Commiphora Extract Mixture Ameliorates Monosodium Iodoacetate-Induced Osteoarthritis
Abstract
:1. Introduction
2. Results
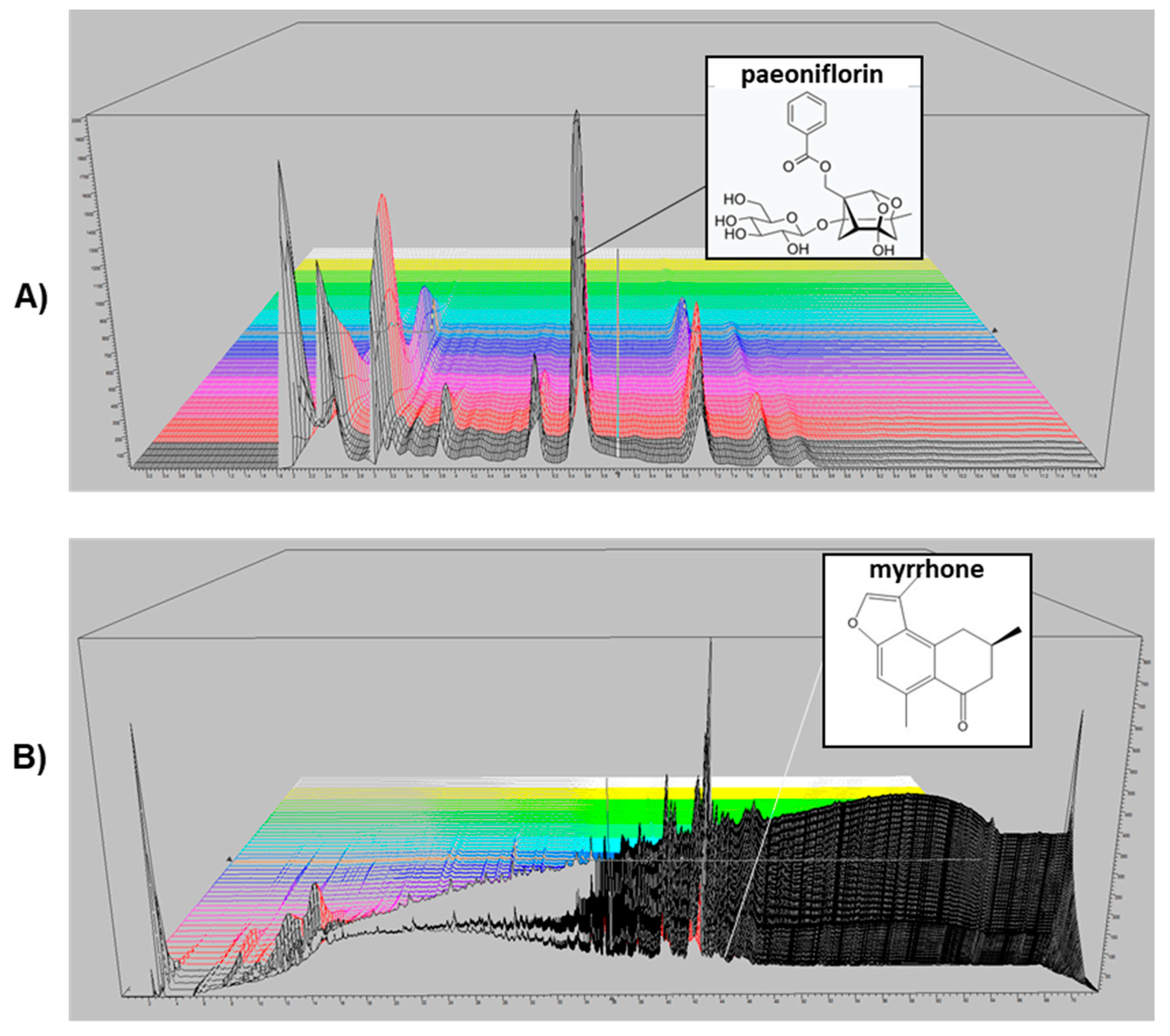
2.1. HPLC Analysis of P. lactiflora and C. myrrha Extracts
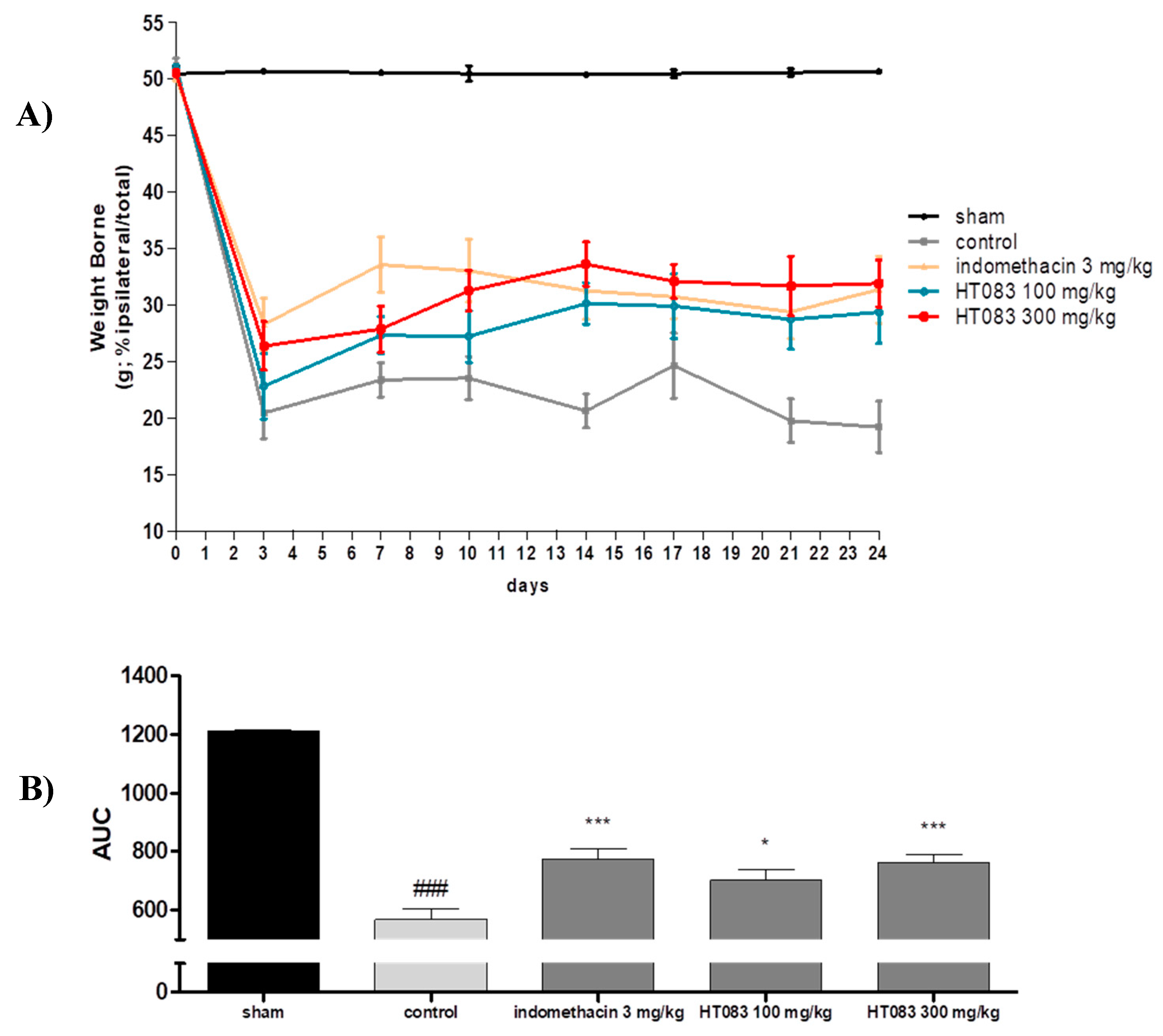
2.2. Weight-Bearing Distribution in Monosodium Iodoacetate (MIA) Rats
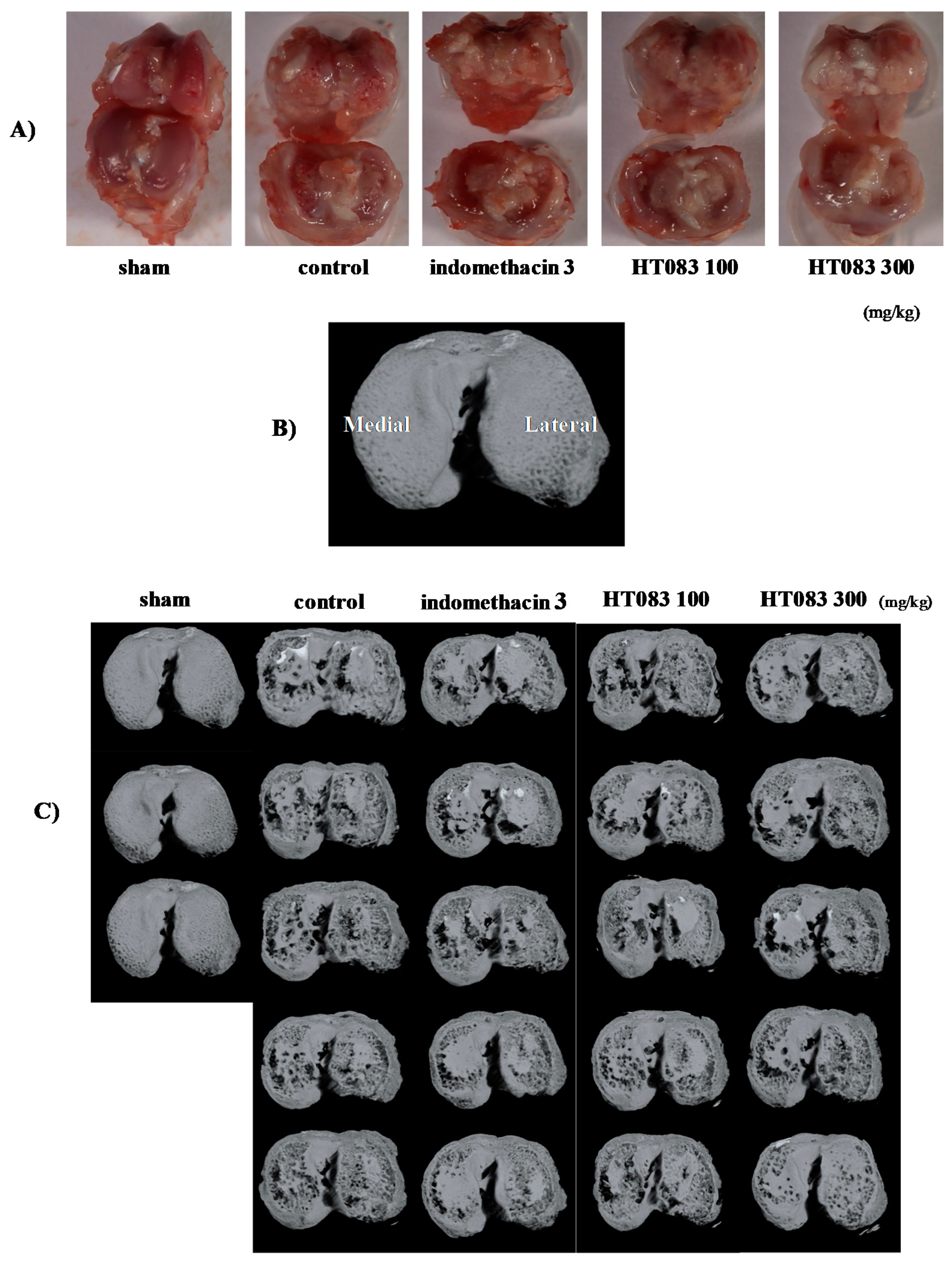
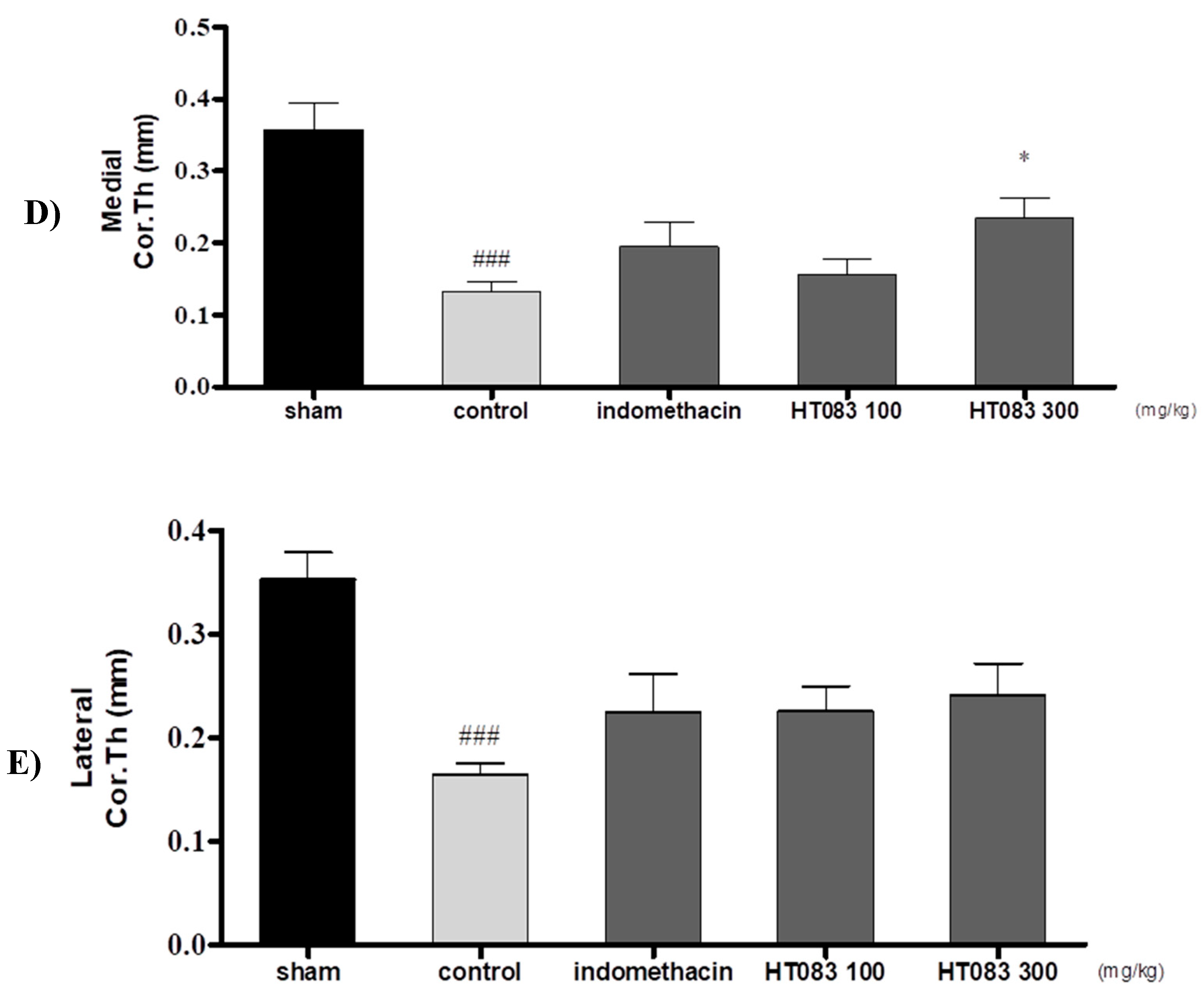
2.3. Knee Joints and Joint Cartilage by HT083
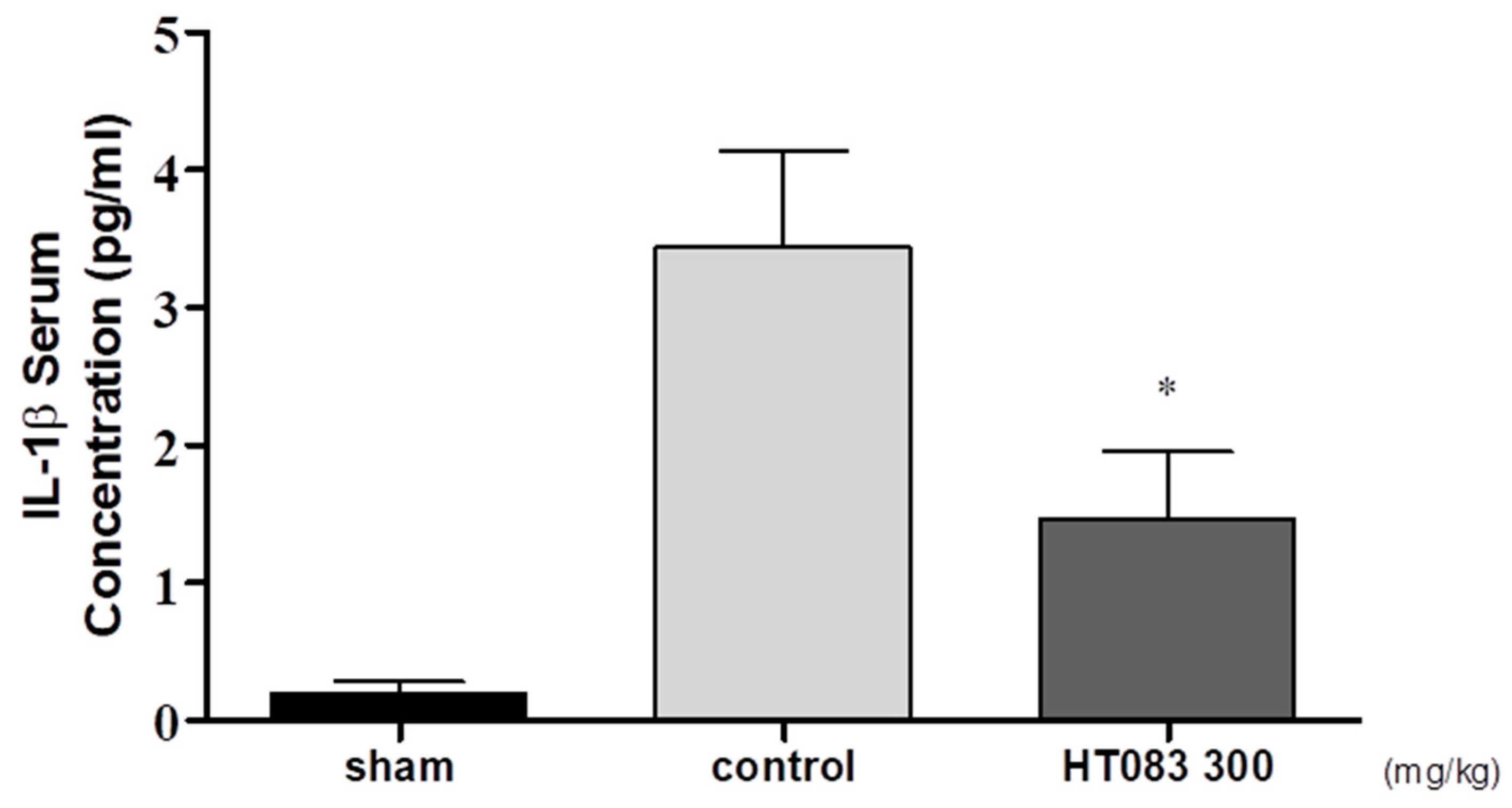
2.4. Serum IL-1β in MIA-Induced Osteoarthritis (OA) Rats
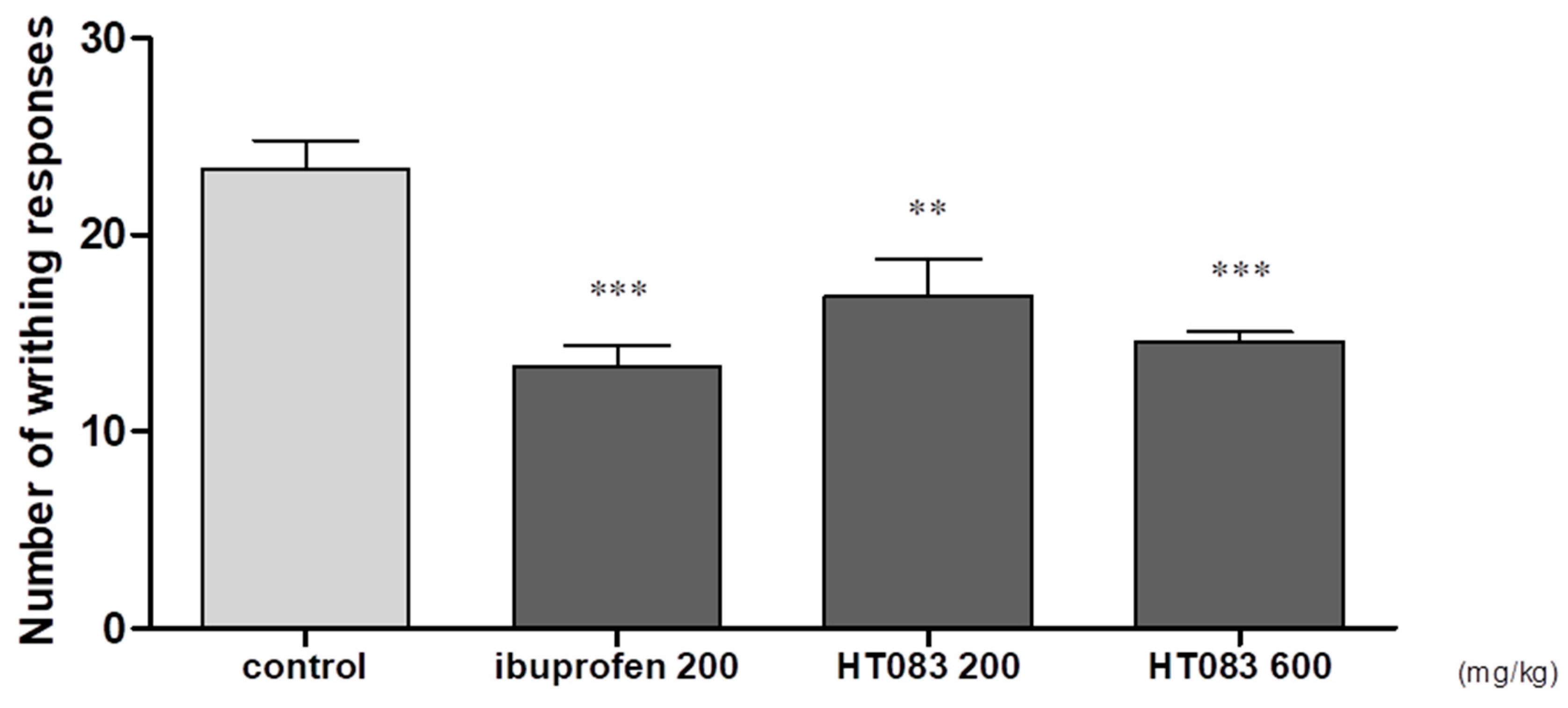
2.5. Effects of HT083 on Acetic Acid-Induced Writhing in Mice
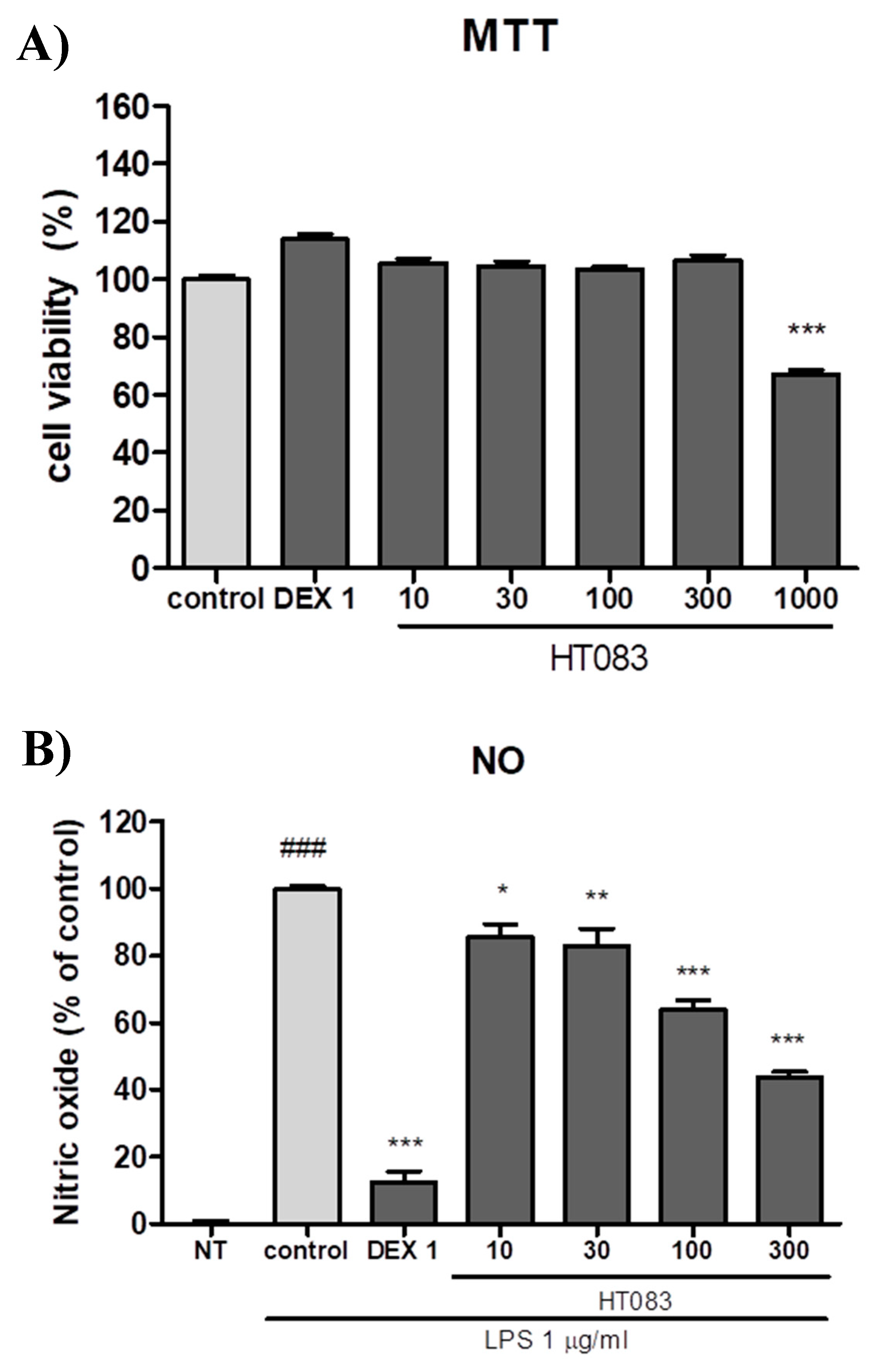
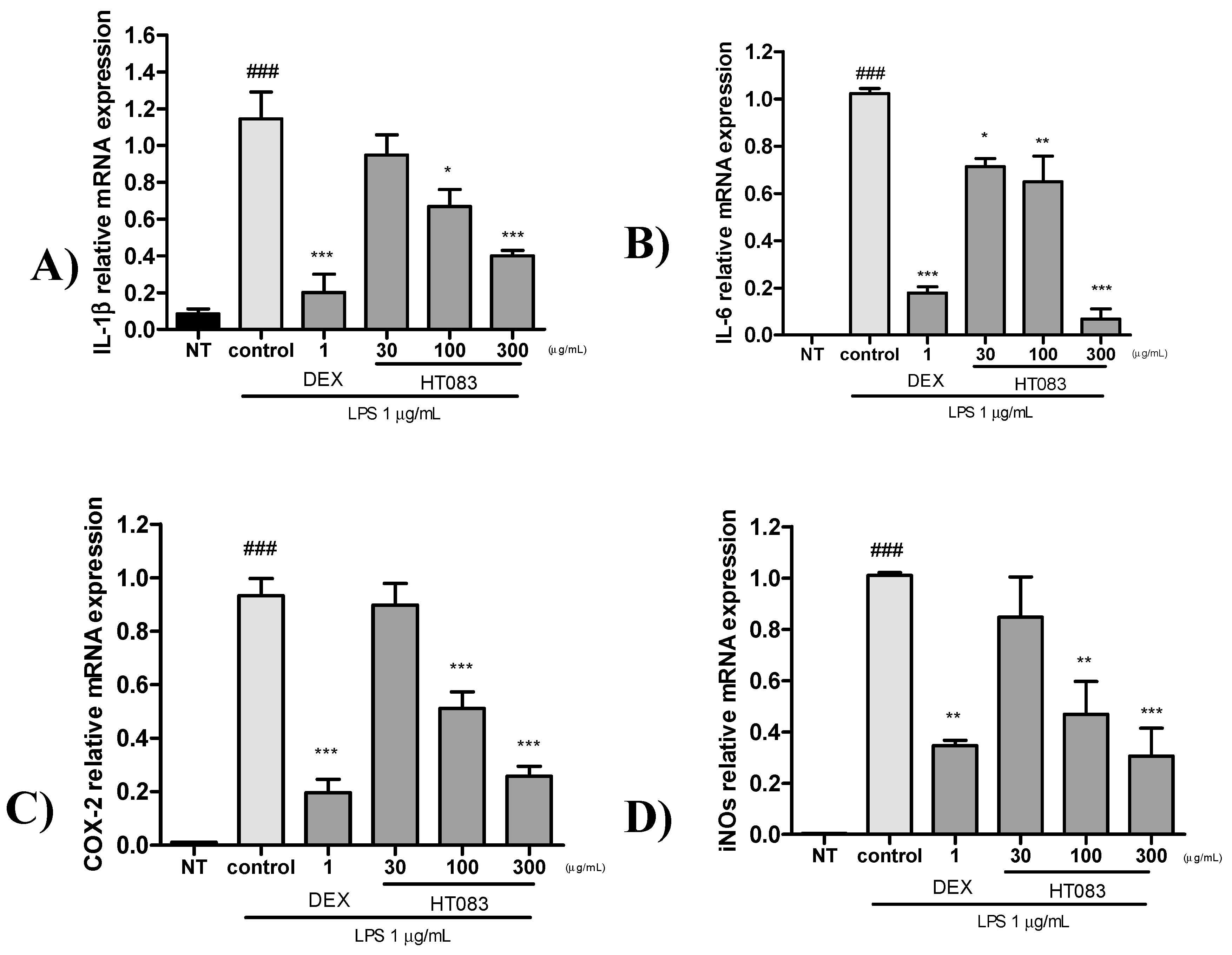
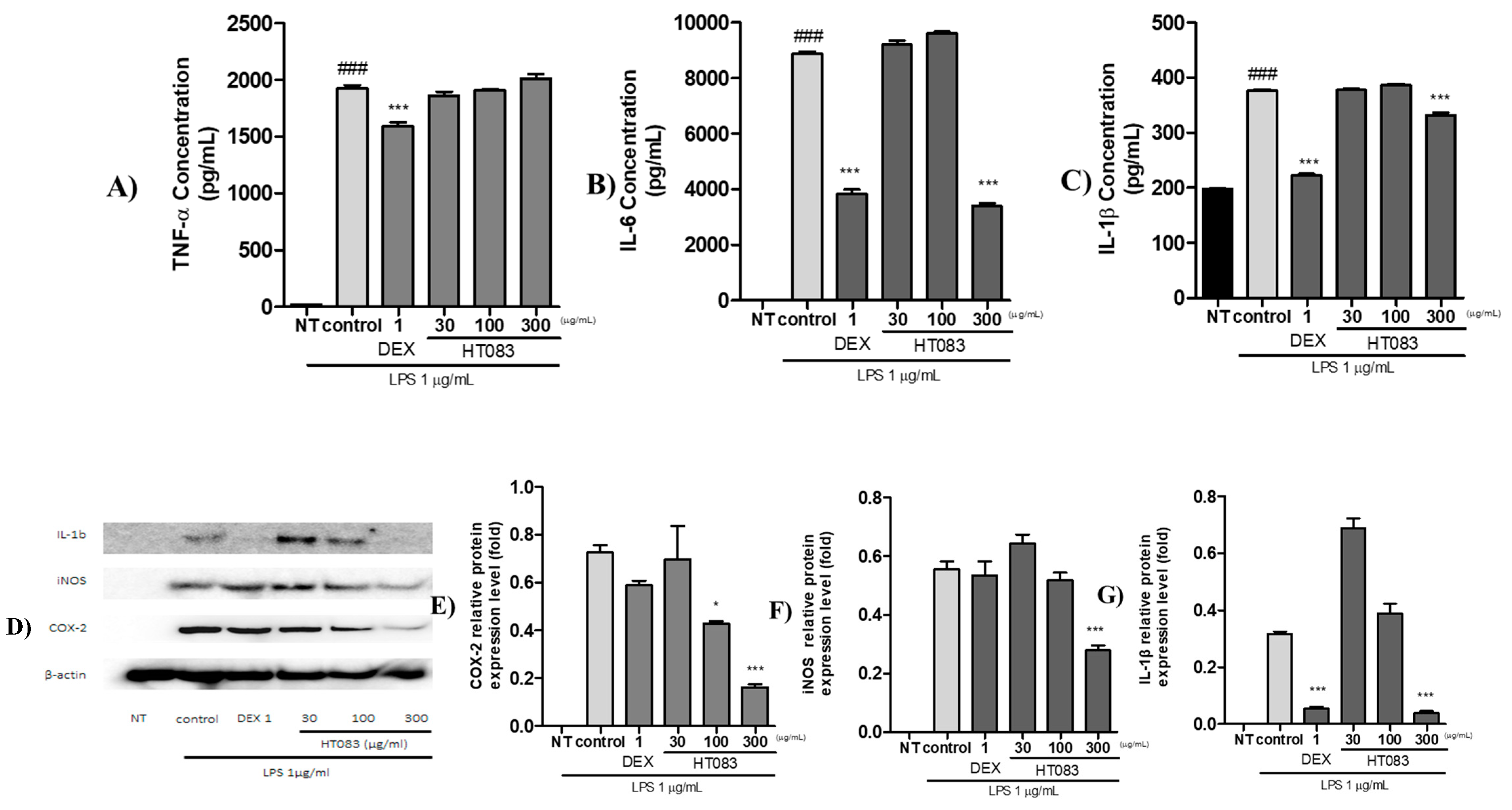
2.6. Effects of HT083 Against Inflammatory Responses in Lipopolysaccharide (LPS)-Stimulated RAW264.7 Cells
3. Discussion
4. Materials and Methods
4.1. Sample Preparation and HPLC Analysis
4.2. Animal Management, MIA Injection, and Diet Preparation
4.3. Weight-Bearing Capacity
4.4. Serum IL-1β Analysis of MIA Rats
4.5. Micro-Computed Tomography (Micro-CT) Analysis
4.6. Measurement of Acetic Acid-Induced Writhing Response
4.7. Cell Culture
4.8. NO Generation and Cell Toxicity Measurement
4.9. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
4.10. Protein Expression Analysis
4.11. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- O’Brien, M.; Philpott, H.T.; McDougall, J.J. Understanding osteoarthritis pain through animal models. Clin. Exp. Rheumatol. 2017, 35, S42–S47. [Google Scholar]
