β2 Adrenergic Regulation of the Phagocytic and Microbicide Capacity of Circulating Monocytes: Influence of Obesity and Exercise
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experiment Design
2.2. Exercise Protocol
2.3. Anaesthesia, Whole Blood Collection, Fasting Glucose, and Lipid-Profile Determination
2.4. Effect of Terbutaline on Monocyte Innate Function
2.4.1. Phagocytic and Oxidative-Burst Assays via Flow Cytometry
2.4.2. Toll-Like Receptor 2 (TLR2) and 4 (TLR4) Surface Expression via Flow Cytometry
2.5. Statistical Analysis
3. Results
3.1. Body Weight, Dietary Intake, Fasting Blood Glucose, and Lipid Profile—Effect of Exercise
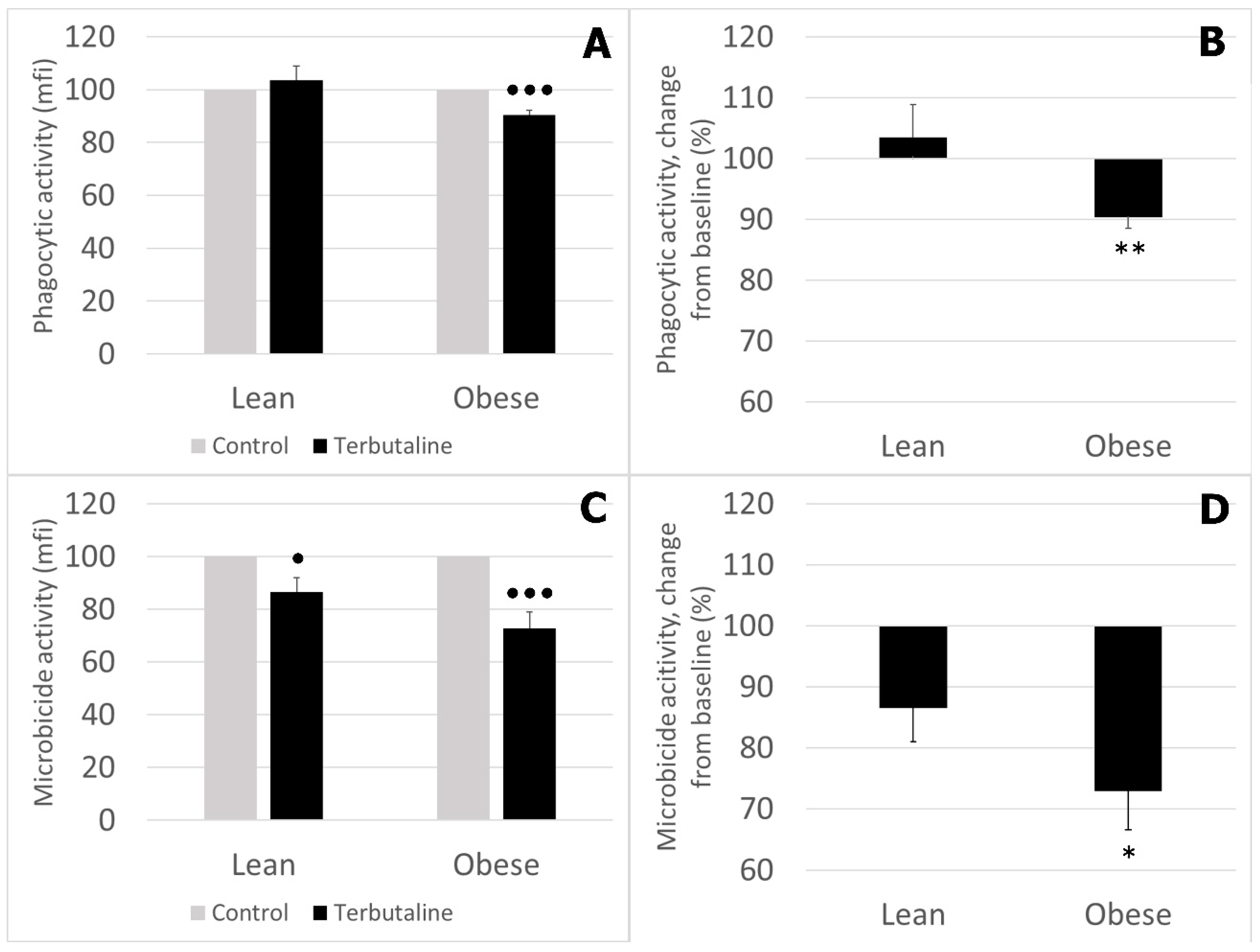
3.2. Effect of β2 Adrenergic Activation by Terbutaline on Phagocytic and Microbicide Capacities of Circulating Monocytes from Lean and Obese Mice
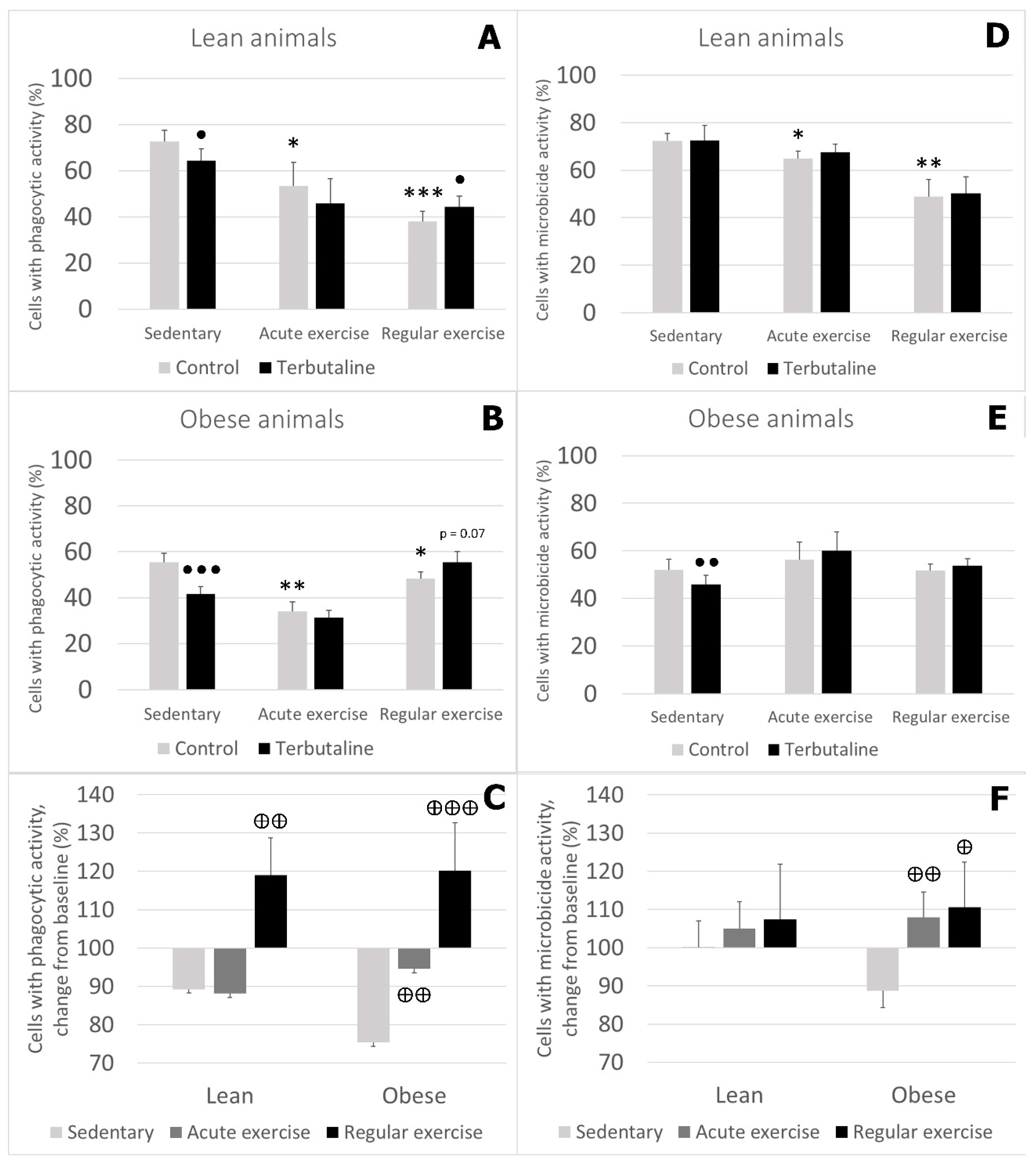
3.3. Effect of Exercise in β2 Adrenergic Regulation of Phagocytic and Microbicide Capacity of Circulating Monocytes from Obese and Lean Mice
3.4. Effect of Exercise in β2 Adrenergic Regulation of TLR2 and TLR4 Expression in Circulating Monocytes from Obese and Lean Mice
4. Discussion
5. Conclusions
- Obese sedentary animals present a reduced monocyte-mediated innate immune response compared to that of lean sedentary animals.
