Apple Flavonols Mitigate Adipocyte Inflammation and Promote Angiogenic Factors in LPS- and Cobalt Chloride-Stimulated Adipocytes, in Part by a Peroxisome Proliferator-Activated Receptor-γ-Dependent Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Differentiation
2.2. Treatments
2.3. RAW 264.7 Macrophages Treated with LPS + CoCl2-Stimulated Culture Conditioned Media
2.4. mRNA and Secreted Protein Analyses
2.5. Secreted Protein Analysis
2.6. ROS Accumulation
2.7. NF-κB p65 Activation
2.8. Cellular Apoptotic Protein Analysis
2.9. Statistical Analysis
3. Results
3.1. PT and PZ Modulate Adipokine mRNA Expression in LPS-Stimulated Adipocytes, in Part by a PPAR-γ-Dependent Mechanism
3.2. PT and PZ Modulate Adipokine mRNA Expression in CoCl2-Stimulated Adipocytes, in Part by a PPAR-γ-Dependent Mechanism
3.3. PT but not PZ Modulates Adipokine mRNA Expression in LPS + CoCl2-Stimulated Adipocytes, in Part by a PPAR-γ-Dependent Mechanism
3.4. PT but not PZ reduced ROS Accumulation and NF-κB Activation in LPS + CoCl2-Stimulated Adipocytes, in Part by a PPAR-γ-Dependent Mechanism
3.5. PT but not PZ Modulates Cellular Regulators of Apoptosis in LPS + CoCl2-Stimulated Adipocytes, in Part by a PPAR-γ-Dependent Mechanism
3.6. PT and PZ Treatment of LPS + CoCl2-Stimulated Adipocytes in Turn Modulate Macrophage Expression of M1 and M2 Polarization Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hammarstedt, A.; Gogg, S.; Hedjazifar, S.; Nerstedt, A.; Smith, U. Impaired Adipogenesis and Dysfunctional Adipose Tissue in Human Hypertrophic Obesity. Physiol. Rev. 2018, 98, 1911–1941. [Google Scholar] [CrossRef] [PubMed]
- Meijer, K.; de Vries, M.; Al-Lahham, S.; Bruinenberg, M.; Weening, D.; Dijkstra, M.; Kloosterhuis, N.; van der Leij, R.J.; van der Want, H.; Kroesen, B.J.; et al. Human primary adipocytes exhibit immune cell function; adipocytes prime inflammation independent of macrophages. PLoS ONE 2011, 6, e17154. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Investig. 2006, 116, 3015–3025. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, U.; Cao, Q.; Yilmaz, E.; Lee, A.H.; Iwakoshi, N.N.; Ozdelen, E.; Tuncman, G.; Görgün, C.; Glimcher, L.H.; Hotamisligil, G.S. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004, 306, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 175–176. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Trayhurn, P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol. Rev. 2013, 93, 1–21. [Google Scholar] [CrossRef]