- Chen, D.; Shen, J.; Zhao, W.; Wang, T.; Han, L.; Hamilton, J.L.; Im, H.J. Osteoarthritis: Toward a comprehensive understanding of pathological mechanism. Bone Res. 2017, 5, 16044. [Google Scholar] [CrossRef] [PubMed]
- Kidd, B. Mechanisms of Pain in Osteoarthritis. HSS J. 2012, 8, 26–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldring, M.B.; Otero, M.; Plumb, D.A.; Dragomir, C.; Favero, M.; El Hachem, K.; Hashimoto, K.; Roach, H.I.; Olivotto, E.; Borzì, R.M.; et al. Roles of inflammatory and anabolic cytokines in cartilage metabolism: Signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur. Cells Mater. 2011, 21, 202–220. [Google Scholar] [CrossRef]
- Tantowi, N.A.C.A.; Lau, S.F.; Mohamed, S. Ficus deltoidea Prevented Bone Loss in Preclinical Osteoporosis/Osteoarthritis Model by Suppressing Inflammation. Calcif. Tissue Int. 2018, 103, 388–399. [Google Scholar] [CrossRef]
- Sharma, V.K.; Mamontov, E.; Tyagi, M. Effects of NSAIDs on the nanoscopic dynamics of lipid membrane. Biochim. Biophys. Acta Biomembr. 2019, 1862, 183100. [Google Scholar] [CrossRef]
- Goudarzi, R.; Reid, A.; McDougall, J.J. Evaluation of the novel avocado/soybean unsaponifiable Arthrocen to alter joint pain and inflammation in a rat model of osteoarthritis. PLoS ONE 2018, 13, e0191906. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.H.; Lee, H.J.; Lee, C.J.; Park, J.S. Natural Products as Sources of Novel Drug Candidates for the Pharmacological Management of Osteoarthritis: A Narrative Review. Biomol. Ther. 2019, 27, 503–523. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, W. Anti-inflammatory and immunoregulatory effects of paeoniflorin and total glucosides of paeony. Pharmacol. Ther. 2019, 107452. [Google Scholar] [CrossRef]
