Protein Distribution and Muscle-Related Outcomes: Does the Evidence Support the Concept?
Abstract
1. Introduction
2. Observational Research
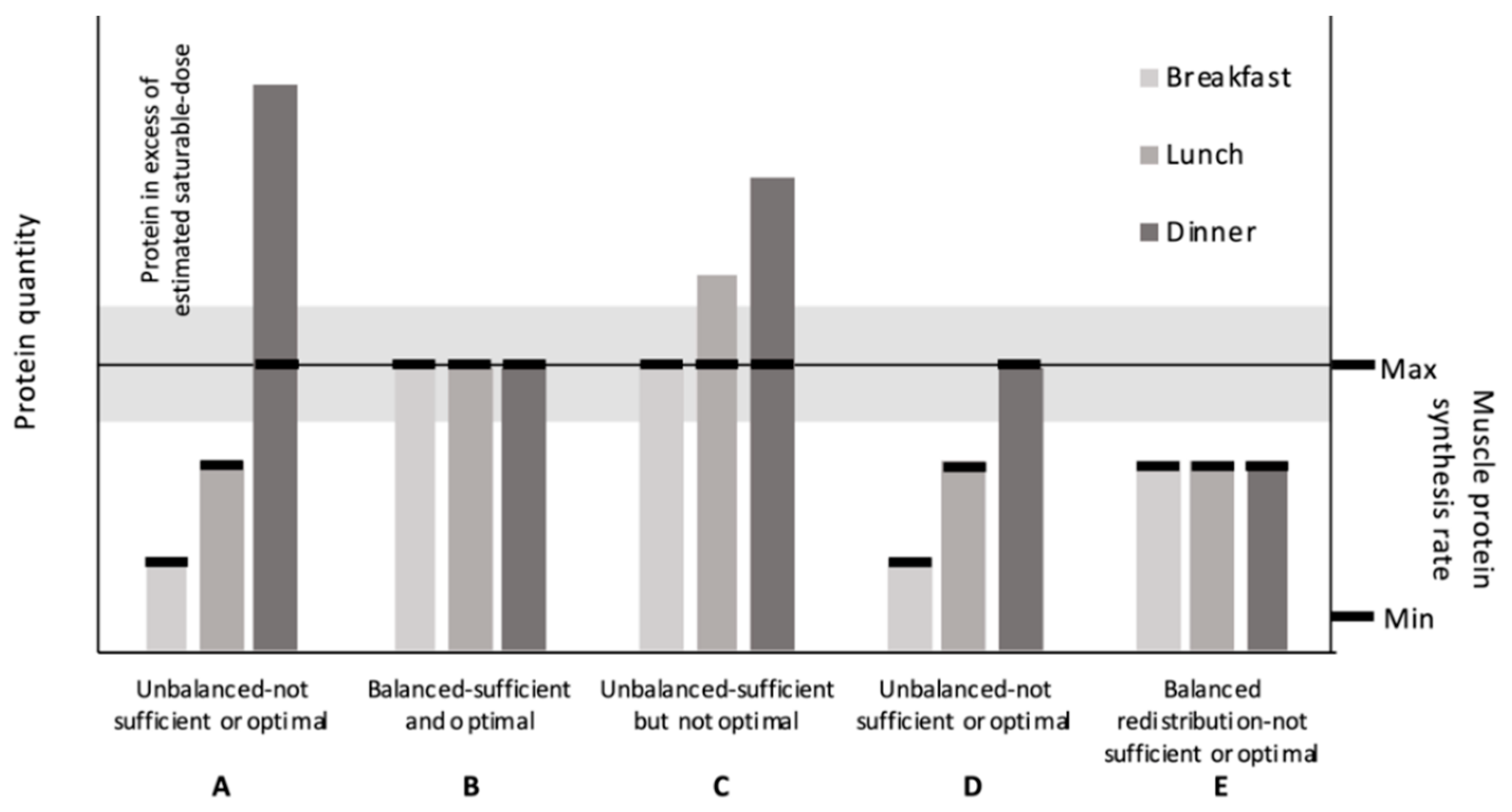
2.1. Degree of Protein Distribution
2.2. Number of Meals Reaching a Target Threshold
2.3. Number of Meals Not Reaching a Target Threshold
2.4. Conclusions from Observational Research
3. Randomized Controlled Research
3.1. Acute Protein Ingestion Research
3.1.1. Future Research Directions for Acute Protein Ingestion Research
3.1.2. Conclusions from the Acute Protein Ingestion Research
3.2. Chronic Protein Ingestion Research
3.2.1. Nitrogen Balance
3.2.2. Body Composition
3.2.3. Future Research Directions for Chronic Protein Ingestion Research
3.2.4. Conclusions from Chronic Protein Ingestion Research
4. Conclusions—Does the Evidence Support the Concept?
Author Contributions
Funding
Conflicts of Interest
References
- Institute of Medicine. Panel on Macronutrients. In Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients); National Academies Press: Washington, DC, USA, 2004. [Google Scholar]
- Waterlow, J.C.; Garlick, P.J.; Mill Ward, D. Protein Turnover in Mammalian Tissues and in the Whole Body; Elsevier/North-Holland Biomedical Press: Amsterdam, The Netherlands, 1978. [Google Scholar]
- Geiger, E. Experiments with delayed supplementation of incomplete amino acid mixtures. J. Nutr. 1947, 34, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Cannon, P.R.; Steffee, C.H. The influence of time of ingestion of essential amino acid upon utilization in tissue-synthesis. Fed. Proc. 1947, 6, 390. [Google Scholar] [PubMed]
- Henry, K.M.; Kon, S.K. 337. The supplementary relationships between the proteins of dairy products and those of bread and potato as affected by the method of feeding. With a note on the value of soya-bean protein. J. Dairy Res. 1946, 14, 330–339. [Google Scholar] [CrossRef]
- Harte, R.A.; Travers, J.J.; Sarich, P. Voluntary caloric intake of the growing rat. J. Nutr. 1948, 36, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Leverton, R.M.; Gram, M.R. Nitrogen excretion of women related to the distribution of animal protein in daily meals. J. Nutr. 1949, 39, 57–65. [Google Scholar] [CrossRef]
- Rennie, M.J.; Wackerhage, H.; Spangenburg, E.E.; Booth, F.W. Control of the size of the human muscle mass. Annu. Rev. Physiol. 2004, 66, 799–828. [Google Scholar] [CrossRef] [PubMed]
- Symons, T.B.; Sheffield-Moore, M.; Wolfe, R.R.; Paddon-Jones, D. A moderate serving of high-quality protein maximally stimulates skeletal muscle protein synthesis in young and elderly subjects. J. Am. Diet. Assoc. 2009, 109, 1582–1586. [Google Scholar] [CrossRef]
- Moore, D.R.; Churchward-Venne, T.A.; Witard, O.; Breen, L.; Burd, N.A.; Tipton, K.D.; Phillips, S.M. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 57–62. [Google Scholar] [CrossRef]
- Deutz, N.E.; Wolfe, R.R. Is there a maximal anabolic response to protein intake with a meal? Clin. Nutr. 2013, 32, 309–313. [Google Scholar] [CrossRef]
- Kim, I.Y.; Deutz, N.E.P.; Wolfe, R.R. Update on maximal anabolic response to dietary protein. Clin. Nutr. 2018, 37, 411–418. [Google Scholar] [CrossRef]
- Gillen, J.B.; Trommelen, J.; Wardenaar, F.C.; Brinkmans, N.Y.; Versteegen, J.J.; Jonvik, K.L.; Kapp, C.; de Vries, J.; van den Borne, J.J.; Gibala, M.J.; et al. Dietary Protein Intake and Distribution Patterns of Well-Trained Dutch Athletes. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Tieland, M.; Borgonjen-Van den Berg, K.J.; van Loon, L.J.; de Groot, L.C. Dietary protein intake in community-dwelling, frail, and institutionalized elderly people: Scope for improvement. Eur. J. Nutr. 2012, 51, 173–179. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture. Nutrient Intakes from Food: Mean Amounts Consumed per Individual, by Gender and Age, What We Eat in America, NHANES 2009–2010; Agricultural Research Service: Washington, DC, USA, 2012.
- Brinkmans, N.Y.J.; Iedema, N.; Plasqui, G.; Wouters, L.; Saris, W.H.M.; van Loon, L.J.C.; van Dijk, J.W. Energy expenditure and dietary intake in professional football players in the Dutch Premier League: Implications for nutritional counselling. J. Sports Sci. 2019, 37, 2759–2767. [Google Scholar] [CrossRef] [PubMed]