- Skurk, T.; Alberti-Huber, C.; Herder, C.; Hauner, H. Relationship between adipocyte size and adipokine expression and secretion. J. Clin. Endocrinol. Metab. 2007, 92, 1023–1033. [Google Scholar] [CrossRef]
- Lolmède, K.; de Saint Front, V.D.; Galitzky, J.; Lafontan, M.; Bouloumié, A. Effects of hypoxia on the expression of proangiogenic factors in differentiated 3T3-F442A adipocytes. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wood, I.S.; Trayhurn, P. Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflugers Arch. 2007, 455, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Gao, Z.; Yin, J.; He, Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E1118–E1128. [Google Scholar] [CrossRef] [PubMed]
- Pasarica, M.; Sereda, O.R.; Redman, L.M.; Albarado, D.C.; Hymel, D.T.; Roan, L.E.; Rood, J.C.; Burk, D.H.; Smith, S.R. Reduced adipose tissue oxygenation in human obesity: Evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 2009, 58, 718–725. [Google Scholar] [CrossRef]
- Giordano, A.; Murano, I.; Mondini, E.; Perugini, J.; Smorlesi, A.; Severi, I.; Barazzoni, R.; Scherer, P.E.; Cinti, S. Obese adipocytes show ultrastructural features of stressed cells and die of pyroptosis. J. Lipid Res. 2013, 54, 2423–2436. [Google Scholar] [CrossRef]
- Cinti, S.; Mitchell, G.; Barbatelli, G.; Murano, I.; Ceresi, E.; Faloia, E.; Wang, S.; Fortier, M.; Greenberg, A.S.; Obin, M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005, 46, 2347–2355. [Google Scholar] [CrossRef]
- Lee, B.C.; Lee, J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim. Biophys. Acta 2014, 1842, 446–462. [Google Scholar] [CrossRef]
- Arts, I.C.; Hollman, P.C. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81, 317S–325S. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef] [PubMed]
- Hyson, D.A. A comprehensive review of apples and apple components and their relationship to human health. Adv. Nutr. 2011, 2, 408–420. [Google Scholar] [CrossRef]