- Kim, K.H.; Shim, J.S.; Kim, H.J.; Son, E.D. Penta-O-galloyl-β-D-glucose from Paeonia lactiflora Pall. root extract enhances the expression of skin barrier genes via EGR3. J. Ethnopharmacol. 2019, 248, 112337. [Google Scholar] [CrossRef]
- Sotoudeh, R.; Hadjzadeh, M.A.R.; Gholamnezhad, Z.; Aghaei, A. The anti-diabetic and antioxidant effects of a combination of Commiphora mukul, Commiphora myrrha and Terminalia chebula in diabetic rats. Avicenna J. Phytomed. 2019, 9, 454–464. [Google Scholar]
- Cao, B.; Wei, X.C.; Xu, X.R.; Zhang, H.Z.; Luo, C.H.; Feng, B.; Xu, R.C.; Zhao, S.Y.; Du, X.J.; Han, L.; et al. Seeing the Unseen of the Combination of Two Natural Resins, Frankincense and Myrrh: Changes in Chemical Constituents and Pharmacological Activities. Molecules 2019, 24, 3076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, S.; Hua, Y.; Wang, Y.; Gu, W.; Zhou, W.; Duan, J.A.; Jiang, H.; Cen, T.; Tang, Y. Evaluation of the anti-inflammatory and analgesic properties ofindividual and combined extracts from Commiphora myrrha, and Boswellia carterii. J. Ethnopharm. 2012, 139, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Fonsi, M.; El Amrani, A.; Gervais, F.; Vincent, P. Intra-Articular Hyaluronic Acid and Chondroitin Sulfate: Pharmacokinetic Investigation in Osteoarthritic Rat Models. Curr. Ther. Res. Clin. Exp. 2019, 9, 100573. [Google Scholar] [CrossRef]
- Chien, T.Y.; Huang, S.K.H.; Lee, C.J.; Tsai, P.W.; Wang, C.C. Antinociceptive and anti-inflammatory effects of zerumbone against mono-iodoacetate-induced arthritis. Int. J. Mol. Sci. 2016, 17, 249. [Google Scholar] [CrossRef] [Green Version]
- Gawade., S.P. Acetic acid induced painful endogenous infliction in writhing test on mice. J. Pharmacol. Pharmacother. 2012, 3, 348. [Google Scholar] [CrossRef] [Green Version]
