-Bochaca, I.; Lubitz, S.; et al. Insulin-like growth factor-I inhibition with pasireotide decreases cell proliferation and increases apoptosis in pre-malignant lesions of the breast: A phase 1 proof of principle trial. Breast Cancer Res. 2014, 16, 463. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.J.; Diem, S.J.; Fabian, C.J.; Neven, P.; Wickerham, D.L.; Cox, D.A.; Muram, D.; Agnusdei, D.; Dowsett, S.A.; Amewou-Atisso, M.; et al. Breast cancer incidence in postmenopausal women with osteoporosis or low bone mass using arzoxifene. Breast Cancer Res. Treat. 2012, 134, 299–306. [Google Scholar] [CrossRef]
- Vinayak, S.; Schwartz, E.J.; Jensen, K.; Lipson, J.; Alli, E.; McPherson, L.; Fernandez, A.M.; Sharma, V.B.; Staton, A.; Mills, M.A.; et al. A clinical trial of lovastatin for modification of biomarkers associated with breast cancer risk. Breast Cancer Res. Treat. 2013, 142, 389–398. [Google Scholar] [CrossRef][Green Version]
- ClinicalTrials.gov: Pilot Study of Bisphosphonates for Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT02781805?term=chemoprevention&recrs=abdf&cond=Breast+Cancer&draw=2&rank= (accessed on 26 March 2020).
- ClinicalTrials.gov: Study of Breast Cancer Prevention by Letrozole in High Risk Women. Available online: https://clinicaltrials.gov/ct2/show/NCT00579826 (accessed on 26 March 2020).
- ClinicalTrials.gov: Deslorelin Combined With Low-Dose Add-Back Estradiol and Testosterone in Preventing Breast Cancer in Premenopausal Women Who Are at High Risk for This Disease. Available online: https://clinicaltrials.gov/ct2/show/NCT00080756?term=chemoprevention&recrs=abdf&cond=Breast+Cancer&draw=2&rank=7 (accessed on 26 March 2020).
- ClinicalTrials.gov: NSAID Effects on Clinical and Imaging Breast Biomarkers. Available online: https://clinicaltrials.gov/ct2/show/NCT01761877?term=chemoprevention&recrs=abdf&cond=Breast+Cancer&draw=2&rank=10 (accessed on 26 March 2020).
- ClinicalTrials.gov: Metformin Hydrochloride in Preventing Breast Cancer in Patients with Atypical Hyperplasia or In Situ Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT01905046?term=chemoprevention&recrs=abdf&cond=Breast+Cancer&draw=2&rank=6 (accessed on 26 March 2020).
- Cuzick, J.; Sestak, I.; Forbes, J.F.; Dowsett, M.; Knox, J.; Cawthorn, S.; Saunders, C.; Roche, N.; Mansel, R.E.; Von Minckwitz, G.; et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): An international, double-blind, randomised placebo-controlled trial. Lancet 2014, 383, 1041–1048. [Google Scholar] [CrossRef]
- Cuzick, J.; Sestak, I.; Forbes, J.F.; Dowsett, M.; Cawthorn, S.; Mansel, R.E.; Loibl, S.; Bonanni, B.; Evans, D.G.; Howell, A. Use of anastrozole for breast cancer prevention (IBIS-II): Long-term results of a randomised controlled trial. Lancet 2020, 395, 117–122. [Google Scholar] [CrossRef]
- ClinicalTrials.gov: Chemoprevention Trial—Anastrozole in Ductal Carcinoma In Situ (DCIS) in Postmenopausal Women. Available online: https://clinicaltrials.gov/ct2/show/NCT00256217?term=chemoprevention%2C+Anastrozole&cond=Breast+Cancer&draw=2&rank=2 (accessed on 26 March 2020).
- Mocellin, S.; Pilati, P.; Briarava, M.; Nitti, D. Breast Cancer Chemoprevention: A Network Meta-Analysis of Randomized Controlled Trials. J. Natl. Cancer Inst. 2016, 108. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Available online: https://www.fda.gov/ (accessed on 26 March 2020).
- ClinicalTrials.gov: Breast Cancer Chemoprevention by SOM230, an IGF-I Action Inhibitor. Available online: https://clinicaltrials.gov/ct2/show/NCT01372618 (accessed on 26 March 2020).