- Smeuninx, B.; Greig, C.A.; Breen, L. Amount, Source and Pattern of Dietary Protein Intake Across the Adult Lifespan: A Cross-Sectional Study. Front. Nutr. 2020, 7, 25. [Google Scholar] [CrossRef]
- Tieland, M.; Borgonjen-Van den Berg, K.J.; Van Loon, L.J.; de Groot, L.C. Dietary Protein Intake in Dutch Elderly People: A Focus on Protein Sources. Nutrients 2015, 7, 9697–9706. [Google Scholar] [CrossRef]
- Berner, L.A.; Becker, G.; Wise, M.; Doi, J. Characterization of Dietary Protein among Older Adults in the United States: Amount, Animal Sources, and Meal Patterns. J. Acad. Nutr. Diet. 2013, 113, 809–815. [Google Scholar] [CrossRef]
- Cardon-Thomas, D.K.; Riviere, T.; Tieges, Z.; Greig, C.A. Dietary Protein in Older Adults: Adequate Daily Intake but Potential for Improved Distribution. Nutrients 2017, 9, 184. [Google Scholar] [CrossRef]
- Rousset, S.; Patureau Mirand, P.; Brandolini, M.; Martin, J.F.; Boirie, Y. Daily protein intakes and eating patterns in young and elderly French. Br. J. Nutr. 2003, 90, 1107–1115. [Google Scholar] [CrossRef]
- Bollwein, J.; Diekmann, R.; Kaiser, M.J.; Bauer, J.M.; Uter, W.; Sieber, C.C.; Volkert, D. Distribution but not amount of protein intake is associated with frailty: A cross-sectional investigation in the region of Nurnberg. Nutr. J. 2013, 12, 109. [Google Scholar] [CrossRef]
- Gingrich, A.; Spiegel, A.; Kob, R.; Schoene, D.; Skurk, T.; Hauner, H.; Sieber, C.C.; Volkert, D.; Kiesswetter, E. Amount, Distribution, and Quality of Protein Intake Are Not Associated with Muscle Mass, Strength, and Power in Healthy Older Adults without Functional Limitations-An enable Study. Nutrients 2017, 9, 1358. [Google Scholar] [CrossRef]
- Ten Haaf, D.S.M.; van Dongen, E.J.I.; Nuijten, M.A.H.; Eijsvogels, T.M.H.; de Groot, L.; Hopman, M.T.E. Protein Intake and Distribution in Relation to Physical Functioning and Quality of Life in Community-Dwelling Elderly People: Acknowledging the Role of Physical Activity. Nutrients 2018, 10, 506. [Google Scholar] [CrossRef] [PubMed]
- Farsijani, S.; Morais, J.A.; Payette, H.; Gaudreau, P.; Shatenstein, B.; Gray-Donald, K.; Chevalier, S. Relation between mealtime distribution of protein intake and lean mass loss in free-living older adults of the NuAge study. Am. J. Clin. Nutr. 2016, 104, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Farsijani, S.; Payette, H.; Morais, J.A.; Shatenstein, B.; Gaudreau, P.; Chevalier, S. Even mealtime distribution of protein intake is associated with greater muscle strength, but not with 3-y physical function decline, in free-living older adults: The Quebec longitudinal study on Nutrition as a Determinant of Successful Aging (NuAge study). Am. J. Clin. Nutr. 2017, 106, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Loenneke, J.P.; Loprinzi, P.D.; Murphy, C.H.; Phillips, S.M. Per meal dose and frequency of protein consumption is associated with lean mass and muscle performance. Clin. Nutr. 2016. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Loenneke, J.P.; Hamilton, D.L. Leisure time sedentary behavior, physical activity and frequency of protein consumption on lower extremity strength and lean mass. Eur. J. Clin. Nutr. 2017, 71, 1399–1404. [Google Scholar] [CrossRef]
- Gaytán-González, A.; Ocampo-Alfaro, M.D.J.; Torres-Naranjo, F.; Arroniz-Rivera, M.; González-Mendoza, R.G.; Gil-Barreiro, M.; López-Taylor, J.R. The Consumption of Two or Three Meals per Day with Adequate Protein Content Is Associated with Lower Risk of Physical Disability in Mexican Adults Aged 60 Years and Older. Geriatrics 2020, 5, 1. [Google Scholar] [CrossRef]
- Mishra, S.; Goldman, J.D.; Sahyoun, N.R.; Moshfegh, A.J. Association between dietary protein intake and grip strength among adults aged 51 years and over: What We Eat in America, National Health and Nutrition Examination Survey 2011–2014. PLoS ONE 2018, 13, e0191368. [Google Scholar] [CrossRef]