- Bayazid, A.B.; Seo Hyun Park, S.Y.; Kim, J.G.; Lim, B.O. Green chicory leaf extract exerts anti-inflammatory effects through suppressing LPS-induced MAPK/NF-κB activation and hepatoprotective activity in vitro. Food Agric. Immunol. 2020, 31, 513–532. [Google Scholar] [CrossRef] [Green Version]
- Eszter, B.; Zsolt, S.; Aliz, S.; Balazs, D.; Nikoletta, K.; Zsuzsanna, T.; Balazs, S.; Ferenc, G.J. Antioxidant and anti-inflammatory effects in RAW264.7 macrophges of Malvidin, a major red wine polyphenol. PLoS ONE 2013, 8, e65355. [Google Scholar]
- Ortega, E.; Galvez, I.; Hinchado, M.D.; Guerrero, J.; Martin-Cordero, L.; Torres-Piles, S. Anti-inflammatory effect as a mechanism of effectiveness underlying the clinical benefits of pelotherapy in osteoarthritis patients: Regulation of the altered inflammatory and stress feedback response. Int. J. Biometeorol. 2017, 61, 1777–1785. [Google Scholar] [CrossRef]
- Stevenson, G.W.; Bilsky, E.J.; Negus, S.S. Targeting Pain-Suppressed Behaviors in Preclinical Assays of Pain and Analgesia: Effects of Morphine on Acetic Acid-Suppressed Feeding in C57BL/6J Mice. J. Pain 2006, 7, 408–416. [Google Scholar] [CrossRef]
- Nakazato-Imasato, E.; Kurebayashi, Y. Pharmacological characteristics of the hind paw weight bearing difference induced by chronic constriction injury of the sciatic nerve in rats. Life Sci. 2009, 84, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Guler, N.; Kurkcu, M.; Duygu, G.; Cam, B. Sodium iodoacetate induced osteoarthrosis model in rabbit temporomandibular joint: CT and histological study (Part I). Int. J. Oral Maxillofac. Surg. 2011, 40, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Valente, J.S. The Pharmacology of Pain Associated With the Monoiodoacetate Model of Osteoarthritis. Front. Pharm. 2019, 10, 974. [Google Scholar] [CrossRef] [PubMed]