- The effect of β2 adrenergic activation on the innate function of monocytes from sedentary animals was globally inhibitory in lean and obese mice, although this inhibition was significantly greater in obese animals, probably reflecting the higher impairment of innate function in obese animals in response to β2 AR stimulation by terbutaline.
- Exercise stimulates phagocytic function in lean and obese mice, and microbicide function only in lean mice. Therefore, the beneficial effects of exercise are stronger in lean animals.
- Exercise, especially regular exercise, modifies β2 adrenergic regulation of the innate response of monocytes from lean and obese mice, with a global stimulatory or neutral effect, thus abolishing the inhibitory effect occurring in sedentary animals. This behaviour was similar in lean and obese animals but was stronger in obese ones.
- Changes in TLR expression seem to partially explain some innate-/inflammatory-response variations related to obesity, exercise, and β2 AR stimulation.
- In general, terbutaline seems to not hinder the effects of regular exercise, but regular exercise does abolish the effects of terbutaline (different effects in sedentary vs. exercised).
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vecchié, A.; Dallegri, F.; Carbone, F.; Bonaventura, A.; Liberale, L.; Portincasa, P.; Frühbeck, G.; Montecucco, F. Obesity phenotypes and their paradoxical association with cardiovascular diseases. Eur. J. Intern. Med. 2018, 48, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.; O’Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Is metabolic syndrome X an inflammatory condition? Exp. Biol. Med. 2002, 227, 989–997. [Google Scholar] [CrossRef]
- Das, U.N. Metabolic syndrome X: An inflammatory condition? Curr. Hypertens. Rep. 2004, 6, 66–73. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Johnson, A.R.; Milner, J.J.; Makowski, L. The inflammation highway: Metabolism accelerates inflammatory traffic in obesity. Immunol. Rev. 2012, 249, 218–238. [Google Scholar] [CrossRef]
- Lumeng, C.N. Innate Immune Activation in Obesity. Mol. Aspects Med. 2013, 34, 12–29. [Google Scholar] [CrossRef]
- Petersen, A.M.; Pedersen, B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef]
- Gálvez, I.; Martín-Cordero, L.; Hinchado, M.D.; Álvarez-Barrientos, A.; Ortega, E. Anti-inflammatory effect of β2 adrenergic stimulation on circulating monocytes with a pro-inflammatory state in high-fat diet-induced obesity. Brain Behav. Immun. 2019, 80, 564–572. [Google Scholar] [CrossRef]
- Wellen, K.E.; Hotamisligil, G.S. Inflammation, stress, and diabetes. J. Clin. Investig. 2005, 115, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Bastard, J.P.; Maachi, M.; Lagathu, C.; Kim, M.J.; Caron, M.; Vidal, H.; Capeau, J.; Feve, B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur. Cytokine Netw. 2006, 17, 4–12. [Google Scholar] [PubMed]
- Mraz, M.; Haluzik, M. The role of adipose tissue immune cells in obesity and low-grade inflammation. J. Endocrinol. 2014, 222, R113–R127. [Google Scholar] [CrossRef] [PubMed]