- Valenzuela, R.E.R.; Ponce, J.A.; Morales-Figueroa, G.G.; Muro, K.A.; Carreón, V.R.; Alemán-Mateo, H. Insufficient amounts and inadequate distribution of dietary protein intake in apparently healthy older adults in a developing country: Implications for dietary strategies to prevent sarcopenia. Clin. Interv. Aging 2013, 8, 1143–1148. [Google Scholar] [CrossRef]
- Gaytan-Gonzalez, A.; Ocampo-Alfaro, M.J.; Arroniz-Rivera, M.; Torres-Naranjo, F.; Gonzalez-Mendoza, R.G.; Gil-Barreiro, M.; Lopez-Taylor, J.R. Inadequate Protein Intake at Specific Meals Is Associated with Higher Risk of Impaired Functionality in Middle to Older Aged Mexican Adults. J. Aging Res. 2019, 2019, 6597617. [Google Scholar] [CrossRef]
- Yasuda, J.; Asako, M.; Arimitsu, T.; Fujita, S. Association of Protein Intake in Three Meals with Muscle Mass in Healthy Young Subjects: A Cross-Sectional Study. Nutrients 2019, 11, 612. [Google Scholar] [CrossRef]
- Mamerow, M.M.; Mettler, J.A.; English, K.L.; Casperson, S.L.; Arentson-Lantz, E.; Sheffield-Moore, M.; Layman, D.K.; Paddon-Jones, D. Dietary protein distribution positively influences 24-h muscle protein synthesis in healthy adults. J. Nutr. 2014, 144, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; Schutzler, S.; Schrader, A.; Spencer, H.; Kortebein, P.; Deutz, N.E.; Wolfe, R.R.; Ferrando, A.A. Quantity of dietary protein intake, but not pattern of intake, affects net protein balance primarily through differences in protein synthesis in older adults. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E21–E28. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.H.; Churchward-Venne, T.A.; Mitchell, C.J.; Kolar, N.M.; Kassis, A.; Karagounis, L.G.; Burke, L.M.; Hawley, J.A.; Phillips, S.M. Hypoenergetic diet-induced reductions in myofibrillar protein synthesis are restored with resistance training and balanced daily protein ingestion in older men. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E734–E743. [Google Scholar] [CrossRef] [PubMed]
- El-Khoury, A.E.; Sanchez, M.; Fukagawa, N.K.; Gleason, R.E.; Tsay, R.H.; Young, V.R. The 24-h kinetics of leucine oxidation in healthy adults receiving a generous leucine intake via three discrete meals. Am. J. Clin. Nutr. 1995, 62, 579–590. [Google Scholar] [CrossRef]
- Arnal, M.A.; Mosoni, L.; Boirie, Y.; Houlier, M.L.; Morin, L.; Verdier, E.; Ritz, P.; Antoine, J.M.; Prugnaud, J.; Beaufrere, B.; et al. Protein pulse feeding improves protein retention in elderly women. Am. J. Clin. Nutr. 1999, 69, 1202–1208. [Google Scholar] [CrossRef]
- Arnal, M.A.; Mosoni, L.; Boirie, Y.; Houlier, M.L.; Morin, L.; Verdier, E.; Ritz, P.; Antoine, J.M.; Prugnaud, J.; Beaufrere, B.; et al. Protein feeding pattern does not affect protein retention in young women. J. Nutr. 2000, 130, 1700–1704. [Google Scholar] [CrossRef]
- Phillips, S.M.; Tipton, K.D.; Aarsland, A.; Wolf, S.E.; Wolfe, R.R. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am. J. Physiol. Endocrinol. Metab. 1997, 273, E99–E107. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Baumgartner, R.N.; Ross, R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J. Appl. Physiol. (1985) 2000, 89, 465–471. [Google Scholar] [CrossRef]
- Kim, I.-Y.; Schutzler, S.; Schrader, A.M.; Spencer, H.J.; Azhar, G.; Wolfe, R.R.; Ferrando, A.A. Protein intake distribution pattern does not affect anabolic response, lean body mass, muscle strength or function over 8 weeks in older adults: A randomized-controlled trial. Clin. Nutr. 2018, 37, 488–493. [Google Scholar] [CrossRef]
- Murphy, C.H.; Shankaran, M.; Churchward-Venne, T.A.; Mitchell, C.J.; Kolar, N.M.; Burke, L.M.; Hawley, J.A.; Kassis, A.; Karagounis, L.G.; Li, K.; et al. Effect of resistance training and protein intake pattern on myofibrillar protein synthesis and proteome kinetics in older men in energy restriction. J. Physiol. 2018, 596, 2091–2120. [Google Scholar] [CrossRef] [PubMed]
- Burd, N.A.; Gorissen, S.H.; Van Loon, L.J.C. Anabolic Resistance of Muscle Protein Synthesis with Aging. Exerc. Sport Sci. Rev. 2013, 41, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.J.; Burd, N.A.; Breen, L.; Rerecich, T.; Yang, Y.; Hector, A.J.; Baker, S.K.; Phillips, S.M. Dose-dependent responses of myofibrillar protein synthesis with beef ingestion are enhanced with resistance exercise in middle-aged men. Appl. Physiol. Nutr. Metab. 2013, 38, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Breen, L.; Burd, N.A.; Hector, A.J.; Churchward-Venne, T.A.; Josse, A.R.; Tarnopolsky, M.A.; Phillips, S.M. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br. J. Nutr. 2012, 108, 1780–1788. [Google Scholar] [CrossRef] [PubMed]