- Guzman, R.E.; Evans, M.G.; Bove, S.; Morenko, B.; Kilgore, K. Mono-iodoacetate–induced histologic changes in subchondral bone and articular cartilage of rat femorotibial joints: An animal model of osteoarthritis. Toxicol. Pathol. 2003, 31, 619–624. [Google Scholar] [CrossRef]
- Zhang, R.; Ren, K.; Dubner, R. Osteoarthritis pain mechanisms: Basic studies in animal models. Osteoarthr. Cartil. 2013, 21, 1308–1315. [Google Scholar] [CrossRef] [Green Version]
- Luceri, C.; Ghelardini, C.; Monserrat, C.; Aiolli, S.; Luceri, F.; Lodovici, M.; Menichetti, S.; Romanelli, M.N. Analgesic effects of myrrh. Nature 1996, 379, 29. [Google Scholar]
- Hu, B.; Xu, G.; Zhang, X.; Xu, L.; Zhou, H.; Ma, Z.; Shen, X.; Zhu, J.; Shen, R. Paeoniflorin attenuates inflammatory pain by inhibiting microglial activation and Akt-NFkB signaling. Cell Physiol. Biochem. 2018, 47, 842–850. [Google Scholar] [CrossRef]
- Lee, Y.M.; Son, E.J.; Kim, S.Y.; Kim, O.S.; Kim, D.S. Anti-inflammatory and anti-osteoarthritis effect of Mollugopentaphylla extract. Pharm. Biol. 2019, 57, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Haywood, A.R.; Hathway, G.J.; Chapman, V. Differential contributions of peripheral and central mechanisms to pain in a rodent model of osteoarthritis. Sci. Rep. 2018, 8, 7122. [Google Scholar] [CrossRef]