- Reilly, S.M.; Saltiel, A.R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 2017, 13, 633–643. [Google Scholar] [CrossRef]
- Pasquali, R.; Vicennati, V.; Cacciari, M.; Pagotto, U. The hypothalamic–pituitary–adrenal axis activity in obesity and the metabolic syndrome. Ann. N. Y. Acad. Sci. 2006, 1083, 111–128. [Google Scholar] [CrossRef]
- Lambert, G.W.; Straznicky, N.E.; Lambert, E.A.; Dixon, J.B.; Schlaich, M.P. Sympathetic nervous activation in obesity and the metabolic syndrome—Causes, consequences and therapeutic implications. Pharmacol. Ther. 2010, 126, 159–172. [Google Scholar] [CrossRef]
- Ortega, E.; Martín-Cordero, L.; García-Roves, P.M.; Chicco, A.J.; González-Franquesa, A.; Marado, D. Diabetes Mellitus and Metabolic Syndrome. In Biomarkers of Cardiometabolic Risk, Inflammation and Disease; Palavra, F., Reis, F., Marado, D., Sena, A., Eds.; Springer: Cham, Switzerland, 2015; pp. 55–80. [Google Scholar]
- Martín-Cordero, L.; García, J.J.; Hinchado, M.D.; Ortega, E. The interleukin-6 and noradrenaline mediated inflammation-stress feedback mechanism is dysregulated in metabolic syndrome: Effect of exercise. Cardiovasc. Diabetol. 2011, 10, 42. [Google Scholar] [CrossRef]
- Martín-Cordero, L.; García, J.J.; Ortega, E. Noradrenaline-mediated inhibition of inflammatory cytokines is altered in macrophages from obese Zucker rats: Effect of habitual exercise. Endocr. Metab. Immune Disord. Drug Targets 2013, 13, 234–239. [Google Scholar] [CrossRef]
- Straznicky, N.E.; Lambert, E.A.; Nestel, P.J.; McGrane, M.T.; Dawood, T.; Schlaich, M.P.; Masuo, K.; Eikelis, N.; de Courten, B.; Mariani, J.A.; et al. Sympathetic Neural Adaptation to Hypocaloric Diet with or without Exercise Training in Obese Metabolic Syndrome Subjects. Diabetes 2010, 59, 71–79. [Google Scholar] [CrossRef]
- Straznicky, N.E.; Lambert, G.W.; Lambert, E.A. Neuroadrenergic Dysfunction in Obesity: An Overview of the Effects of Weight Loss. Curr. Opin. Lipidol. 2010, 21, 21–30. [Google Scholar] [CrossRef]
- Ortega, E.; García, J.J.; De la Fuente, M. Modulation of adherence and chemotaxis of macrophages by norepinephrine. Influ. Ageing Mol. Cell. Biochem. 2000, 203, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Elenkov, I.J.; Chrousos, G.P. Stress hormones, proinflammatory and anti-inflammatory cytokines, and autoimmunity. Ann. N. Y. Acad. Sci. 2002, 966, 290–303. [Google Scholar] [CrossRef] [PubMed]
- García, J.J.; Sáez, M.C.; De la Fuente, M.; Ortega, E. Regulation of phagocytic process of macrophages by noradrenaline and its end metabolite 4-hydroxy-3-metoxyphenyl-glycol. Role of alpha- and beta-adrenoreceptors. Mol. Cell. Biochem. 2003, 254, 299–304. [Google Scholar] [CrossRef]
- Besedovsky, H.O.; Del Rey, A. Physiology of psychoneuroimmunology: A personal view. Brain Behav. Immun. 2007, 21, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, D.L.; Lorton, D. Autonomic regulation of cellular immune function. Auton. Neurosci. 2014, 182, 15–41. [Google Scholar] [CrossRef]