- Pennings, B.; Groen, B.; de Lange, A.; Gijsen, A.P.; Zorenc, A.H.; Senden, J.M.; van Loon, L.J. Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E992–E999. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.L.; Paddon-Jones, D.; Campbell, W.W. Whey protein supplementation 2 hours after a lower protein breakfast restores plasma essential amino acid availability comparable to a higher protein breakfast in overweight adults. Nutr. Res. 2017, 47, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Winter, J.A.; Cameron-Smith, D.; Enslen, M.; Farnfield, M.; Decombaz, J. Effect of Intake of Different Dietary Protein Sources on Plasma Amino Acid Profiles at Rest and After Exercise. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 452–462. [Google Scholar] [CrossRef]
- Conley, T.B.; Apolzan, J.W.; Leidy, H.J.; Greaves, K.A.; Lim, E.; Campbell, W.W. Effect of food form on postprandial plasma amino acid concentrations in older adults. Br. J. Nutr. 2011, 106, 203–207. [Google Scholar] [CrossRef]
- West, D.W.; Burd, N.A.; Coffey, V.G.; Baker, S.K.; Burke, L.M.; Hawley, J.A.; Moore, D.R.; Stellingwerff, T.; Phillips, S.M. Rapid aminoacidemia enhances myofibrillar protein synthesis and anabolic intramuscular signaling responses after resistance exercise. Am. J. Clin. Nutr. 2011, 94, 795–803. [Google Scholar] [CrossRef]
- Howarth, N.C.; Huang, T.T.; Roberts, S.B.; Lin, B.H.; McCrory, M.A. Eating patterns and dietary composition in relation to BMI in younger and older adults. Int. J. Obes. (Lond.) 2007, 31, 675–684. [Google Scholar] [CrossRef]
- Hector, A.J.; McGlory, C.; Damas, F.; Mazara, N.; Baker, S.K.; Phillips, S.M. Pronounced energy restriction with elevated protein intake results in no change in proteolysis and reductions in skeletal muscle protein synthesis that are mitigated by resistance exercise. FASEB J. 2018, 32, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Tomé, D.; Bos, C.C. Dietary Protein and Nitrogen Utilization. J. Nutr. 2000, 130, 1868S–1873S. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Energy and protein requirements. In Report of a Joint FAO/WHO/UNU Expert Consultation; World Health Organization Technical Report Series; World Health Organization: Geneva, Switzerland, 1985; Volume 724, pp. 1–206. [Google Scholar]
- Hegsted, D.M. Balance Studies. J. Nutr. 1976, 106, 307–311. [Google Scholar] [CrossRef]
- Waterlow, J.C. The mysteries of nitrogen balance. Nutr. Res. Rev. 1999, 12, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Young, V.R. Nutritional balance studies: Indicators of human requirements or of adaptive mechanisms? J. Nutr. 1986, 116, 700–703. [Google Scholar] [CrossRef]
- Millward, D.J. Methodological considerations. Proc. Nutr. Soc. 2001, 60, 3–5. [Google Scholar] [CrossRef]
- Cuthbertson, D.P.; Munro, H.N. The relationship of carbohydrate metabolism to protein metabolism: The roles of total dietary carbohydrate and of surfeit carbohydrate in protein metabolism. Biochem. J. 1939, 33, 128–142. [Google Scholar] [CrossRef]
- Adechian, S.; Balage, M.; Remond, D.; Migne, C.; Quignard-Boulange, A.; Marset-Baglieri, A.; Rousset, S.; Boirie, Y.; Gaudichon, C.; Dardevet, D.; et al. Protein feeding pattern, casein feeding, or milk-soluble protein feeding did not change the evolution of body composition during a short-term weight loss program. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E973–E982. [Google Scholar] [CrossRef]
- Bortz, W.M.; Wroldsen, A.; Issekutz, B., Jr.; Rodahl, K. Weight loss and frequency of feeding. N. Engl. J. Med. 1966, 274, 376–379. [Google Scholar] [CrossRef]
- Irwin, M.I.; Feeley, R.M. Frequency and size of meals and serum lipids, nitrogen and mineral retention, fat digestibility, and urinary thiamine and riboflavin in young women. Am. J. Clin. Nutr. 1967, 20, 816–824. [Google Scholar] [CrossRef]