- Wu, A.H.; Spicer, D.; Garcia, A.; Tseng, C.-C.; Hovanessian-Larsen, L.; Sheth, P.; Martin, S.E.; Hawes, D.; Russell, C.; MacDonald, H.; et al. Double-blind randomized 12-month soy intervention had no effects on breast MRI fibroglandular tissue density or mammographic density. Cancer Prev. Res. 2015, 8, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Somasundaram, S.; Edmund, N.A.; Moore, D.T.; Small, G.W.; Shi, Y.Y.; Orlowski, R.Z. Dietary curcumin inhibits chemotherapy-induced apoptosis in models of human breast cancer. Cancer Res. 2002, 62, 3868–3875. [Google Scholar] [PubMed]
- Choudhuri, T.; Pal, S.; Agwarwal, M.L.; Das, T.; Sa, G. Curcumin induces apoptosis in human breast cancer cells through p53-dependent Bax induction. FEBS Lett. 2002, 512, 334–340. [Google Scholar] [CrossRef]
- Li, Y.; Upadhyay, S.; Bhuiyan, M.; Sarkar, F.H. Induction of apoptosis in breast cancer cells MDA-MB-231 by genistein. Oncogene 1999, 18, 3166–3172. [Google Scholar] [CrossRef] [PubMed]
- Pozo-Guisado, E.; Merino, J.M.; Mulero-Navarro, S.; Lorenzo-Benayas, M.J.; Centeno, F.; Alvarez-Barrientos, A.; Fernandez Salguero, P.M. Resveratrol-induced apoptosis in MCF-7 human breast cancer cells involves a caspase-independent mechanism with downregulation of Bcl-2 and NF-κB. Int. J. Cancer 2005, 115, 74–84. [Google Scholar] [CrossRef]
- Baker, K.M.; Bauer, A.C. Green Tea Catechin, EGCG, Suppresses PCB 102-Induced Proliferation in Estrogen-Sensitive Breast Cancer Cells. Int. J. Breast Cancer 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Zong, H.; Wang, F.; Fan, Q.X.; Wang, L.X. Curcumin inhibits metastatic progression of breast cancer cell through suppression of urokinase-type plasminogen activator by NF-kappa B signaling pathways. Mol. Biol. Rep. 2012, 39, 4803–4808. [Google Scholar] [CrossRef]
- Lee, H.P.; Lee, J.; Gourley, L.; Duffy, S.W.; Day, N.E.; Estève, J. Dietary effects on breast-cancer risk in Singapore. Lancet 1991, 337, 1197–1200. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Liu, X.; Xing, K.; Wang, Y.; Li, F.; Yao, L. Resveratrol-induced cell inhibition of growth and apoptosis in MCF7 human breast cancer cells are associated with modulation of phosphorylated Akt and caspase-9. Appl. Biochem. Biotechnol. 2006, 135, 181–192. [Google Scholar] [CrossRef]
- Von Schmid, H.; Karrer, P. Synthese der racemischen und der optisch aktiven Formen des Sulforaphans. Helv. Chim. Acta 1948, 31, 1497–1505. [Google Scholar] [CrossRef]
- Mithen, R.; Faulkner, K.; Magrath, R.; Rose, P.; Williamson, G.; Marquez, J. Development of isothiocyanate-enriched broccoli, and its enhanced ability to induce phase 2 detoxification enzymes in mammalian cells. Theor. Appl. Genet. 2003, 106, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Procházka, Ž. Isolation of sulforaphane from hoary cress (Lepidium draba L.). Collect. Czechoslov. Chem. Commun. 1959, 24, 2429–2430. [Google Scholar] [CrossRef]
- Zhang, Y.; Talalay, P.; Cho, C.G.; Posner, G.H. A major inducer of anticarcinogenic protective enzymes from broccoli: Isolation and elucidation of structure. Proc. Natl. Acad. Sci. USA 1992, 89, 2399–2403. [Google Scholar] [CrossRef] [PubMed]
- Kushad, M.M.; Brown, A.F.; Kurilich, A.C.; Juvik, J.A.; Klein, B.P.; Wallig, M.A.; Jeffery, E.H. Variation of glucosinolates in vegetable crops of Brassica oleracea. J. Agric. Food Chem. 1999, 47, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, M.; Klöpping-Ketelaars, I.W.A.A.; Van Den Berg, R.; Vaes, W.H.J. Bioavailability and kinetics of sulforaphane in humans after consumption of cooked versus raw broccoli. J. Agric. Food Chem. 2008, 56, 10505–10509. [Google Scholar] [CrossRef] [PubMed]
- Chung, F.-L.; Jiao, D.; Getahun, S.M.; Yu, M.C. A urinary biomarker for uptake of dietary isothiocyanates in humans. Cancer Epidemiol. Biomark. Prev. 1998, 7, 103–108. [Google Scholar]