- Germano, A.; Occhipinti, A.; Francesca, B.; Maffei, M.E. A Pilot study on bioactive constituents and analgesic effects of MyrLiq®, a Commiphora myrrha extract with a high furanodiene content. Biomed. Res. Int. 2017. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Dai, S. Mechanisms involved in the therapeutic effects of Paeonia lactiflora Pallas in rheumatoid arthritis. Int. Immunopharmacol. 2012, 14, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Moilanen, L.J.; Hamalainen, M.; Ilmarinen, P.; Vuoleteenaho, K.; Nieminen, R.M.; Lehtimaki, L.; Moilanen, E. Monosodium iodoacetate-induced inflammation and joint pain are reduced in TRPA1 deficient mice – potential role of TRPA1 in osteoarthritis. Osteoarthr. Cartil. 2015, 23, 2017–2026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugita, R.; Kuwabara, H.; Kubota, K.; Sugimoto, K.; Kiho, T.; Tengeiji, a.; Kawakami, K.; Shimada, K. Simultaneous Inhibition of PGE2 and PGI2 Signals Is Necessary to Suppress Hyperalgesia in Rat Inflammatory Pain Models. Mediat. Inflamm. 2016, 2016, 9847840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, Y.J.; Joo, Y.B.; Jung, Y.O.; Ju, J.H.; la Cho, M.; Oh, H.J.; Jhun, J.Y.; Park, M.K.; Park, J.S.; Kang, C.M.; et al. Grape seed proanthocyanidin extract ameliorates monosodium iodoacetate-induced osteoarthritis. Exp. Mol. Med. 2011, 43, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Oyajobi, B.O.; Frazer, A.; Kozai, L.D.; Russell, R.G.; Hollander, A.P. Effects of growth factors and interleukin-1α on proteoglycan and type II collagen turnover in bovine nasal and articular chondrocyte pellet cultures. Endocrinology 1996, 137, 3557–3565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philp, A.M.; Davis, E.T.; Jones, S.W. Developing anti-inflammatory therapeutics for patients with osteoarthritis. Rheumatology 2017, 56, 869–881. [Google Scholar] [CrossRef] [Green Version]