- Leverton, R.M.; Gram, M.R.; Chaloupka, M. Effect of the time factor and calorie level on nitrogen utilization of young women. J. Nutr. 1951, 44, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Swindells, Y.E.; Holmes, S.A.; Robinson, M.F. The metabolic response of young women to changes in the frequency of meals. Br. J. Nutr. 1968, 22, 667–680. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Taylor, Y.S.; Young, V.R.; Murray, E.; Pencharz, P.B.; Scrimshaw, N.S. Daily protein and meal patterns affecting young men fed adequate and restricted energy intakes. Am. J. Clin. Nutr. 1973, 26, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wu, D.Y. Influence of feeding schedule on nitrogen utilization and excretion. Proc. Soc. Exp. Biol. Med. 1950, 74, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Young, C.M.; Scanlan, S.S.; Topping, C.M.; Simko, V.; Lutwak, L. Frequency of feeding, weight reduction, and body composition. J. Am. Diet. Assoc. 1971, 59, 466–472. [Google Scholar]
- Scrimshaw, N.S. Criteria for valid nitrogen balance measurement of protein requirements. Eur. J. Clin. Nutr. 1996, 50 (Suppl. 1), S196–S197. [Google Scholar]
- Symons, T.B.; Sheffield-Moore, M.; Mamerow, M.M.; Wolfe, R.R.; Paddon-Jones, D. The anabolic response to resistance exercise and a protein-rich meal is not diminished by age. J. Nutr. Health Aging 2011, 15, 376–381. [Google Scholar] [CrossRef]
- Bouillanne, O.; Curis, E.; Hamon-Vilcot, B.; Nicolis, I.; Chretien, P.; Schauer, N.; Vincent, J.P.; Cynober, L.; Aussel, C. Impact of protein pulse feeding on lean mass in malnourished and at-risk hospitalized elderly patients: A randomized controlled trial. Clin. Nutr. 2013, 32, 186–192. [Google Scholar] [CrossRef]
- Yasuda, J.; Tomita, T.; Arimitsu, T.; Fujita, S. Evenly Distributed Protein Intake over 3 Meals Augments Resistance Exercise–Induced Muscle Hypertrophy in Healthy Young Men. J. Nutr. 2020. [Google Scholar] [CrossRef]
- Hudson, J.L.; Kim, J.E.; Paddon-Jones, D.; Campbell, W.W. Within-day protein distribution does not influence body composition responses during weight loss in resistance-training adults who are overweight. Am. J. Clin. Nutr. 2017. [Google Scholar] [CrossRef]
- Morley, J.E.; Abbatecola, A.M.; Argiles, J.M.; Baracos, V.; Bauer, J.; Bhasin, S.; Cederholm, T.; Coats, A.J.S.; Cummings, S.R.; Evans, W.J.; et al. Sarcopenia with limited mobility: An international consensus. J. Am. Med. Dir. Assoc. 2011, 12, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Paddon-Jones, D.; Campbell, W.W.; Jacques, P.F.; Kritchevsky, S.B.; Moore, L.L.; Rodriguez, N.R.; van Loon, L.J. Protein and healthy aging. Am. J. Clin. Nutr. 2015, 101, 1339s–1345s. [Google Scholar] [CrossRef] [PubMed]
- Dillon, E.L.; Sheffield-Moore, M.; Paddon-Jones, D.; Gilkison, C.; Sanford, A.P.; Casperson, S.L.; Jiang, J.; Chinkes, D.L.; Urban, R.J. Amino acid supplementation increases lean body mass, basal muscle protein synthesis, and insulin-like growth factor-I expression in older women. J. Clin. Endocrinol. Metab. 2009, 94, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.L.; Wootton, S.A.; Jackson, A.A. Variability of fecal energy content measured in healthy women. Am. J. Clin. Nutr. 1993, 58, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Mittendorfer, B.; Andersen, J.L.; Plomgaard, P.; Saltin, B.; Babraj, J.A.; Smith, K.; Rennie, M.J. Protein synthesis rates in human muscles: Neither anatomical location nor fibre-type composition are major determinants. J. Physiol. 2005, 563, 203–211. [Google Scholar] [CrossRef]
- Lohman, T.G.; Going, S.B. Multicomponent models in body composition research: Opportunities and pitfalls. Basic Life Sci. 1993, 60, 53–58. [Google Scholar] [CrossRef]
- Wilson, J.P.; Mulligan, K.; Fan, B.; Sherman, J.L.; Murphy, E.J.; Tai, V.W.; Powers, C.L.; Marquez, L.; Ruiz-Barros, V.; Shepherd, J.A. Dual-energy X-ray absorptiometry-based body volume measurement for 4-compartment body composition. Am. J. Clin. Nutr. 2012, 95, 25–31. [Google Scholar] [CrossRef]
- Phillips, S.M.; Martinson, W. Nutrient-rich, high-quality, protein-containing dairy foods in combination with exercise in aging persons to mitigate sarcopenia. Nutr. Rev. 2019, 77, 216–229. [Google Scholar] [CrossRef]