- Vrhovsek, U.; Rigo, A.; Tonon, D.; Mattivi, F. Quantitation of polyphenols in different apple varieties. J. Agric. Food Chem. 2004, 52, 6532–6538. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Yazidi, C.E.; Landrier, J.F.; Lairon, D.; Margotat, A.; Amiot, M.J. Phloretin enhances adipocyte differentiation and adiponectin expression in 3T3-L1 cells. Biochem. Biophys. Res. Commun. 2007, 361, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Gealekman, O.; Burkart, A.; Chouinard, M.; Nicoloro, S.M.; Straubhaar, J.; Corvera, S. Enhanced angiogenesis in obesity and in response to PPARgamma activators through adipocyte VEGF and ANGPTL4 production. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1056–E1064. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Yazidi, C.E.; Malezet-Desmoulins, C.; Amiot, M.J.; Margotat, A. Gene expression profiling of 3T3-L1 adipocytes exposed to phloretin. J. Nutr. Biochem. 2010, 21, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.T.; Huang, W.C.; Liou, C.J. Evaluation of the anti-inflammatory effects of phloretin and phlorizin in lipopolysaccharide-stimulated mouse macrophages. Food Chem. 2012, 134, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Chang, W.T.; Wu, S.J.; Xu, P.Y.; Ting, N.C.; Liou, C.J. Phloretin and phlorizin promote lipolysis and inhibit inflammation in mouse 3T3-L1 cells and in macrophage-adipocyte co-cultures. Mol. Nutr. Food Res. 2013, 57, 1803–1813. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, C.; Lai, X.; Hou, S.; Zeng, X.; Li, X. Immunomodulatory activities of phlorizin metabolites in lipopolysaccharide-stimulated RAW264.7 cells. Biomed. Pharmacother. 2017, 91, 49–53. [Google Scholar] [CrossRef]
- Shin, S.K.; Cho, S.J.; Jung, U.J.; Ryu, R.; Choi, M.S. Phlorizin supplementation attenuates obesity, inflammation, and hyperglycemia in diet-induced obese mice fed a high-fat diet. Nutrients 2016, 8, 92. [Google Scholar] [CrossRef]
- Alsanea, S.; Gao, M.; Liu, D. Phloretin prevents high-fat diet-induced obesity and improves metabolic homeostasis. AAPS J. 2017, 19, 797–805. [Google Scholar]
- Cranmer-Byng, M.M.; Liddle, D.M.; De Boer, A.A.; Monk, J.M.; Robinson, L.E. Proinflammatory effects of arachidonic acid in a lipopolysaccharide-induced inflammatory microenvironment in 3T3-L1 adipocytes in vitro. Appl. Physiol. Nutr. Metab. 2015, 40, 142–154. [Google Scholar] [CrossRef]
- Crespy, V.; Aprikian, O.; Morand, C.; Besson, C.; Manach, C.; Demigné, C.; Rémésy, C. Bioavailability of phloretin and phloridzin in rats. J. Nutr. 2001, 131, 3227–3230. [Google Scholar] [CrossRef] [PubMed]