- Hayer, S.; Bauer, G.; Willburger, M.; Sinn, K.; Alasti, F.; Plasenzotti, R.; Shvets, T.; Niederreiter, B.; Aschauer, C.; Steiner, G.; et al. Cartilage damage and bone erosion are more prominent determinants of functional impairment in longstanding experimental arthritis than synovial inflammation. DMM Dis. Model. Mech. 2016, 9, 1329–1338. [Google Scholar] [CrossRef] [Green Version]
- An, S.; Heo, D. Effects of Kyejiinsam-tang in MIA-Induced Osteoarthritis Rats. J. Kor. Med. 2013, 40, 35–58. [Google Scholar] [CrossRef]
- Kim, S.E.; Lee, J.Y.; Shim, K.S.; Lee, S.; Min, K.; Bae, J.H.; Kim, H.J.; Park, K.; Song, H.R. Attenuation of inflammation and cartilage degradation by sulfasalazine-containing hyaluronic acid on osteoarthritis rat model. Int. J. Biol. Macromol. 2018, 114, 341–348. [Google Scholar] [CrossRef]
- Pauly, H.M.; Larson, B.E.; Coatney, G.A.; Button, K.D.; DeCamp, C.E.; Fajardo, R.S.; Haul, R.C.; Donahue, T.L.H.M. Assessment of Cortical and Trabecular Bone Changes in Two Models of Post Traumatic Osteoarthritis. J. Orthop. Res. 2015, 33, 1835–1845. [Google Scholar] [CrossRef] [Green Version]
- Syx, D.; Tran, P.B.; Miller, R.E. Peripheral mechanisms contributing to osteoarthritis pain. Curr. Rheumatol. Rep. 2018, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Deraedt, R.; Jouquey, S.; Delevallée, F.; Flahaut, M. Release of prostaglandins E and F in an algogenic reaction and its inhibition. Eur. J. Pharmacol. 1980, 61, 17–24. [Google Scholar] [CrossRef]