- Kim, J.E.; O’Connor, L.E.; Sands, L.P.; Slebodnik, M.B.; Campbell, W.W. Effects of dietary protein intake on body composition changes after weight loss in older adults: A systematic review and meta-analysis. Nutr. Rev. 2016, 74, 210–224. [Google Scholar] [CrossRef]
- Morton, R.W.; Murphy, K.T.; McKellar, S.R.; Schoenfeld, B.J.; Henselmans, M.; Helms, E.; Aragon, A.A.; Devries, M.C.; Banfield, L.; Krieger, J.W. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports Med. 2018, 52, 376–384. [Google Scholar] [CrossRef]
- Wycherley, T.P.; Moran, L.J.; Clifton, P.M.; Noakes, M.; Brinkworth, G.D. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2012, 96, 1281–1298. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.L.; Wang, Y.; Bergia, R.E., III; Campbell, W.W. Protein Intake Greater than the RDA Differentially Influences Whole-Body Lean Mass Responses to Purposeful Catabolic and Anabolic Stressors: A Systematic Review and Meta-analysis. Adv. Nutr. 2019. [Google Scholar] [CrossRef] [PubMed]
- Schaap, L.A.; van Schoor, N.M.; Lips, P.; Visser, M. Associations of Sarcopenia Definitions, and Their Components, With the Incidence of Recurrent Falling and Fractures: The Longitudinal Aging Study Amsterdam. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
| Authors | Population | Techniques | Outcome |
|---|---|---|---|
| Bollwein et al. [22] | 194 adults from Nurnberg, Germany (≥75 y) | Dietary intake: food frequency questionnaire. Frailty: definition by Fried et al. [41] | Non-frail participants reported a more balanced protein distribution [median CV (min–max); 0.68 au (0.15–1.24)] than pre-frail [0.74 (0.07–1.29)] and frail participants [0.76 (0.18–1.33); total protein intakes were comparable (~1.1 g·kg−1·d−1) |
| Gingrich et al. [23] | 97 adults without functional limitations from Nuremburg, Germany (75–85 y) | Dietary intake: 7-d food records. Body composition: BIA. Skeletal muscle mass: estimated [42]. Leg strength, leg power, and hand grip strength: dynamometers | No association between daily protein intake quantity, balance of within-day distribution (CV), number of meals containing ≥0.4 g·kg−1, and number of meals containing ≥2.5 g leucine with leg strength, leg power, and hand grip strength |
| Ten Haaf et al. [24] | 140 community-dwelling adults from the Netherlands (81 ± 6 y) | Dietary intake: average of 2, 24-h recalls or from 3-d food records. Hand grip strength: dynamometer. Physical function: Short Physical Performance Battery tests. Quality of life: EQ-5D-5L. | Among the five outcomes, a more balanced protein distribution (spread CV < 0.43) was associated only with greater gait speed (β = −0.42) when compared to the intermediate CV (0.43–0.62) |
| Farsijani et al. [25] | 2-y follow up in 351 men and 361 women from the Quebec Longitudinal Study on Nutrition as a Determinant of Successful Aging study (67–84 y) | Dietary intake: average of 3, 24-h food recalls collected at baseline and at 2 y. Body composition: DXA | At baseline, men with the most balanced protein distribution (CV ≤ 0.33 au) had higher whole-body and appendicular lean mass at baseline than did those with the most unbalanced distribution (CV ≥ 0.67 au). No differences among women. At 2-y, a more balanced distribution was negatively associated with higher whole-body and appendicular lean mass in both men and women. Protein distribution was not associated with changes in lean tissue over 2 y |
| Farsijani et al. [26] | 3-y follow up in 827 men and 914 women from the Quebec Longitudinal Study on Nutrition as a Determinant of Successful Aging study (67–84 y) | Dietary intake: data from the 2-y follow-up. Hand, leg, and arm strength: dynamometers. Mobility: timed-up-and-go, chair stand, and walking speed tests | A more balanced distribution was associated with a higher muscle strength score at 2-y in men and women (β ± SE = −0.73 6 ± 0.20 and −0.66 ± 0.20, respectively). Similar negative associations were observed between protein distribution with handgrip and arm strengths. These associations were significant before and after adjustment for covariates in women and only before adjustment for covariates in men with a trend toward significance after adjustment. The association between leg strength and protein distribution was not significant in either sex. Protein distribution was not associated with the decline in composite and component mobility scores |