- Creely, S.J.; McTernan, P.G.; Kusminski, C.M.; Fisher, F.M.; Da Silva, N.F.; Khanolkar, M.; Evans, M.; Harte, A.L.; Kumar, S. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E740–E747. [Google Scholar] [CrossRef] [PubMed]
- Oster, R.T.; Tishinsky, J.M.; Yuan, Z.; Robinson, L.E. Docosahexaenoic acid increases cellular adiponectin mRNA and secreted adiponectin protein, as well as PPARγ mRNA, in 3T3-L1 adipocytes. Appl. Physiol. Nutr. Metab. 2010, 35, 783–789. [Google Scholar] [CrossRef] [PubMed]
- De Boer, A.A.; Monk, J.M.; Robinson, L.E. Docosahexaenoic acid decreases pro-inflammatory mediators in an in vitro murine adipocyte macrophage co-culture model. PLoS ONE 2014, 9, e85037. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, J.W.; Osborne, O.; Oh, D.Y.; Sasik, R.; Schenk, S.; Chen, A.; Chung, H.; Murphy, A.; Watkins, S.M.; et al. Increased adipocyte O2 consumption triggers HIF-1α, causing inflammation and insulin resistance in obesity. Cell 2014, 157, 1339–1352. [Google Scholar] [CrossRef]
- Sekhon-Loodu, S.; Ziaullah; Rupasinghe, H. P. Docosahexaenoic acid ester of phloridzin inhibit lipopolysaccharide-induced inflammation in THP-1 differentiated macrophages. Int. Immunopharmacol. 2015, 25, 199–206. [Google Scholar] [CrossRef]
- Lauren, D.R.; Smith, W.A.; Adaim, A.; Cooney, J.M.; Wibisono, R.; Jensen, D.J.; Zhang, J.; Skinner, M.A. Chemical composition and in vitro anti-inflammatory activity of apple phenolic extracts and of their sub-fractions. Int. J. Food Sci. Nutr. 2009, 60 (Suppl. 7), 188–205. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Maury, E.; Noël, L.; Detry, R.; Brichard, S.M. In vitro hyperresponsiveness to tumor necrosis factor-alpha contributes to adipokine dysregulation in omental adipocytes of obese subjects. J. Clin. Endocrinol. Metab. 2009, 94, 1393–1400. [Google Scholar] [CrossRef]
- Jung, S.H.; Park, S.Y.; Kim-Pak, Y.; Lee, H.K.; Park, K.S.; Shin, K.H.; Ohuchi, K.; Shin, H.K.; Keum, S.R.; Lim, S.S. Synthesis and PPAR-gamma ligand-binding activity of the new series of 2’-hydroxychalcone and thiazolidinedione derivatives. Chem. Pharm. Bull. (Tokyo) 2006, 54, 368–371. [Google Scholar] [CrossRef]
- Rezk, B.M.; Haenen, G.R.; van der Vijgh, W.J.; Bast, A. The antioxidant activity of phloretin: The disclosure of a new antioxidant pharmacophore in flavonoids. Biochem. Biophys. Res. Commun. 2002, 295, 9–13. [Google Scholar] [CrossRef]