- Mennicke, W.H.; Kral, T.; Krumbiegel, G.; Rittmann, N. Determination of N-acetyl-S-(N-alkylthiocarbamoyl)-l-cysteine, a principal metabolite of alkyl isothiocyanates, in rat urine. J. Chromatogr. B Biomed. Sci. Appl. 1987, 414, 19–24. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, Q.H.; Xu, K. Are isothiocyanates potential anti-cancer drugs? Acta Pharmacol. Sin. 2009, 30, 501–512. [Google Scholar] [CrossRef]
- Ye, L.; Dinkova-Kostova, A.T.; Wade, K.L.; Zhang, Y.; Shapiro, T.A.; Talalay, P. Quantitative determination of dithiocarbamates in human plasma, serum, erythrocytes and urine: Pharmacokinetics of broccoli sprout isothiocyanates in humans. Clin. Chim. Acta 2002, 316, 43–53. [Google Scholar] [CrossRef]
- Cristiano, M.C.; Froiio, F.; Spaccapelo, R.; Mancuso, A.; Nisticò, S.P.; Udongo, B.P.; Fresta, M.; Paolino, D. Sulforaphane-Loaded Ultradeformable Vesicles as A Potential Natural Nanomedicine for the Treatment of Skin Cancer Diseases. Pharmaceutics 2019, 12, 6. [Google Scholar] [CrossRef]
- Soni, K.; Rizwanullah, M.; Kohli, K. Development and optimization of sulforaphane-loaded nanostructured lipid carriers by the Box-Behnken design for improved oral efficacy against cancer: In vitro, ex vivo and in vivo assessments. Artif. Cells Nanomed. Biotechnol. 2018, 46, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Kheiri Manjili, H.; Sharafi, A.; Attari, E.; Danafar, H. Pharmacokinetics and in vitro and in vivo delivery of sulforaphane by PCL–PEG–PCL copolymeric-based micelles. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1728–1739. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.L.; Kong, A.N. Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention. AAPS J. 2010, 12, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Smith, T.J.; Hong, J.Y. Cytochrome P-450 enzymes as targets for chemoprevention against chemical carcinogenesis and toxicity: Opportunities and limitations. Cancer Res. 1994, 54, 1982s–1986s. [Google Scholar] [PubMed]
- Shimada, T.; Inoue, K.; Suzuki, Y.; Kawai, T.; Azuma, E.; Nakajima, T.; Shindo, M.; Kurose, K.; Sugie, A.; Yamagishi, Y.; et al. Arylhydrocarbon receptor-dependent induction of liver and lung cytochromes P450 1A1, 1A2, and 1B1 by polycyclic aromatic hydrocarbons and polychlorinated biphenyls in genetically engineered C57BL/6J mice. Carcinogenesis 2002, 23, 1199–1207. [Google Scholar] [CrossRef]
- Skupinska, K.; Misiewicz-Krzeminska, I.; Lubelska, K.; Kasprzycka-Guttman, T. The effect of isothiocyanates on CYP1A1 and CYP1A2 activities induced by polycyclic aromatic hydrocarbons in Mcf7 cells. Toxicol. Vitr. 2009, 23, 763–771. [Google Scholar] [CrossRef]
- Licznerska, B.; Szaefer, H.; Matuszak, I.; Murias, M.; Baer-Dubowska, W. Modulating Potential of L -Sulforaphane in the Expression of Cytochrome P450 to Identify Potential Targets for Breast Cancer Chemoprevention and Therapy Using Breast Cell Lines. Phyther. Res. 2014, 29, 93–99. [Google Scholar] [CrossRef]
- Rushmore, T.H.; Tony Kong, A. Pharmacogenomics, Regulation and Signaling Pathways of Phase I and II Drug Metabolizing Enzymes. Curr. Drug Metab. 2002, 3, 481–490. [Google Scholar] [CrossRef]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Holtzclaw, W.D.; Cole, R.N.; Itoh, K.; Wakabayashi, N.; Katoh, Y.; Yamamoto, M.; Talalay, P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. USA 2002, 99, 11908–11913. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kang, M.-I.; Watai, Y.; Tong, K.I.; Shibata, T.; Uchida, K.; Yamamoto, M. Oxidative and Electrophilic Stresses Activate Nrf2 through Inhibition of Ubiquitination Activity of Keap1. Mol. Cell. Biol. 2006, 26, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zahid, M.; Liao, Y.; Rogan, E.G.; Cavalieri, E.L.; Davidson, N.E.; Yager, J.D.; Visvanathan, K.; Groopman, J.D.; Kensler, T.W. Reduced formation of depurinating estrogen-DNA adducts by sulforaphane or KEAP1 disruption in human mammary epithelial MCF-10A cells. Carcinogenesis 2013, 34, 2587–2592. [Google Scholar] [CrossRef] [PubMed]