- Ortega, E.; Gálvez, I.; Martín-Cordero, L. Adrenergic Regulation of Macrophage-Mediated Innate/Inflammatory Responses in Obesity and Exercise in this Condition: Role of β2 Adrenergic Receptors. Endocr. Metab. Immune Disord. Drug Targets 2019. [Google Scholar] [CrossRef]
- Ortega, E. Neuroendocrine Mediators in the Modulation of Phagocytosis by Exercise: Physiological Implications. Exerc. Immunol. Rev. 2003, 9, 70–93. [Google Scholar]
- Ortega, E. The “Bioregulatory Effect of Exercise” on the Innate/Inflammatory Responses. J. Physiol. Biochem. 2016, 72, 361–369. [Google Scholar] [CrossRef]
- Ortega, E.; Giraldo, E.; Hinchado, M.D.; Martín, L.; García, J.J.; De La Fuente, M. Neuroimmunomodulation during Exercise: Role of Catecholamines as “stress Mediator” and/or “Danger Signal” for the Innate Immune Response. Neuroimmunomodulation 2007, 14, 206–212. [Google Scholar] [CrossRef]
- Landmann, R. Beta-adrenergic receptors in human leukocyte subpopulations. Eur. J. Clin. Investig. 1992, 22, 30–36. [Google Scholar]
- Elenkov, I.J.; Wilder, R.L.; Chrousos, G.P.; Vizi, E.S. The sympathetic nerve—An integrative interface between two supersystems: The brain and the immune system. Pharmacol. Rev. 2000, 52, 595–638. [Google Scholar] [PubMed]
- Sanders, V.M.; Kavelaars, A. Adrenergic regulation of immunity. In Psychoneuroimmunology; Ader, R., Ed.; Academic Press: Amsterdam, The Netherlands, 2007; pp. 63–84. [Google Scholar]
- Scanzano, A.; Cosentino, M. Adrenergic regulation of innate immunity: A review. Front. Pharmacol. 2015, 6, 171. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, I.; Martín-Cordero, L.; Hinchado, M.D.; Álvarez-Barrientos, A.; Ortega, E. Obesity Affects β2 Adrenergic Regulation of the Inflammatory Profile and Phenotype of Circulating Monocytes from Exercised Animals. Nutrients 2019, 11, 2630. [Google Scholar] [CrossRef] [PubMed]
- Martín-Cordero, L.; Gálvez, I.; Hinchado, M.D.; Ortega, E. β2 Adrenergic Regulation of the Phagocytic and Microbicide Capacity of Macrophages from Obese and Lean Mice: Effects of Exercise. Nutrients 2019, 11, 2721. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef]
- Dimitrov, S.; Hulteng, E.; Hong, S. Inflammation and exercise: Inhibition of monocytic intracellular TNF production by acute exercise via β(2)-adrenergic activation. Brain Behav. Immun. 2017, 61, 60–68. [Google Scholar] [CrossRef]
- Kim, T.W.; Choi, H.H.; Chung, Y.R. Treadmill exercise alleviates impairment of cognitive function by enhancing hippocampal neuroplasticity in the high-fat diet-induced obese mice. J. Exerc. Rehabil. 2016, 12, 156–162. [Google Scholar] [CrossRef]
- Petrosino, J.M.; Heiss, V.J.; Maurya, S.K.; Kalyanasundaram, A.; Periasamy, M.; LaFountain, R.A.; Wilson, J.M.; Simonetti, O.P.; Ziouzenkova, O. Graded Maximal Exercise Testing to Assess Mouse Cardio-Metabolic Phenotypes. PLoS ONE 2016, 11, e0148010. [Google Scholar] [CrossRef]
- Martín-Cordero, L.; Reis, F.; García, J.J.; Teixeira, F.; Ortega, E. Effect of exercise without diet on functional capacity of peritoneal macrophages and TNF-a levels in blood and in adipose tissue in the obese Zucker rat model of the metabolic syndrome. Proc. Nutr. Soc. 2013, 72, E76. [Google Scholar] [CrossRef]