- Yang, Q.; Han, L.; Li, J.; Xu, H.; Liu, X.; Wang, X.; Pan, C.; Lei, C.; Chen, H.; Lan, X. Activation of nrf2 by phloretin attenuates palmitic acid-induced endothelial cell oxidative stress via ampk-dependent signaling. J. Agric. Food Chem. 2019, 67, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Halberg, N.; Khan, T.; Trujillo, M.E.; Wernstedt-Asterholm, I.; Attie, A.D.; Sherwani, S.; Wang, Z.V.; Landskroner-Eiger, S.; Dineen, S.; Magalang, U.J.; et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol. Cell. Biol. 2009, 29, 4467–4483. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, L.; Pereira, J.A.D.S.; Palhinha, L.; Moraes-Vieira, P.M.M. Leptin in the regulation of the immunometabolism of adipose tissue-macrophages. J. Leukoc. Biol. 2019, 106, 703–716. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, R.W.; White, A.E.; Metcalf, M.D.; Olivas, A.S.; Mitra, P.; Larison, W.G.; Cheang, E.C.; Varlamov, O.; Corless, C.L.; Roberts, C.T.; et al. Hypoxia-induced inflammatory cytokine secretion in human adipose tissue stromovascular cells. Diabetologia 2011, 54, 1480–1490. [Google Scholar] [CrossRef]
- Fujisaka, S.; Usui, I.; Ikutani, M.; Aminuddin, A.; Takikawa, A.; Tsuneyama, K.; Mahmood, A.; Goda, N.; Nagai, Y.; Takatsu, K.; et al. Adipose tissue hypoxia induces inflammatory M1 polarity of macrophages in an HIF-1α-dependent and HIF-1α-independent manner in obese mice. Diabetologia 2013, 56, 1403–1412. [Google Scholar] [CrossRef]
- Jetten, N.; Verbruggen, S.; Gijbels, M.J.; Post, M.J.; De Winther, M.P.; Donners, M.M. Anti-inflammatory M2; but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis 2014, 17, 109–118. [Google Scholar] [CrossRef]
- Tamori, Y.; Masugi, J.; Nishino, N.; Kasuga, M. Role of peroxisome proliferator-activated receptor-gamma in maintenance of the characteristics of mature 3T3-L1 adipocytes. Diabetes 2002, 51, 2045–2055. [Google Scholar] [CrossRef]
- Suganami, T.; Nishida, J.; Ogawa, Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: Role of free fatty acids and tumor necrosis factor alpha. Arter. Thromb. Vasc. Biol. 2005, 25, 2062–2068. [Google Scholar] [CrossRef]

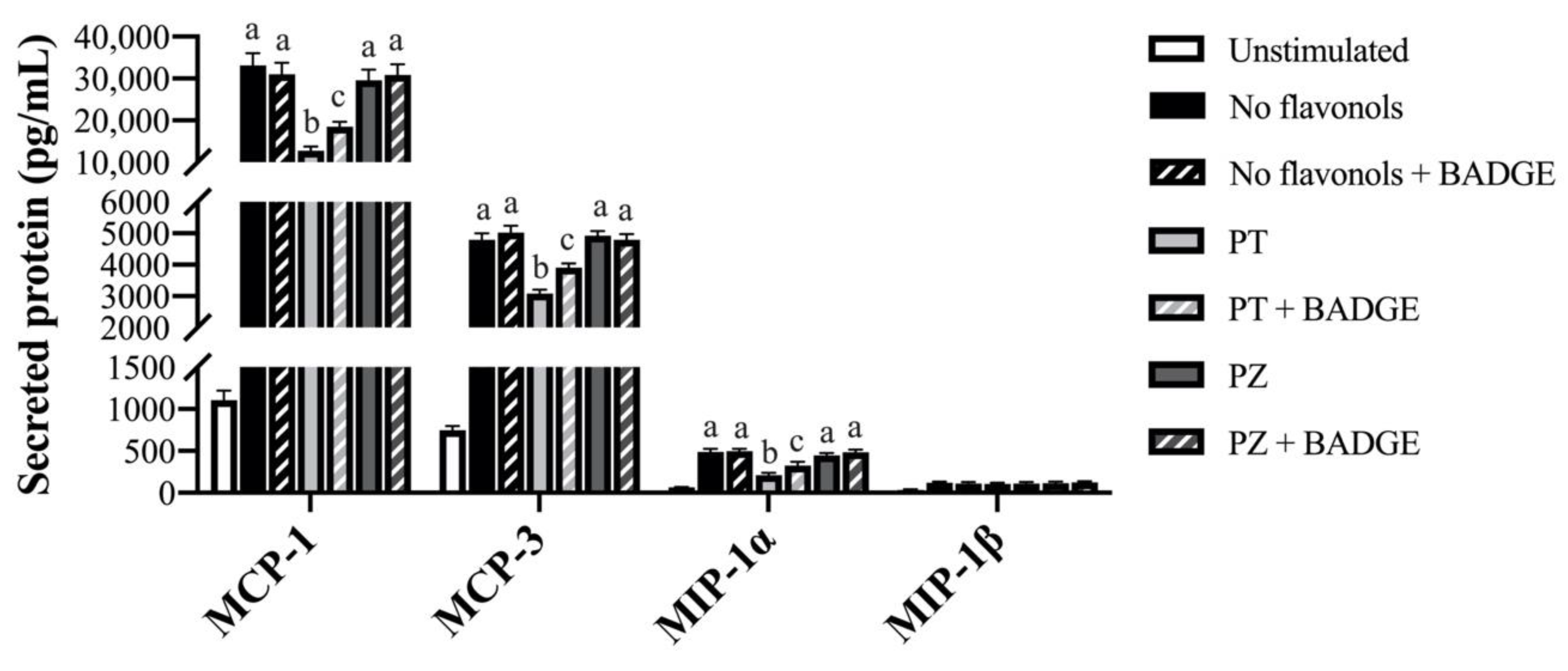


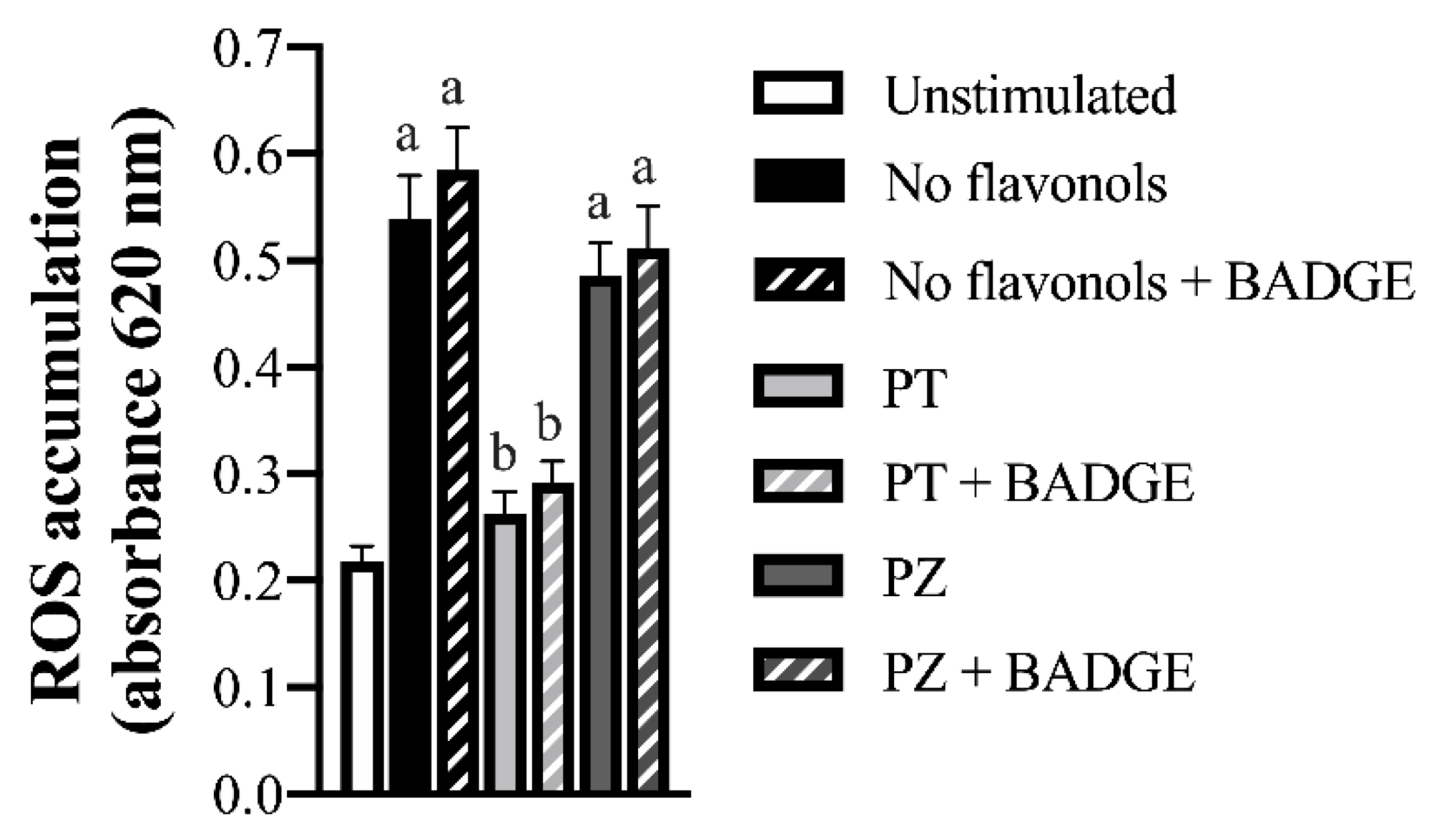