- Agyeman, A.S.; Chaerkady, R.; Shaw, P.G.; Davidson, N.E.; Visvanathan, K.; Pandey, A.; Kensler, T.W. Transcriptomic and proteomic profiling of KEAP1 disrupted and sulforaphane-treated human breast epithelial cells reveals common expression profiles. Breast Cancer Res. Treat. 2012, 132, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.Q.; Chen, C.; Yang, B.; Hebbar, V.; Kong, A.N.T. Differential responses from seven mammalian cell lines to the treatments of detoxifying enzyme inducers. Life Sci. 2003, 72, 2243–2253. [Google Scholar] [CrossRef]
- Lubelska, K.; Wiktorska, K.; Mielczarek, L.; Milczarek, M.; Zbroińska-Bregisz, I.; Chilmonczyk, Z. Sulforaphane Regulates NFE2L2/Nrf2-Dependent Xenobiotic Metabolism Phase II and Phase III Enzymes Differently in Human Colorectal Cancer and Untransformed Epithelial Colon Cells. Nutr. Cancer 2016, 68, 1338–1348. [Google Scholar] [CrossRef]
- Bose, C.; Awasthi, S.; Sharma, R.; Beneš, H.; Hauer-Jensen, M.; Boerma, M.; Singh, S.P. Sulforaphane potentiates anticancer effects of doxorubicin and attenuates its cardiotoxicity in a breast cancer model. PLoS ONE 2018, 13, e0193918. [Google Scholar] [CrossRef]
- Hu, L.; Miao, W.; Loignon, M.; Kandouz, M.; Batist, G. Putative chemopreventive molecules can increase Nrf2-regulated cell defense in some human cancer cell lines, resulting in resistance to common cytotoxic therapies. Cancer Chemother. Pharmacol. 2010, 66, 467–474. [Google Scholar] [CrossRef]
- Zhang, H.S.; Zhang, Z.G.; Du, G.Y.; Sun, H.L.; Liu, H.Y.; Zhou, Z.; Gou, X.M.; Wu, X.H.; Yu, X.Y.; Huang, Y.H. Nrf2 promotes breast cancer cell migration via up-regulation of G6PD/HIF-1α/Notch1 axis. J. Cell. Mol. Med. 2019, 23, 3451–3463. [Google Scholar] [CrossRef]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP–dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef]
- Sibhatu, M.B.; Smitherman, P.K.; Townsend, A.J.; Morrow, C.S. Expression of MRP1 and GSTP1-1 modulate the acute cellular response to treatment with the chemopreventive isothiocyanate, sulforaphane. Carcinogenesis 2008, 29, 807–815. [Google Scholar] [CrossRef][Green Version]
- Zimta, A.A.; Cenariu, D.; Irimie, A.; Magdo, L.; Nabavi, S.M.; Atanasov, A.G.; Berindan-Neagoe, I. The role of Nrf2 activity in cancer development and progression. Cancers 2019, 11, 1755. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Lu, H.; Bai, Y. Nrf2 in cancers: A double-edged sword. Cancer Med. 2019, 8, 2252–2267. [Google Scholar] [CrossRef] [PubMed]
- Rojo de la Vega, M.; Chapman, E.; Zhang, D.D. NRF2 and the Hallmarks of Cancer. Cancer Cell 2018, 34, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.C.; Singletary, K. Regulation of estrogen receptor α expression in human breast cancer cells by sulforaphane. J. Nutr. Biochem. 2009, 20, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Lewinska, A.; Adamczyk-Grochala, J.; Deregowska, A.; Wnuk, M. Sulforaphane-induced cell cycle arrest and senescence are accompanied by DNA hypomethylation and changes in microRNA profile in breast cancer cells. Theranostics 2017, 7, 3461–3477. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, A.; Wiczk, A.; Kaczyńska, A.; Antosiewicz, J.; Herman-Antosiewicz, A. Sulforaphane inhibits growth of phenotypically different breast cancer cells. Eur. J. Nutr. 2013, 52, 1949–1958. [Google Scholar] [CrossRef]