- Strandberg, L.; Verdrengh, M.; Enge, M.; Andersson, N.; Amu, S.; Onnheim, K.; Benrick, A.; Brisslert, M.; Bylund, J.; Bokarewa, M.; et al. Mice Chronically Fed High-Fat Diet Have Increased Mortality and Disturbed Immune Response in Sepsis. PLoS ONE 2009, 4, e7605. [Google Scholar] [CrossRef]
- Amar, S.; Zhou, Q.; Shaik-Dasthagirisaheb, Y.; Leeman, S. Diet-Induced Obesity in Mice Causes Changes in Immune Responses and Bone Loss Manifested by Bacterial Challenge. Proc. Natl. Acad. Sci. USA 2007, 104, 20466–20471. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.J.; Murphy, K.E.; Fernandez, M.L. Impact of Obesity and Metabolic Syndrome on Immunity. Adv. Nutr. 2016, 7, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Schopf, R.E.; Lemmel, E.M. Control of the production of oxygen intermediates of human polymorphonuclear leukocytes and monocytes by beta-adrenergic receptors. J. Immunopharmacol. 1983, 5, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Ortega, E.; Giraldo, E.; Hinchado, M.D.; Martín-Cordero, L.; García, J.J. 72 KDa Extracellular Heat Shock Protein (EHsp72), Norepinephrine (NE), and the Innate Immune Response Following Moderate Exercise. In Heat Shock Proteins and Whole Body Physiology; Asea, A., Pederser, B.-K., Eds.; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; London, UK; New York, NY, USA, 2010; pp. 327–350. [Google Scholar]
- Galvan, D.L.; Danesh, F.R. Β2-Adrenergic Receptors in Inflammation and Vascular Complications of Diabetes. Kidney Int. 2017, 92, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.; Yu, M.R.; Kim, H.J.; Lee, J.H.; Park, B.-W.; Wu, I.-H.; Matsumoto, M.; King, G.L. Beta 2-Adrenergic Receptor Agonists Are Novel Regulators of Macrophage Activation in Diabetic Renal and Cardiovascular Complications. Kidney Int. 2017, 92, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, D.B.; Shepherd, S.O.; Wilson, O.J.; Adlan, A.M.; Wagenmakers, A.J.M.; Shaw, C.S.; Lord, J.M. Neutrophil and Monocyte Bactericidal Responses to 10 Weeks of Low-Volume High-Intensity Interval or Moderate-Intensity Continuous Training in Sedentary Adults. Oxid. Med. Cell Longev. 2017, 2017, 8148742. [Google Scholar] [CrossRef]
- Ortega Rincón, E. Physiology and biochemistry: Influence of exercise on phagocytosis. Int. J. Sports Med. 1994, 15, S172–S178. [Google Scholar] [CrossRef]
- Ortega, E.; Collazos, M.E.; Barriga, C.; de la Fuente, M. Effect of Physical Activity Stress on the Phagocytic Process of Peritoneal Macrophages from Old Guinea Pigs. Mech. Ageing Dev. 1992, 65, 157–165. [Google Scholar] [CrossRef]
- Ortega, E.; Forner, M.A.; Barriga, C.; De La Fuente, M. Effect of Age and of Swimming-Induced Stress on the Phagocytic Capacity of Peritoneal Macrophages from Mice. Mech. Ageing Dev. 1993, 70, 53–63. [Google Scholar] [CrossRef]