| Loenneke et al. [27] and Loprinzi et al. [28] | 1081 adults from the 1999–2002 NHANES cohort of (50–85 y) | Dietary intake: a 24-h dietary recall method. Leg lean mass: DXA. Knee extensor strength: dynamometer | Compared to 0 meals, consuming 1 and 2+ meals/d with ≥30 g of protein was associated with greater leg lean mass (1 vs. 0, β = 23.6; 2+ vs. 0, β = 51.1) and knee extensor strength (1 vs. 0, β = 1160; 2+ vs. 0, β = 2389) |
| Gayatán Gonález et al. [29] | 187 adults from Mexico (60–97 y) | Dietary intake: a 24-h dietary recall method on a single day. Functionality: questionnaire to determine ADL and IADL scores | Compared to 0 meals, consuming 2 or 3, but not 1, meals/d with ≥30 g protein was associated with lower risk of physical disability on transportation (OR [95% CI]: 0.06 [0.01–0.50]), shopping (0.05[0.01–0.40]), feeding (0.06 [0.01–0.74]), and transfer (0.09 [0.01–0.98]). Consuming 2 or 3, but not 1, meals/d with ≥0.4 g/kg was associated with lower risk of physical disability on shopping (0.21 [0.05–0.89]) and transportation (0.12 [0.03–0.48]) |
| Mishra et al. [30] | 4123 adults from the 2011–2014 NHANES cohort (≥51 y) | Dietary intake: a 24-h dietary recall method. Grip strength: hand dynamometer | Compared to 1 meal, consuming 2 and ≥3 meals containing ≥ 25 g protein was not associated with grip strength |
| Valenzuela et al. [31] | 78 adults from Mexico (68.7 ± 6.3 y) | Dietary intake: a 24-h dietary recall method on 3 non-consecutive days. Appendicular lean mass: DXA | After adjusting for weight, sex, and height, appendicular lean mass was not different between groups that consumed at least one meal containing ≥25 g of protein and those who did not |
| Gayatán Gonález et al. [32] | 190 adults from Mexico (53–97 y) | Dietary intake: a 24-h dietary recall method on a single day. Functionality: questionnaire to determine ADL and IADL | 30 g criterion: Low and middle ADL scores were associated with “inadequate” protein intake at lunch (low scores, OR = 3.82 [95% CI, 1.15–12.65]; middle scores, OR = 2.40 [1.03–5.62]). 0.4 g·kg−1 criterion: “Inadequate” protein intake at dinner was associated with middle IADL scores (OR = 7.64, [1.27–45.85]) |
| Yasuda et al. [33] | 233 adults from Japan (21.4 ± 2.4 y) | Dietary intake: photography on 3 non-consecutive days. Body composition: DXA | Total fat-free mass % was greater in those that consumed ≥0.24 g·kg−1 at three meals compared to those that did not consume ≥0.24 g·kg−1 in at least one meal (77.0 ± 0.5% vs. 75.2 ± 0.4%) |
| Protein, g (g/kg) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors | Study Design | Group 1 | n (f) | Age, y | Duration | Energy Status | Exercise Status | Meal Type | Protein Source (s) | Total | Breakfast | Lunch | Dinner | 4th Meal | Outcome |
| Mamerow et al. [34] | Cross-over | EVEN | 8 | 36.9 ± 3.1 | 24 h | EB | No RT | Whole-food | Animal and plant | 90 (1.17) | 30 (0.39) | 30 (0.39) | 30 (0.39) | 25% greater MPS in EVEN | |
| SKEW | 90 (1.17) | 10 (0.13) | 15 (0.20) | 65 (0.85) | |||||||||||
| Murphy et al. [36] | Cross-over within parallel | BAL | 10 | 65 ± 3 | 12 h | EB and ER phases | RT and No RT phases | Isolated intact protein | Whey micellar | 75 (0.77) | 25 (0.26) | 25 (0.26) | 25 (0.26) | 19% greater MPS in BAL in ER and ER + RT; no effect in EB | |
| SKEW | 10 | 66 ± 4 | 75 (0.78) | 10 (0.10) | 15 (0.16) | 50 (0.52) | |||||||||
| Kim et al. [35] | Parallel | RDA Even | 5 (4) | 66.4 ± 1.7 | 22 h | EB | No RT | Whole-food | Animal and plant | 65.8 (0.8) | 22.3 (0.3) | 21.5 (0.2) | 22 (0.3) | No effect | |
| RDA Uneven | 4 (1) | 64.0 ± 3.6 | 73.7 (0.8) | 11.1 (0.1) | 14.9 (0.2) | 47.8 (0.5) | |||||||||
| 2RDA Even | 5 (3) | 64.0 ± 2.7 | 112.4 (1.5) | 38 (0.5) | 36.5 (0.5) | 37.9 (0.5) | |||||||||