- Ko, W.K.; Lee, S.H.; Kim, S.J.; Jo, M.J.; Han, I.B.; Sohn, S. Anti-inflammatory effects of ursodeoxycholic acid by lipopolysaccharide-stimulated inflammatory responses in RAW 264.7 macrophages. PLoS ONE 2017, 12, e0180673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parente, L. Pros and cons of selective inhibition of cyclooxygenase-2 versus dual lipoxygenase/cyclooxygenase inhibition: Is two better than One? J. Rheumatol. 2001, 28, 2375–2382. [Google Scholar]
- Dahesia, M.; Yao, J.Q. The interleukin 1beta pathway in the pathogenesis of osteoarthritis. J. Rheumatol. 2008, 35, 1306–1312. [Google Scholar]
| IL-6 | F | 5′-ACCAGAGGAAATTTTCAATAGG-3′ |
| R | 5′-TGATGCACTTGCAGAAAACA-3′ | |
| COX-2 | F | 5′-AACCGCATTGCCTCTGAAT-3′ |
| R | 5′-CATGTTCCAGGAGGATGGAG-3′ | |
| IL-1β | F | 5′-CCTAAAGTATGGGCTGGACTGT-3′ |
| R | 5′-GACTAAGGAGTCCCCTGGAGAT-3′ | |
| iNOS | F | 5′-CCCTTCCGAAGTTTCTGGCAGCAGC-3′ |
| R | 5′-GGCTGTCAGAGCCTCGTGGCTTTGG-3′ | |
| GAPDH | F | 5′-TGGCCTCCAAGGAGTAAGAAAC-3′ |
| R | 5′-CAGCAACTGAGGGCCTCTCT-3′ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Ju, M.-K.; Kim, H. Commiphora Extract Mixture Ameliorates Monosodium Iodoacetate-Induced Osteoarthritis. Nutrients 2020, 12, 1477. https://doi.org/10.3390/nu12051477
Lee D, Ju M-K, Kim H. Commiphora Extract Mixture Ameliorates Monosodium Iodoacetate-Induced Osteoarthritis. Nutrients. 2020; 12(5):1477. https://doi.org/10.3390/nu12051477
Chicago/Turabian StyleLee, Donghun, Mi-Kyoung Ju, and Hocheol Kim. 2020. "Commiphora Extract Mixture Ameliorates Monosodium Iodoacetate-Induced Osteoarthritis" Nutrients 12, no. 5: 1477. https://doi.org/10.3390/nu12051477
APA StyleLee, D., Ju, M.-K., & Kim, H. (2020). Commiphora Extract Mixture Ameliorates Monosodium Iodoacetate-Induced Osteoarthritis. Nutrients, 12(5), 1477. https://doi.org/10.3390/nu12051477