- Hussain, A.; Mohsin, J.; Prabhu, S.A.; Begum, S.; Nusri, Q.E.A.; Harish, G.; Javed, E.; Khan, M.A.; Sharma, C. Sulforaphane inhibits growth of human breast cancer cells and augments the therapeutic index of the chemotherapeutic drug, gemcitabine. Asian Pac. J. Cancer Prev. 2013, 14, 5855–5860. [Google Scholar] [CrossRef]
- Azarenko, O.; Okouneva, T.; Singletary, K.W.; Jordan, M.A.; Wilson, L. Suppression of microtubule dynamic instability and turnover in MCF7 breast cancer cells by sulforaphane. Carcinogenesis 2008, 29, 2360–2368. [Google Scholar] [CrossRef]
- Cierpiał, T.; Łuczak, J.; Kwiatkowska, M.; Kiełbasiński, P.; Mielczarek, L.; Wiktorska, K.; Chilmonczyk, Z.; Milczarek, M.; Karwowska, K. Organofluorine Isoselenocyanate Analogues of Sulforaphane: Synthesis and Anticancer Activity. ChemMedChem 2016, 11, 2398–2409. [Google Scholar] [CrossRef]
- Pledgie-Tracy, A.; Sobolewski, M.D.; Davidson, N.E. Sulforaphane induces cell type-specific apoptosis in human breast cancer cell lines. Mol. Cancer Ther. 2007, 6, 1013–1021. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, T.; Korkaya, H.; Liu, S.; Lee, H.F.; Newman, B.; Yu, Y.; Clouthier, S.G.; Schwartz, S.J.; Wicha, M.S.; et al. Sulforaphane, a Dietary Component of Broccoli/Broccoli Sprouts, Inhibits Breast Cancer Stem Cells. Clin. Cancer Res. 2010, 16, 2580–2590. [Google Scholar] [CrossRef] [PubMed]
- Tseng, E.; Ramsay, E.A.S.; Morris, M.E. Dietary Organic Isothiocyanates are Cytotoxic in Human Breast Cancer MCF-7 and Mammary Epithelial MCF-12A Cell Lines. Exp. Biol. Med. 2004, 229, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Kanematsu, S.; Uehara, N.; Miki, H.; Yoshizawa, K.; Kawanaka, A.; Yuri, T.; Tsubura, A. Autophagy inhibition enhances sulforaphane-induced apoptosis in human breast cancer cells. Anticancer Res. 2010, 30, 3381–3390. [Google Scholar] [PubMed]
- Yang, F.; Wang, F.; Liu, Y.; Wang, S.; Li, X.; Huang, Y.; Xia, Y.; Cao, C. Sulforaphane induces autophagy by inhibition of HDAC6-mediated PTEN activation in triple negative breast cancer cells. Life Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Burnett, J.P.; Lim, G.; Li, Y.; Shah, R.B.; Lim, R.; Paholak, H.J.; McDermott, S.P.; Sun, L.; Tsume, Y.; Bai, S.; et al. Sulforaphane enhances the anticancer activity of taxanes against triple negative breast cancer by killing cancer stem cells. Cancer Lett. 2017, 394, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Kanematsu, S.; Yoshizawa, K.; Uehara, N.; Miki, H.; Sasaki, T.; Kuro, M.; Lai, Y.C.; Kimura, A.; Yuri, T.; Tsubura, A. Sulforaphane inhibits the growth of KPL-1 human breast cancer cells in vitro and suppresses the growth and metastasis of orthotopically transplanted KPL-1 cells in female athymic mice. Oncol. Rep. 2011, 26, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Cierpiał, T.; Kiełbasiński, P.; Kwiatkowska, M.; Łyżwa, P.; Lubelska, K.; Kuran, D.; Dąbrowska, A.; Kruszewska, H.; Mielczarek, L.; Chilmonczyk, Z.; et al. Fluoroaryl analogs of sulforaphane—A group of compounds of anticancer and antimicrobial activity. Bioorg. Chem. 2020, 94. [Google Scholar] [CrossRef]
- Lubecka-Pietruszewska, K.; Kaufman-Szymczyk, A.; Stefanska, B.; Cebula-Obrzut, B.; Smolewski, P.; Fabianowska-Majewska, K. Sulforaphane Alone and in Combination with Clofarabine Epigenetically Regulates the Expression of DNA Methylation-Silenced Tumour Suppressor Genes in Human Breast Cancer Cells. J. Nutrigenet. Nutr. 2015, 8, 91–101. [Google Scholar] [CrossRef]
- Fotakis, G.; Timbrell, J.A. In vitro cytotoxicity assays: Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Śliwka, L.; Wiktorska, K.; Suchocki, P.; Milczarek, M.; Mielczarek, S.; Lubelska, K.; Cierpiał, T.; Łyzwa, P.; Kiełbasiński, P.; Jaromin, A.; et al. The comparison of MTT and CVS assays for the assessment of anticancer agent interactions. PLoS ONE 2016, 11, e0155772. [Google Scholar] [CrossRef]