| Gene | No Flavonols | PT | PZ | No Flavonols | PT | PZ |
|---|---|---|---|---|---|---|
| −BADGE | +BADGE | |||||
| Inflammatory adipokines | ||||||
| Il1β | 2.25 ± 0.07 a | 1.95 ± 0.07 b | 1.98 ± 0.03 b | 2.27 ± 0.08 a | 2.16 ± 0.04 b,* | 2.24 ± 0.05 a |
| Il6 | 2.69 ± 0.08 a | 0.94 ± 0.06 b | 1.25 ± 0.05 c | 2.71 ± 0.09 | 2.58 ± 0.05 * | 2.67 ± 0.06 * |
| Mcp1 | 5.40 ± 0.16 a | 1.89 ± 0.11 b | 2.52 ± 0.10 c | 5.43 ± 0.18 | 5.36 ± 0.13 * | 5.36 ± 0.13 * |
| Tnfα | 1.06 ± 0.03 | 0.99 ± 0.03 | 1.00 ± 0.04 | 1.01 ± 0.03 | 1.04 ± 0.05 | 1.00 ± 0.03 |
| Anti-inflammatory adipokines | ||||||
| Adiponectin | 0.77 ± 0.03 a | 1.39 ± 0.02 b | 1.21 ± 0.04 c | 0.79 ± 0.03 | 0.84 ± 0.02 * | 0.83 ± 0.03 * |
| Il10 | 1.06 ± 0.04 | 1.01 ± 0.02 | 0.98 ± 0.03 | 1.01 ± 0.02 | 1.01 ± 0.03 | 0.97 ± 0.03 |
| Angiogenic factors | ||||||
| Vegfa | 1.23 ± 0.05 | 1.27 ± 0.04 | 1.24 ± 0.04 | 1.26 ± 0.04 | 1.26 ± 0.08 | 1.25 ± 0.05 |
| Angptl4 | 1.21 ± 0.05 | 1.20 ± 0.03 | 1.20 ± 0.02 | 1.21 ± 0.03 | 1.21 ± 0.06 | 1.21 ± 0.04 |
| Leptin | 2.56 ± 0.13 a | 1.72 ± 0.11 b | 2.51 ± 0.13 a | 2.93 ± 0.14 * | 2.88 ± 0.09 * | 2.84 ± 0.05 * |
| Transcription factors | ||||||
| Hif1α | 1.05 ± 0.03 | 0.98 ± 0.03 | 1.00 ± 0.04 | 1.00 ± 0.03 | 1.03 ± 0.05 | 0.99 ± 0.03 |
| Nfκb | 1.28 ± 0.07 | 1.31 ± 0.05 | 1.36 ± 0.05 | 1.34 ± 0.07 | 1.30 ± 0.10 | 1.38 ± 0.07 |
| Pparγ | 1.08 ± 0.04 | 1.10 ± 0.03 | 1.03 ± 0.02 | 1.05 ± 0.03 | 1.03 ± 0.02 | 0.99 ± 0.02 |
| Gene | No Flavonols | PT | PZ | No Flavonols | PT | PZ |
|---|---|---|---|---|---|---|
| −BADGE | +BADGE | |||||
| Inflammatory adipokines | ||||||
| Il1β | 5.96 ± 0.24 a | 2.93 ± 0.09 b | 5.39 ± 0.16 c | 5.96 ± 0.14 a | 3.78 ± 0.06 b,* | 5.95 ± 0.13 a,* |
| Il6 | 5.03 ± 0.20 a | 2.48 ± 0.07 b | 3.70 ± 0.11 c | 5.03 ± 0.12 a | 3.48 ± 0.08 b,* | 5.03 ± 0.11 a,* |
| Mcp1 | 10.5 ± 0.32 a | 4.98 ± 0.15 b | 7.42 ± 0.22 c | 10.1 ± 0.24 a | 6.99 ± 0.16 b,* | 10.1 ± 0.21 a,* |
| Tnfα | 1.07 ± 0.03 | 1.01 ± 0.03 | 0.99 ± 0.03 | 1.03 ± 0.02 | 1.03 ± 0.03 | 1.00 ± 0.03 |
| Anti-inflammatory adipokines | ||||||
| Adiponectin | 0.56 ± 0.02 a | 0.91 ± 0.02 b | 0.66 ± 0.02 c | 0.58 ± 0.02 | 0.60 ± 0.02 * | 0.57 ± 0.02 * |
| Il10 | 0.72 ± 0.02 | 0.73 ± 0.03 | 0.69 ± 0.02 | 0.70 ± 0.03 | 0.74 ± 0.03 | 0.72 ± 0.03 |
| Angiogenic factors | ||||||
| Vegfa | 3.25 ± 0.16 a | 5.30 ± 0.26 b | 3.97 ± 0.16 c | 3.33 ± 0.12 a | 4.15 ± 0.11 b,* | 3.28 ± 0.16 a,* |