- Girard, M.T.; Hjaltadottir, S.; Fejes-Toth, A.N.; Guyre, P.M. Glucocorticoids enhance the gamma interferon augmentation of human monocyte immunoglobulin G Fc receptor expression. J. Immunol. 1987, 138, 3235–3241. [Google Scholar]
- Petroni, K.C.; Shen, L.; Guyre, P.M. Modulation of human polymorphonuclear leukocyte IgG Fc receptors and Fc receptor-mediated functions by IFN-gamma and glucocorticoids. J. Immunol. 1988, 140, 3467–3472. [Google Scholar] [PubMed]
- Backman, K.A.; Guyre, P.M. Gamma-interferon inhibits Fc receptor II-mediated phagocytosis of tumor cells by human macrophages. Cancer Res. 1994, 54, 2456–2461. [Google Scholar] [PubMed]
- Jialal, I.; Kaur, H.; Devaraj, S. Toll-like receptor status in obesity and metabolic syndrome: A translational perspective. J. Clin. Endocrinol. Metab. 2014, 99, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Rogero, M.M.; Calder, P.C. Obesity, Inflammation, Toll-Like Receptor 4 and Fatty Acids. Nutrients 2018, 10, 432. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, E.; Martin-Cordero, L.; Garcia, J.J.; Gehrmann, M.; Multhoff, G.; Ortega, E. Exercise-induced extracellular 72 kDa heat shock protein (Hsp72) stimulates neutrophil phagocytic and fungicidal capacities via TLR-2. Eur. J. Appl. Physiol. 2010, 108, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Schimunek, L.; Serve, R.; Teuben, M.; Störmann, P.; Auner, B.; Woschek, M.; Pfeifer, R.; Horst, K.; Simon, T.P.; Kalbitz, M.; et al. Early decreased TLR2 expression on monocytes is associated with their reduced phagocytic activity and impaired maturation in a porcine polytrauma model. PLoS ONE 2017, 12, e0187404. [Google Scholar] [CrossRef]
- Doyle, S.E.; O’Connell, R.M.; Miranda, G.A.; Vaidya, S.A.; Chow, E.K.; Liu, P.T.; Suzuki, S.; Suzuki, N.; Modlin, R.L.; Yeh, W.C.; et al. Toll-like receptors induce a phagocytic gene program through p38. J. Exp. Med. 2004, 199, 81–90. [Google Scholar] [CrossRef]
- Asea, A.; Rehli, M.; Kabingu, E.; Boch, J.A.; Bare, O.; Auron, P.E.; Stevenson, M.A.; Calderwood, S.K. Novel signal transduction pathway utilized by extracellular HSP70: Role of toll-like receptor (TLR) 2 and TLR4. J. Biol. Chem. 2002, 277, 15028–15034. [Google Scholar] [CrossRef]
- Collao, N.; Rada, I.; Francaux, M.; Deldicque, L.; Zbinden-Foncea, H. Anti-Inflammatory Effect of Exercise Mediated by Toll-Like Receptor Regulation in Innate Immune Cells—A Review. Int. Rev. Immunol. 2020, 39, 39–52. [Google Scholar] [CrossRef]
- Rada, I.; Deldicque, L.; Francaux, M.; Zbinden-Foncea, H. Toll like receptor expression induced by exercise in obesity and metabolic syndrome: A systematic review. Exerc. Immunol. Rev. 2018, 24, 60–71. [Google Scholar]
- Grisanti, L.A.; Evanson, J.; Marchus, E.; Jorissen, H.; Woster, A.P.; DeKrey, W.; Sauter, E.R.; Combs, C.K.; Porter, J.E. Pro-inflammatory responses in human monocytes are beta1-adrenergic receptor subtype dependent. Mol. Immunol. 2010, 47, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
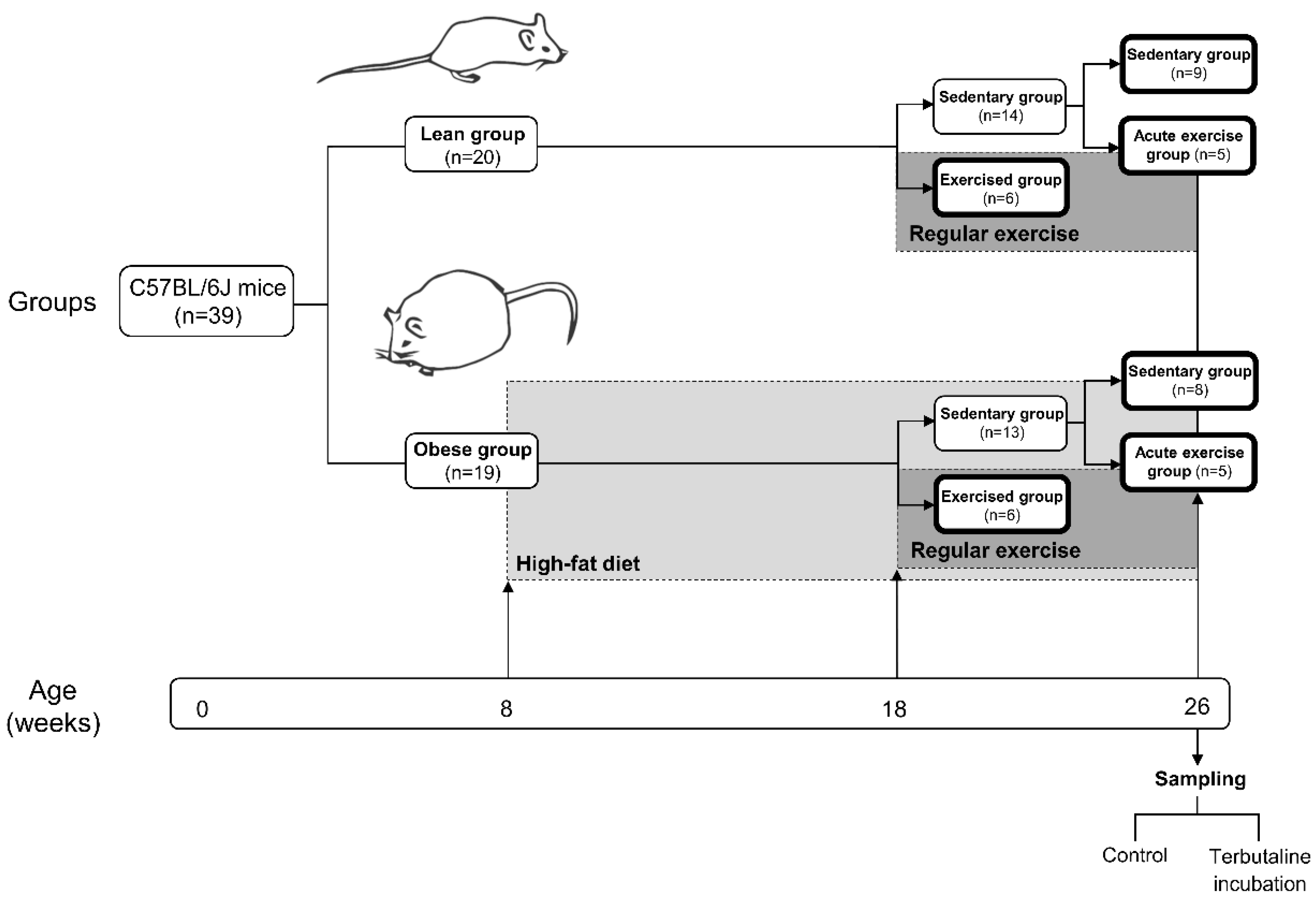

| Lean | Obese | |||||
|---|---|---|---|---|---|---|
| Sedentary | Acute Exercise | Regular Exercise | Sedentary | Acute Exercise | Regular Exercise | |