- Wright Muelas, M.; Ortega, F.; Breitling, R.; Bendtsen, C.; Westerhoff, H.V. Rational cell culture optimization enhances experimental reproducibility in cancer cells. Sci. Rep. 2018, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Royston, K.J.; Udayakumar, N.; Lewis, K.; Tollefsbol, T.O. A novel combination of withaferin A and sulforaphane inhibits epigenetic machinery, cellular viability and induces apoptosis of breast cancer cells. Int. J. Mol. Sci. 2017, 18, 1092. [Google Scholar] [CrossRef] [PubMed]
- Milczarek, M.; Wiktorska, K.; Mielczarek, L.; Koronkiewicz, M.; Dąbrowska, A.; Lubelska, K.; Matosiuk, D.; Chilmonczyk, Z. Autophagic cell death and premature senescence: New mechanism of 5-fluorouracil and sulforaphane synergistic anticancer effect in MDA-MB-231 triple negative breast cancer cell line. Food Chem. Toxicol. 2018, 111, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mielczarek, L.; Krug, P.; Mazur, M.; Milczarek, M.; Chilmonczyk, Z.; Wiktorska, K. In the triple-negative breast cancer MDA-MB-231 cell line, sulforaphane enhances the intracellular accumulation and anticancer action of doxorubicin encapsulated in liposomes. Int. J. Pharm. 2019, 558, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Mielczarek, L.; Chilmonczyk, Z.; Suchocki, P.; Wroczyński, P.; Wiktorska, K. Combinations of isothiocyanates with drugs—A chance or threat to chemoprevention and cancer treatment? Acta Pol. Pharm. Drug Res. 2018, 75, 829–841. [Google Scholar] [CrossRef]
- Ricci, M.S.; Zong, W.-X. Chemotherapeutic Approaches for Targeting Cell Death Pathways. Oncologist 2006, 11, 342–357. [Google Scholar] [CrossRef]
- Adams, J.M.; Cory, S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 2007, 26, 1324–1337. [Google Scholar] [CrossRef]
- Jackson, S.J.T.; Singletary, K.W. Sulforaphane Inhibits Human MCF-7 Mammary Cancer Cell Mitotic Progression and Tubulin Polymerization. J. Nutr. 2004, 134, 2229–2236. [Google Scholar] [CrossRef]
- Jackson, S.J.T.; Singletary, K.W. Sulforaphane: A naturally occurring mammary carcinoma mitotic inhibitor, which disrupts tubulin polymerization. Carcinogenesis 2004, 25, 219–227. [Google Scholar] [CrossRef]
- Pawlik, A.; Słomińska-Wojewódzka, M.; Herman-Antosiewicz, A. Sensitization of estrogen receptor-positive breast cancer cell lines to 4-hydroxytamoxifen by isothiocyanates present in cruciferous plants. Eur. J. Nutr. 2016, 55, 1165–1180. [Google Scholar] [CrossRef]
- Sarkar, R.; Mukherjee, S.; Biswas, J.; Roy, M. Sulphoraphane, a naturally occurring isothiocyanate induces apoptosis in breast cancer cells by targeting heat shock proteins. Biochem. Biophys. Res. Commun. 2012, 427, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Badireenath Konkimalla, V. Hormetic Potential of Sulforaphane (SFN) in Switching Cells’ Fate Towards Survival or Death. Mini-Rev. Med. Chem. 2016, 16, 980–995. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Levine, B.; Cuervo, A.M.; Klionsky, D.J. Autophagy fights disease through cellular self-digestion. Nature 2008, 451, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Glaser, K.B.; Staver, M.J.; Waring, J.F.; Stender, J.; Ulrich, R.G.; Davidsen, S.K. Gene expression profiling of multiple histone deacetylase (HDAC) inhibitors: Defining a common gene set produced by HDAC inhibition in T24 and MDA carcinoma cell lines. Mol. Cancer Ther. 2003, 2, 151–163. [Google Scholar]
- Yang, X.; Ferguson, A.T.; Nass, S.J.; Phillips, D.L.; Butash, K.A.; Wang, S.M.; Herman, J.G.; Davidson, N.E. Transcriptional activation of estrogen receptor α in human breast cancer cells by histone deacetylase inhibition. Cancer Res. 2000, 60, 6890–6894. [Google Scholar]