| 2RDA Uneven | 6 (2) | 68.4 ± 2.2 | 120.8 (1.4) | 18.1 (0.2) | 24.3 (0.3) | 78.4 (0.9) | |||||||||
| Kim et al. [43] | Parallel | Even | 7 (3) | 58.1 ± 2.4 | 23 h | EB | No RT | Whole-food | Animal and plant | (1.1) | (0.37) | (0.37) | (0.37) | No effect | |
| Uneven | 7 (5) | 60.3 ± 2.4 | (1.1) | (0.17) | (0.22) | (0.72) | |||||||||
| Murphy et al. [44] | Parallel | BAL | 10 | 66 ± 4 | 2 wk | ER | RT and No RT phases | Whole-food | Animal and plant | (1.3) | (0.33) | (0.33) | (0.33) | (0.33) | No effect |
| SKEW | 10 | (1.3) | (0.09) | (0.22) | (0.94) | (0.05) | |||||||||
| Authors | n (f) | Age, y | Protein, g·kg−1·d−1 | Meals, Number/d (g/kg/Meal) | Protein Sources | Adaptation/ Collection, d | Results |
|---|---|---|---|---|---|---|---|
| Arnal et al. [38] | 15 | 68 ± 1 | 1.05 | 3 (0.1/0.8/0.15) vs. 4 (0.22/0.33/0.2/0.3) | Whole-food animal and plant | 15/14 | Nitrogen balance was higher in 3 meals (unbalanced, 54 ± 7 mg/fat-free mass) vs. 4 meals (balanced, 27 ± 6 mg/fat-free mass) |
| Arnal et al. [39] | 16 | 26 ± 1 | 1.2 | 3 (0.08/0.95/0.17) vs. 4 (0.26/0.37/0.23/0.34) | Whole-food animal and plant | 15/14 | Nitrogen balance was 60% lower in 3 (unbalanced, 36 ± 8 mg/fat-free mass) vs. 4 meals (balanced, 59 ± 12 mg/fat-free mass) (p = 0.16) |
| Authors | Group 1 | n (f) | Age, y | Duration | Energy Status | Exercise Status | Protein Source(s) | Protein, g (g/kg) | Results | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Breakfast | Lunch | Snack | Dinner | |||||||||
| Bouillanne et al. [72] | Spread | 34 (23) | 85.7 (83.5–87.9) | 6 wk | None | None | Animal and plant | 69 (1.27) | 12.2 (0.25) | 21 (0.38) | 13.5 (0.25) | 21.1 (0.38) | Pulse feeding increased lean mass (0.91 [0–1.48]); spread feeding decreased lean mass (−0.41 [1.53–0.49]) |
| Pulse | 29 (23) | 84.1 (81.8–86.4) | 66 (1.31) | 4.5 (0.08) | 47.8 (1.02) | 2.3 (0.03) | 10.9 (0.14) | ||||||
| Kim et al. [43] | EVEN | 7 (3) | 58.1 ± 2.4 | 8 wk | EB | No RT | Animal and plant | 87.8 (1.1) | 29.3 (0.37) | 29.3 (0.37) | — | 29.2 (0.37) | No effect |
| UNEVEN | 7 (5) | 60.3 ± 2.4 | 86.4 (1.1) | 13.1 (0.16) | 17.7 (0.22) | — | 55.6 (0.7) | ||||||
| Adechian et al. [62] | Casein spread | 10 (8) | 35.1 ± 1.5 | 6 wk | ER | None | >80% casein | 87 (0.94) | 22 (0.24) | 22 (0.24) | 22 (0.24) | 22 (0.24) | No effect |
| Casein pulse | 10 (8) | 34.6 ± 1.4 | >80% casein | 87 (0.96) | 7 (0.08) | 70 (0.77) | 3 (0.04) | 7 (0.08) | |||||
| MSP spread | 11 (7) | 33.6 ± 1.8 | >80% MSP | 87 (0.93) | 22 (0.23) | 22 (0.23) | 22 (0.23 | 22 (0.23 | |||||
| MSP pulse | 10 (9) | 30.6 ± 2.3 | >80% MSP | 87 (1.01) | 7 (0.09) | 70 (0.80) | 3 (0.04) | 7 (0.09) | |||||
| Hudson et al. [74] | EVEN | 21 | 33 | 16 wk | ER | RT | 70% animal; 30% plant | 90 (1.1) | 30 (0.36) | 30 (0.36) | — | 30 (0.36) | No effect |
| SKEW | 20 | 36 | 90 (1.1) | 10 (0.12) | 20 (0.24) | — | 60 (0.71) | ||||||
| Yasuda [73] | Low-protein breakfast | 14 | 20.8 ± 0.4 | 12 wk | None | RT | Animal, plant, and supplement | 97.1 (1.45) | 7.7 (0.12) | 30 (0.45) | — | 55.4 (0.83) | Lean mass increases tended to be greater after consuming the high-protein breakfast (2.5 ± 0.3 kg) than after consuming the low-protein breakfast (1.8 ± 0.3 kg) (p = 0.06) |
| High-protein breakfast | 12 | 89.4 (1.3) | 22.6 (0.33) | 31.8 (0.46) | — | 32.4 (0.48) | |||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hudson, J.L.; Bergia, R.E.; Campbell, W.W. Protein Distribution and Muscle-Related Outcomes: Does the Evidence Support the Concept? Nutrients 2020, 12, 1441. https://doi.org/10.3390/nu12051441
Hudson JL, Bergia RE, Campbell WW. Protein Distribution and Muscle-Related Outcomes: Does the Evidence Support the Concept? Nutrients. 2020; 12(5):1441. https://doi.org/10.3390/nu12051441
Chicago/Turabian StyleHudson, Joshua L., Robert E. Bergia, and Wayne W. Campbell. 2020. "Protein Distribution and Muscle-Related Outcomes: Does the Evidence Support the Concept?" Nutrients 12, no. 5: 1441. https://doi.org/10.3390/nu12051441
APA StyleHudson, J. L., Bergia, R. E., & Campbell, W. W. (2020). Protein Distribution and Muscle-Related Outcomes: Does the Evidence Support the Concept? Nutrients, 12(5), 1441. https://doi.org/10.3390/nu12051441