| Body weight (g) | 29.28 ± 1.17 | 30.15 ± 2.49 | 25.7 ± 1.27 | 42.28 ± 1.15*** | 40.37 ± 2.93 | 36.14 ± 2.98● |
| Dietary intake (g/day) | 3.96 ± 0.22 | 4.16 ± 0.09 | 4.05 ± 0.06 | 2.68 ± 0.11*** | 2.62 ± 0.08 | 2.49 ± 0.03●● |
| Energy intake (kJ/day) | 55.32 ± 3.15 | 58.16 ± 1.38 | 56.63 ± 0.86 | 62.01 ± 2.67*** | 60.50 ± 1.90 | 57.63 ± 0.71●● |
| Glucose (mg/dL) | 218.90 ± 13.26 | 174.45 ± 32.06● | 196.37 ± 25.53 | 311.50 ± 30.93** | 222.75 ± 23.12● | 282.5 ± 27.85 |
| Cholesterol (mg/dL) | ||||||
| TC | 103.69 ± 2.22 | <99† | 106.75 ± 2.90 | 172.70 ± 19.28*** | 175 ± 41.82 | 178.12 ± 24.68 |
| HDL-C | 42.15 ± 2.93 | - | 51.75 ± 3.91● | 59.70 ± 5.69** | 55.5 ± 2.72 | 75.25 ± 4.39● |
| cLDL-C | 50.75 ± 3.49 | - | 39.4 ± 1.74● | 88.83 ± 16.05* | 78 ± 12 | 38.5 ± 1.5● |
| TG (mg/dL) | 86.80 ± 1.86 | 88 ± 0.01 | 76.62 ± 0.73● | 91.55 ± 1.99* | 98.75 ± 7 | 80 ± 1.43●●● |
| Control | Terbutaline | Terbutaline + Butaxamine | ||
|---|---|---|---|---|
| Phagocytic percentage (%) | Lean | 72.54 ± 4.98 | 64.42 ± 4.98• | 70.02 ± 6.43◊◊ |
| Obese | 55.41 ± 3.95** | 41.63 ± 3.26••• | 48.85 ± 3.15◊ | |
| Microbicide percentage (%) | Lean | 72.32 ± 3.07 | 72.60 ± 6.35 | 70.61 ± 5.95 |
| Obese | 52.07 ± 4.48*** | 45.92 ± 3.90•• | 59.59 ± 4.05◊◊ | |
| Without Terbutaline (Control) | With Terbutaline | ||||||
|---|---|---|---|---|---|---|---|
| Sedentary | Acute Exercise | Regular Exercise | Sedentary | Acute Exercise | Regular Exercise | ||
| TLR2+ monocytes (%) | Lean | 78.35 ± 3.62 | 67.77 ± 1.36•• | 63.59 ± 6.21• | 64.64 ± 6.07◊ | 66.09 ± 1.40 | 61.59 ± 6.84 |
| Obese | 64.18 ± 8.01* | 77.86 ± 1.41• | 62.35 ± 7.78 | 64.57 ± 11.30 | 69.28 ± 8.90 | 63.91 ± 6.71 | |
| TLR4+ monocytes (%) | Lean | 79.50 ± 5.10 | 88.58 ± 3.28 | 71.89 ± 4.87 | 55.35 ± 10.77◊ | 74.01 ± 5.10◊◊◊ | 83.35 ± 3.06◊ |
| Obese | 91.16 ± 1.76** | 85.26 ± 2.74• | 76.53 ± 5.35•• | 67.33 ± 10.81◊ | 82.11 ± 1.45 | 79.66 ± 5.98 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gálvez, I.; Martín-Cordero, L.; Hinchado, M.D.; Ortega, E. β2 Adrenergic Regulation of the Phagocytic and Microbicide Capacity of Circulating Monocytes: Influence of Obesity and Exercise. Nutrients 2020, 12, 1438. https://doi.org/10.3390/nu12051438
Gálvez I, Martín-Cordero L, Hinchado MD, Ortega E. β2 Adrenergic Regulation of the Phagocytic and Microbicide Capacity of Circulating Monocytes: Influence of Obesity and Exercise. Nutrients. 2020; 12(5):1438. https://doi.org/10.3390/nu12051438
Chicago/Turabian StyleGálvez, Isabel, Leticia Martín-Cordero, María Dolores Hinchado, and Eduardo Ortega. 2020. "β2 Adrenergic Regulation of the Phagocytic and Microbicide Capacity of Circulating Monocytes: Influence of Obesity and Exercise" Nutrients 12, no. 5: 1438. https://doi.org/10.3390/nu12051438
APA StyleGálvez, I., Martín-Cordero, L., Hinchado, M. D., & Ortega, E. (2020). β2 Adrenergic Regulation of the Phagocytic and Microbicide Capacity of Circulating Monocytes: Influence of Obesity and Exercise. Nutrients, 12(5), 1438. https://doi.org/10.3390/nu12051438