| Angptl4 | 2.35 ± 0.11 a | 4.89 ± 0.13 b | 2.30 ± 0.03 a | 2.38 ± 0.07 a | 3.84 ± 0.07 b,* | 2.28 ± 0.06 a |
| Leptin | 3.60 ± 0.18 a | 2.71 ± 0.13 b | 3.03 ± 0.07 c | 4.14 ± 0.10 a,* | 3.63 ± 0.15 b,* | 3.43 ± 0.15 b* |
| Transcription factors | ||||||
| Hif1α | 1.07 ± 0.03 | 1.01 ± 0.03 | 1.00 ± 0.03 | 1.03 ± 0.02 | 1.04 ± 0.03 | 1.00 ± 0.03 |
| Nfκb | 1.62 ± 0.08 a | 1.39 ± 0.09 b | 1.61 ± 0.06 a | 1.84 ± 0.09 * | 1.69 ± 0.07 * | 1.84 ± 0.10 * |
| Pparγ | 0.60 ± 0.03 a | 0.86 ± 0.03 b | 0.63 ± 0.03 a | 0.64 ± 0.04 | 0.69 ± 0.02 * | 0.63 ± 0.02 |
| Gene | No Flavonols | PT | PZ | No Flavonols | PT | PZ |
|---|---|---|---|---|---|---|
| −BADGE | +BADGE | |||||
| Inflammatory adipokines | ||||||
| Il1β | 9.04 ± 0.23 a | 4.51 ± 0.12 b | 8.81 ± 0.17 a | 8.97 ± 0.19 a | 6.41 ± 0.11 b,* | 8.85 ± 0.13 a |
| Il6 | 9.07 ± 0.26 a | 4.52 ± 0.12 b | 8.84 ± 0.17 a | 9.00 ± 0.19 a | 7.91 ± 0.14 b,* | 8.88 ± 0.13 a |
| Mcp1 | 22.8 ± 0.50 a | 11.2 ± 0.31 b | 21.9 ± 0.43 a | 22.2 ± 0.5 a | 18.4 ± 0.33 b,* | 22.0 ± 0.32 a |
| Tnfα | 2.45 ± 0.07 a | 1.61 ± 0.04 b | 2.42 ± 0.06 a | 2.40 ± 0.06 a | 2.08 ± 0.06 b,* | 2.48 ± 0.06 a |
| Anti-inflammatory adipokines | ||||||
| Adiponectin | 0.59 ± 0.02 a | 0.78 ± 0.02 b | 0.55 ± 0.03 a | 0.59 ± 0.02 | 0.57 ± 0.03 * | 0.56 ± 0.02 |
| Il10 | 0.70 ± 0.02 | 0.73 ± 0.01 | 0.70 ± 0.03 | 0.72 ± 0.01 | 0.71 ± 0.02 | 0.73 ± 0.03 |
| Angiogenic factors | ||||||
| Vegfa | 1.69 ± 0.08 a | 2.39 ± 0.14 b | 2.08 ± 0.08 c | 1.62 ± 0.08 a | 1.90 ± 0.08 b,* | 1.61 ± 0.04 a,* |
| Angptl4 | 1.92 ± 0.09 a | 3.41 ± 0.21 b | 1.89 ± 0.06 a | 1.84 ± 0.09 a | 2.90 ± 0.08 b,* | 1.87 ± 0.06 a |
| Leptin | 7.14 ± 0.19 a | 4.13 ± 0.10 b | 7.10 ± 0.14 a | 7.19 ± 0.11 a | 5.10 ± 0.16 b,* | 7.05 ± 0.11 a |
| Transcription factors | ||||||
| Hif1α | 2.88 ± 0.08 a | 1.90 ± 0.05 b | 2.86 ± 0.07 a | 2.83 ± 0.07 a | 2.45 ± 0.07 b,* | 2.92 ± 0.08 a |
| Nfκb | 4.13 ± 0.12 a | 2.56 ± 0.13 b | 4.08 ± 0.26 a | 4.17 ± 0.08 a | 3.67 ± 0.11 b,* | 4.15 ± 0.10 a |
| Pparγ | 0.50 ± 0.04 a | 0.68 ± 0.02 b | 0.49 ± 0.01 a | 0.45 ± 0.03 a | 0.68 ± 0.05 b | 0.48 ± 0.04 a |
| Gene | No Flavonols | PT | PZ | No Flavonols | PT | PZ |
|---|---|---|---|---|---|---|
| −BADGE | +BADGE | |||||
| M1 polarization markers | ||||||
| Cd11b | 2.89 ± 0.05 | 2.73 ± 0.08 | 2.81 ± 0.13 | 2.94 ± 0.19 | 2.93 ± 0.12 | 2.87 ± 0.12 |