- Cornblatt, B.S.; Ye, L.; Dinkova-Kostova, A.T.; Erb, M.; Fahey, J.W.; Singh, N.K.; Chen, M.S.A.; Stierer, T.; Garrett-Mayer, E.; Argani, P.; et al. Preclinical and clinical evaluation of sulforaphane for chemoprevention in the breast. Carcinogenesis 2007, 28, 1485–1490. [Google Scholar] [CrossRef]
- Atwell, L.L.; Zhang, Z.; Mori, M.; Farris, P.E.; Vetto, J.T.; Naik, A.M.; Oh, K.Y.; Thuillier, P.; Ho, E.; Shannon, J. Sulforaphane Bioavailability and Chemopreventive Activity in Women Scheduled for Breast Biopsy. Cancer Prev. Res. 2015, 8, 1184–1191. [Google Scholar] [CrossRef]
- Atwell, L.L.; Hsu, A.; Wong, C.P.; Stevens, J.F.; Bella, D.; Yu, T.-W.; Pereira, C.B.; Löhr, C.V.; Christensen, J.M.; Dashwood, R.H.; et al. Absorption and chemopreventive targets of sulforaphane in humans following consumption of broccoli sprouts or a myrosinase-treated broccoli sprout extract. Mol. Nutr. Food Res. 2015, 59, 424–433. [Google Scholar] [CrossRef]
- Yagishita, Y.; Fahey, J.W.; Dinkova-Kostova, A.T.; Kensler, T.W. Broccoli or Sulforaphane: Is It the Source or Dose That Matters? Molecules 2019, 24, 3593. [Google Scholar] [CrossRef] [PubMed]
- Melchini, A.; Costa, C.; Traka, M.; Miceli, N.; Mithen, R.; De Pasquale, R.; Trovato, A. Erucin, a new promising cancer chemopreventive agent from rocket salads, shows anti-proliferative activity on human lung carcinoma A549 cells. Food Chem. Toxicol. 2009, 47, 1430–1436. [Google Scholar] [CrossRef]
- Pawlik, A.; Wała, M.; Hać, A.; Felczykowska, A.; Herman-Antosiewicz, A. Sulforaphene, an isothiocyanate present in radish plants, inhibits proliferation of human breast cancer cells. Phytomedicine 2017, 29, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Prełowska, M.; Kaczyńska, A.; Herman-Antosiewicz, A. 4-(Methylthio)butyl isothiocyanate inhibits the proliferation of breast cancer cells with different receptor status. Pharmacol. Rep. 2017, 69, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Skupinska, K.; Misiewicz-Krzeminska, I.; Stypulkowski, R.; Lubelska, K.; Kasprzycka-Guttman, T. Sulforaphane and its analogues inhibit CYP1A1 and CYP1A2 activity induced by benzopyrene. J. Biochem. Mol. Toxicol. 2009, 23, 18–28. [Google Scholar] [CrossRef] [PubMed]
| Name of Compound | Chemical Structure | References |
|---|---|---|
| Anastrozole | 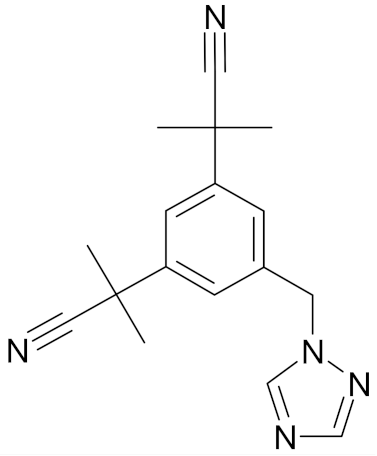 | [47] |
| Exemestane | 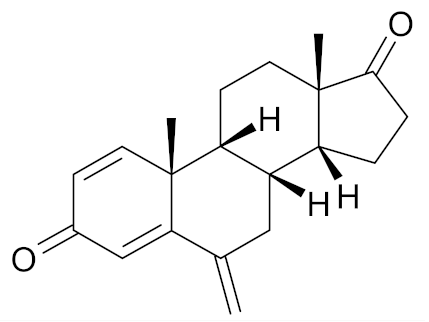 | [48] |
| Lasofoxifene |  | [49] |
| Pasireotide |  | [50] |
| Arzoxifene |  | [51] |
| Lovastatin |  | [52] |
| Alendronate | 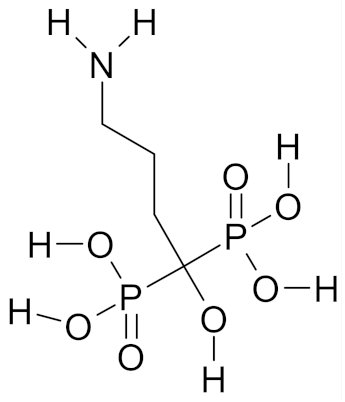 | [53] |
| Letrozole | 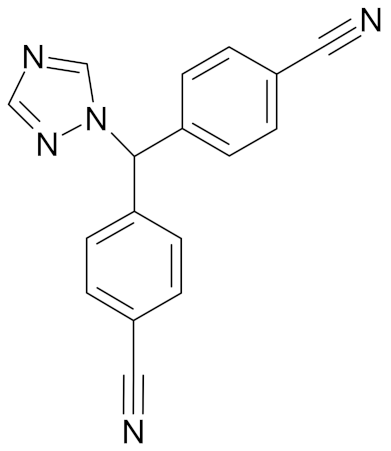 | [54] |
| Deslorelin | 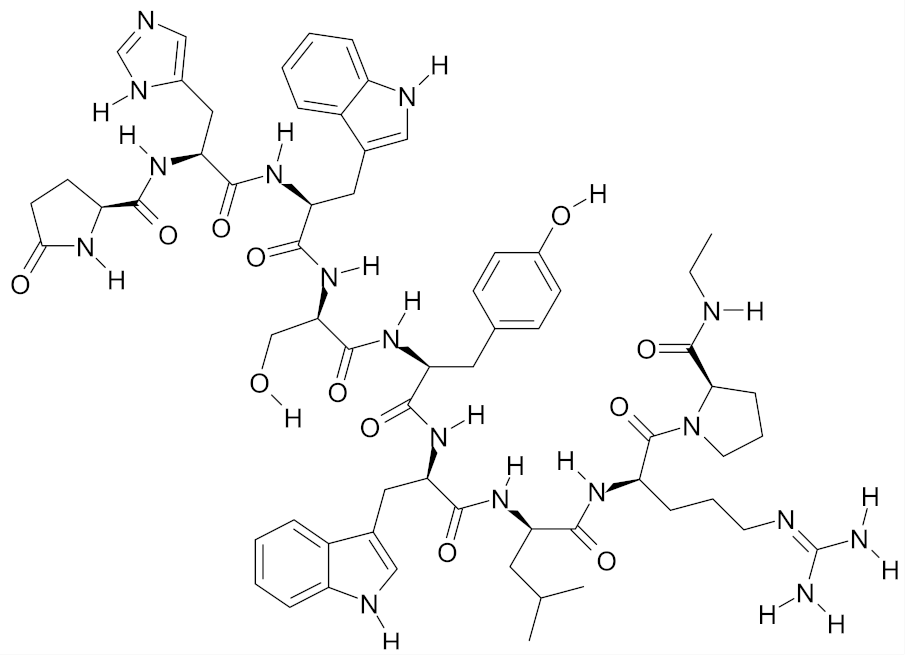 | [55] |
| Sulindac | 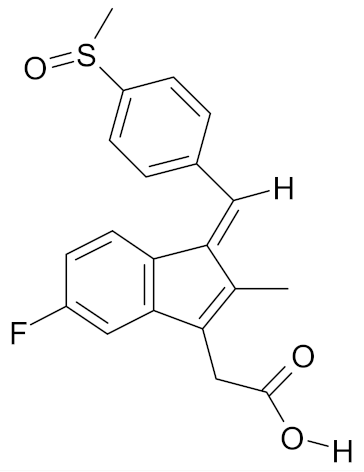 | [56] |
| Metformin hydrochloride | 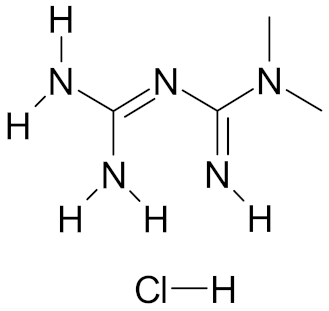 | [57] |
| Name of Compound | Chemical Structure | References |
|---|---|---|
| Curcumin | 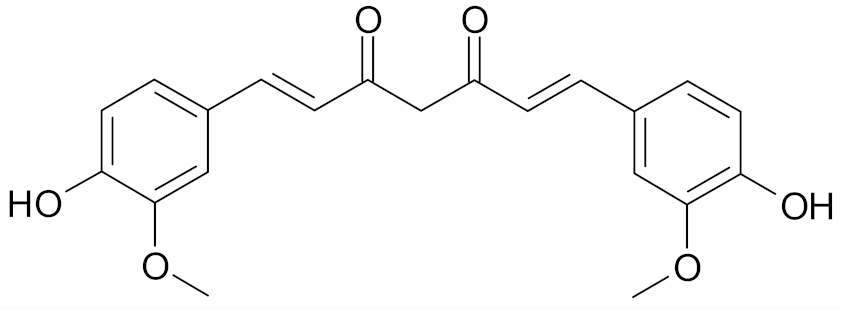 | [66] |
| Genistein | 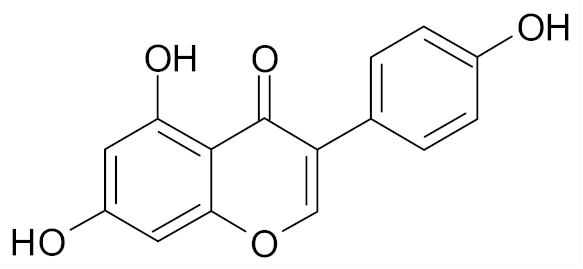 | [67] |
| Resveratrol | 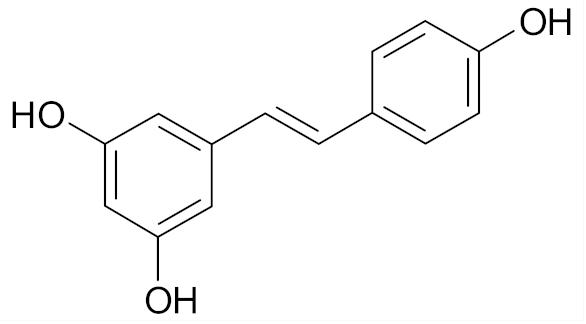 | [68] |
| Epigallocatechin gallate | 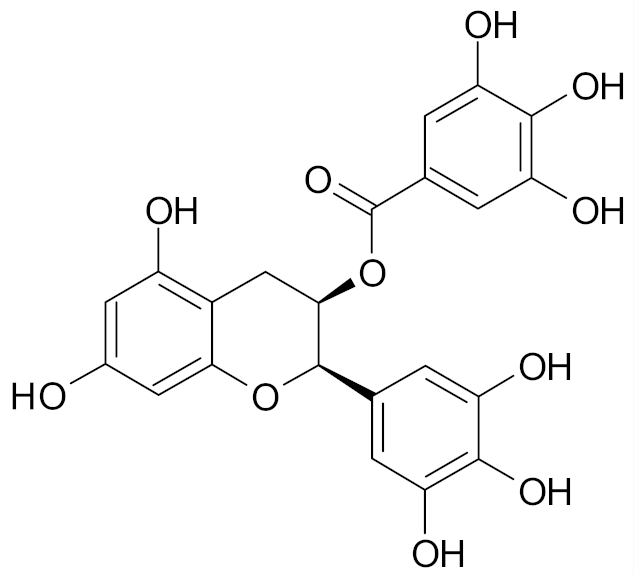 | [69] |
| Receptor Status | Subtype | ||||
|---|---|---|---|---|---|
| Cell Lines | Estrogen Receptor (ER) | Progesterone Receptor (PR) | Human Epithelial Receptor (HER2) | Histologic | Molecular |
| MCF-7 | + | + | - | invasive ductal carcinoma | Luminal A |
| MDA-MB-231 | - | - | - | adenocarcinoma | Triple negative B |
| MDA-MB-468 | - | - | - | adenocarcinoma | Triple negative A |
| BT549 | - | - | - | invasive ductal carcinoma | Triple negative B |
| SUM149 | - | - | - | inflammatory ductal carcinoma | Triple negative B |
| SUM159 | - | - | - | anaplastic carcinoma | Triple negative B |
| BT-20 | - | - | - | invasive ductal carcinoma | Triple negative A |
| SK-BR-3 | - | - | + | adenocarcinoma | HER2 positive |
| T47D | + | + | - | invasive ductal carcinoma | Luminal A |
| ZR-75-1 | + | - | - | invasive ductal carcinoma | Luminal A |
| MCF-12A | - | - | - | non-tumorigenic fibrocystic breast epithelium | basal |
| MCF-10A/F | - | - | - | non-tumorigenic fibrocystic breast epithelium | basal |
| Cell Line | 24 h | 48 h | 72 h |
|---|---|---|---|
| MCF-7 | 12.5 µm [107] 14.05 µm [108] 19 µm [109] 25 µm [110] 26 µm [111] 38.92 µm [112] 54 µm [112] | 7.5 µm [107] 9.2 µm [113] 12.38 µm [112] 13 µm [111] 16 µm [114] 20 µm [110] 20.06 µm [112] 27.9 µm [115] | 6.73 µm [112] 9 µm [111] 10.15 µm [112] 33.8 µm [116] |
| MCF-7/ADR 13.7 µm [115] | |||
| MDA-MB-231 | 19.35 µm [108] 21 µm [109] 21.76 µm [117] 27.40 µm [112] 115.7 µm [112] | 8.3 µm [113] 9.6 µm [112] 16.04 µm [112] | 7.26 µm [112] 11.55 µm [112] 31.5 µm [116] |
| MDA-MB-468 | 20 µm [109] 21.93 µm [117] | 8.1 µm [113] | - |
| SK-BR-3 | 16.64 µm [108] 25 µm [109] | - | - |
| SUM159 | - | 10 µm [114] | 7.8 µm [118] |
| SUM149 | - | - | 7.5 µm [118] |
| BT549 | 20.47 µm [117] | - | - |
| T47D KPL-1 | - - | 9.5 µm [113] 19.1 µm [119] | - 17.8 µm [119] |
| MCF-12A | - | 40.5 µm [115] | - |
| MCF-10A | - | - | 12.4 µm [120] |
| Phase of Cell Cycle Arrest | Time of Incubation with SFN | Cell Line | SFN Concentration |
|---|---|---|---|
| G0/G1 | 24 h | SK-BR-3 | 5 µm [108] |
| 10 µm [108] | |||
| G2/M | 24 h | MCF-7 | 5 µm [108,111] |
| 10 µm [108] | |||
| 15 µm [111,113,131] | |||
| 25 µm [111] | |||
| 24 h | MDA-MB-231 | 5 µm [108] | |
| 10 µm [108] | |||
| 15 µm [113,131] | |||
| 24 h | ZR-75 | 15 µm [131] | |
| 24 h | BT-20 | 15 µm [131] | |
| 72 h | MDA-MB-231 | 30 µm [116] | |
| S | 72 h | MDA-MB-231 | 30 µm [116] |
| Name of Compound | Chemical Structure | Reference |
|---|---|---|
| 1-isothiocyanato-4-methylsulfinylbutane (sulforaphane, SFN) | 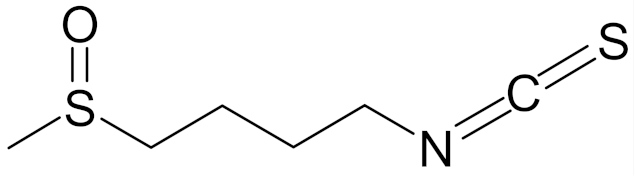 | [89] |
| erucin ((4-methylthio)butyl ITC, ERN) | 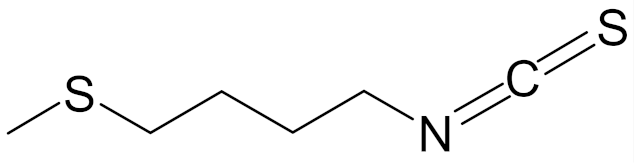 | [143] |
| sulforaphene (4-isothiocyanato-1-methylsulfinylbut-1-ene; SF) | 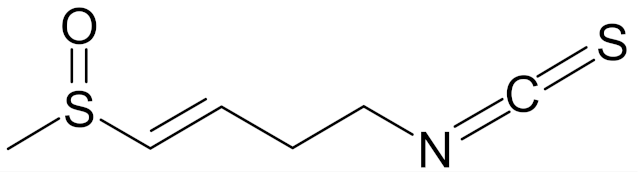 | [144] |
| allysin (isothiocyanate 5-methylsulfinyl-n-amyl) | 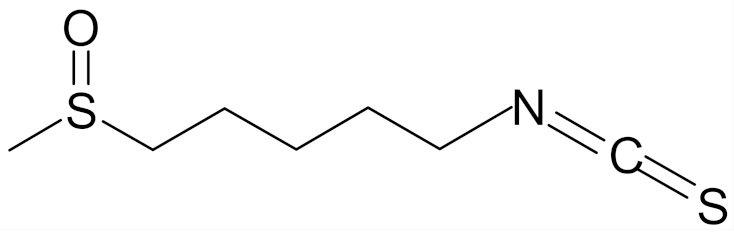 | [89] |
| ITC-2-oxohexyl (isothiocyanate 2-oxoheksyl) | 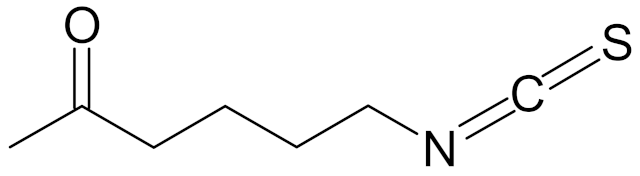 | [89] |
| 4-isoselenocyanato-1-butyl 4′-fluorobenzyl sulfoxide | 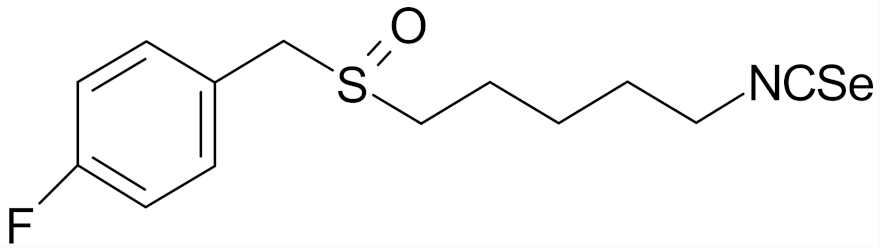 | [112] |
| 4-isoselenocyanato-1-butyl 3′,5′-bis-(trifluoromethyl)phenyl sulfoxide | 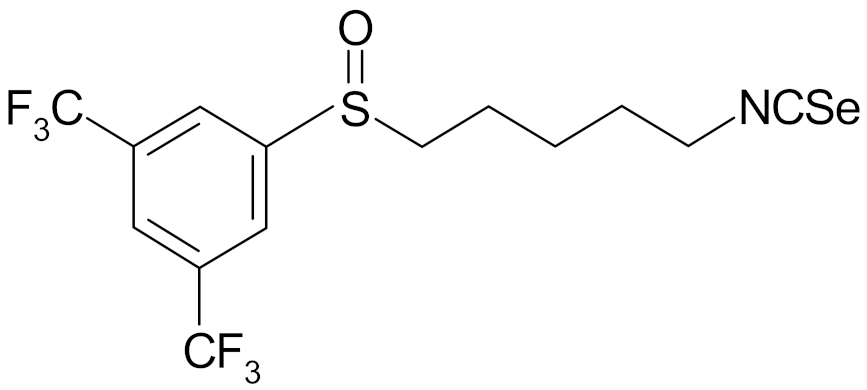 | [112] |
| 4′-(trifluoromethyl)-2′,3′,5′,6′-pentafluorophenyl-4-isothiocyanato-1-butyl sulfoxide | 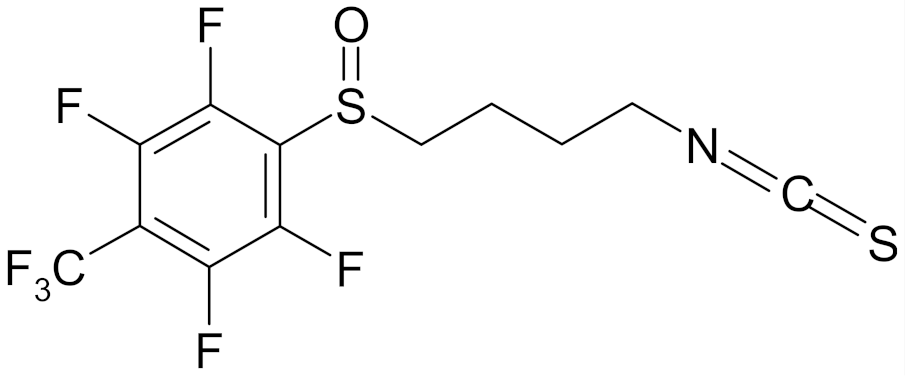 | [113] |
| 4′-(trifluoromethyl)-2′,3′,5′,6′-pentafluorophenyl-5-isothiocyanato-1-pentyl sulfoxide | 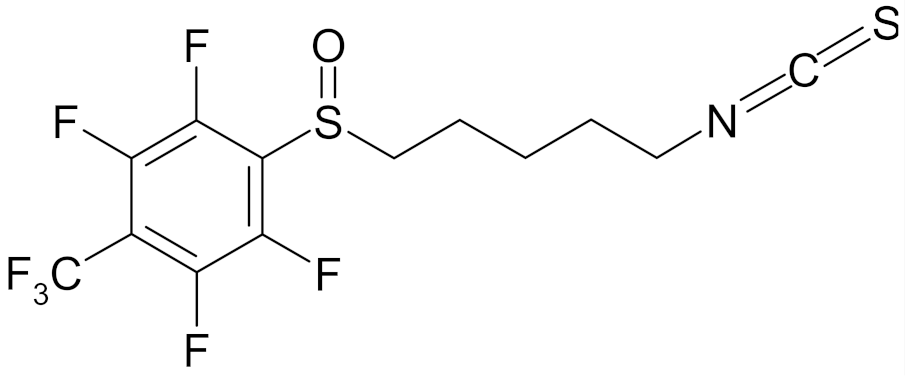 | [113] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuran, D.; Pogorzelska, A.; Wiktorska, K. Breast Cancer Prevention-Is there a Future for Sulforaphane and Its Analogs? Nutrients 2020, 12, 1559. https://doi.org/10.3390/nu12061559
Kuran D, Pogorzelska A, Wiktorska K. Breast Cancer Prevention-Is there a Future for Sulforaphane and Its Analogs? Nutrients. 2020; 12(6):1559. https://doi.org/10.3390/nu12061559
Chicago/Turabian StyleKuran, Dominika, Anna Pogorzelska, and Katarzyna Wiktorska. 2020. "Breast Cancer Prevention-Is there a Future for Sulforaphane and Its Analogs?" Nutrients 12, no. 6: 1559. https://doi.org/10.3390/nu12061559
APA StyleKuran, D., Pogorzelska, A., & Wiktorska, K. (2020). Breast Cancer Prevention-Is there a Future for Sulforaphane and Its Analogs? Nutrients, 12(6), 1559. https://doi.org/10.3390/nu12061559