| Cd11c | 3.45 ± 0.12 | 3.41 ± 0.13 | 3.55 ± 0.12 | 3.59 ± 0.11 | 3.50 ± 0.11 | 3.59 ± 0.10 |
| iNos | 10.1 ± 0.27 a | 6.49 ± 0.29 b | 10.8 ± 0.32 a | 13.4 ± 0.61 a,* | 10.7 ± 0.46 b,* | 12.8 ± 0.39 a,* |
| Il6 | 6.16 ± 0.12 a | 4.31 ± 0.11 b | 5.60 ± 0.08 c | 9.46 ± 0.28 a,* | 6.53 ± 0.20 b,* | 9.20 ± 0.19 a,* |
| Tnfα | 9.10 ± 0.14 a | 5.42 ± 0.11 b | 7.61 ± 0.17 c | 14.9 ± 0.13 a,* | 9.18 ± 0.18 b,* | 14.6 ± 0.12 a,* |
| M2 polarization markers | ||||||
| Cd206 | 0.96 ± 0.02 | 1.05 ± 0.04 | 0.99 ± 0.02 | 0.98 ± 0.02 | 1.01 ± 0.03 | 1.01 ± 0.02 |
| ‘Arg1 | 0.51 ± 0.03 a | 0.73 ± 0.01 b | 0.57 ± 0.03 a | 0.53 ± 0.03 a | 0.66 ± 0.03 b,* | 0.58 ± 0.02 a |
| Il10 | 0.61 ± 0.01 a | 0.91 ± 0.02 b | 0.64 ± 0.02 a | 0.58 ± 0.01 a | 0.84 ± 0.02 b,* | 0.61 ± 0.01 a |
| Tgfβ | 0.65 ± 0.01 a | 0.89 ± 0.02 b | 0.68 ± 0.01 a | 0.69 ± 0.01 a | 0.84 ± 0.03 b | 0.71 ± 0.01 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liddle, D.M.; Kavanagh, M.E.; Wright, A.J.; Robinson, L.E. Apple Flavonols Mitigate Adipocyte Inflammation and Promote Angiogenic Factors in LPS- and Cobalt Chloride-Stimulated Adipocytes, in Part by a Peroxisome Proliferator-Activated Receptor-γ-Dependent Mechanism. Nutrients 2020, 12, 1386. https://doi.org/10.3390/nu12051386
Liddle DM, Kavanagh ME, Wright AJ, Robinson LE. Apple Flavonols Mitigate Adipocyte Inflammation and Promote Angiogenic Factors in LPS- and Cobalt Chloride-Stimulated Adipocytes, in Part by a Peroxisome Proliferator-Activated Receptor-γ-Dependent Mechanism. Nutrients. 2020; 12(5):1386. https://doi.org/10.3390/nu12051386
Chicago/Turabian StyleLiddle, Danyelle M., Meaghan E. Kavanagh, Amanda J. Wright, and Lindsay E. Robinson. 2020. "Apple Flavonols Mitigate Adipocyte Inflammation and Promote Angiogenic Factors in LPS- and Cobalt Chloride-Stimulated Adipocytes, in Part by a Peroxisome Proliferator-Activated Receptor-γ-Dependent Mechanism" Nutrients 12, no. 5: 1386. https://doi.org/10.3390/nu12051386
APA StyleLiddle, D. M., Kavanagh, M. E., Wright, A. J., & Robinson, L. E. (2020). Apple Flavonols Mitigate Adipocyte Inflammation and Promote Angiogenic Factors in LPS- and Cobalt Chloride-Stimulated Adipocytes, in Part by a Peroxisome Proliferator-Activated Receptor-γ-Dependent Mechanism. Nutrients, 12(5), 1386. https://doi.org/10.3390/nu12051386






