Poria Cocos Ameliorates Bone Loss in Ovariectomized Mice and Inhibits Osteoclastogenesis In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Osteoclast Differentiation and Resorption Pit Assay
2.4. Animal Study and Micro-computed Tomography (μ-CT)
2.5. Real-time Quantitative PCR and Western Blotting
2.6. Ultrahigh-performance Liquid Chromatography-diode Array Detector–tandem Mass Spectrometry (UHPLC-DAD–MS/MS) analysis
2.7. Statistical Analysis
3. Results and Discussion
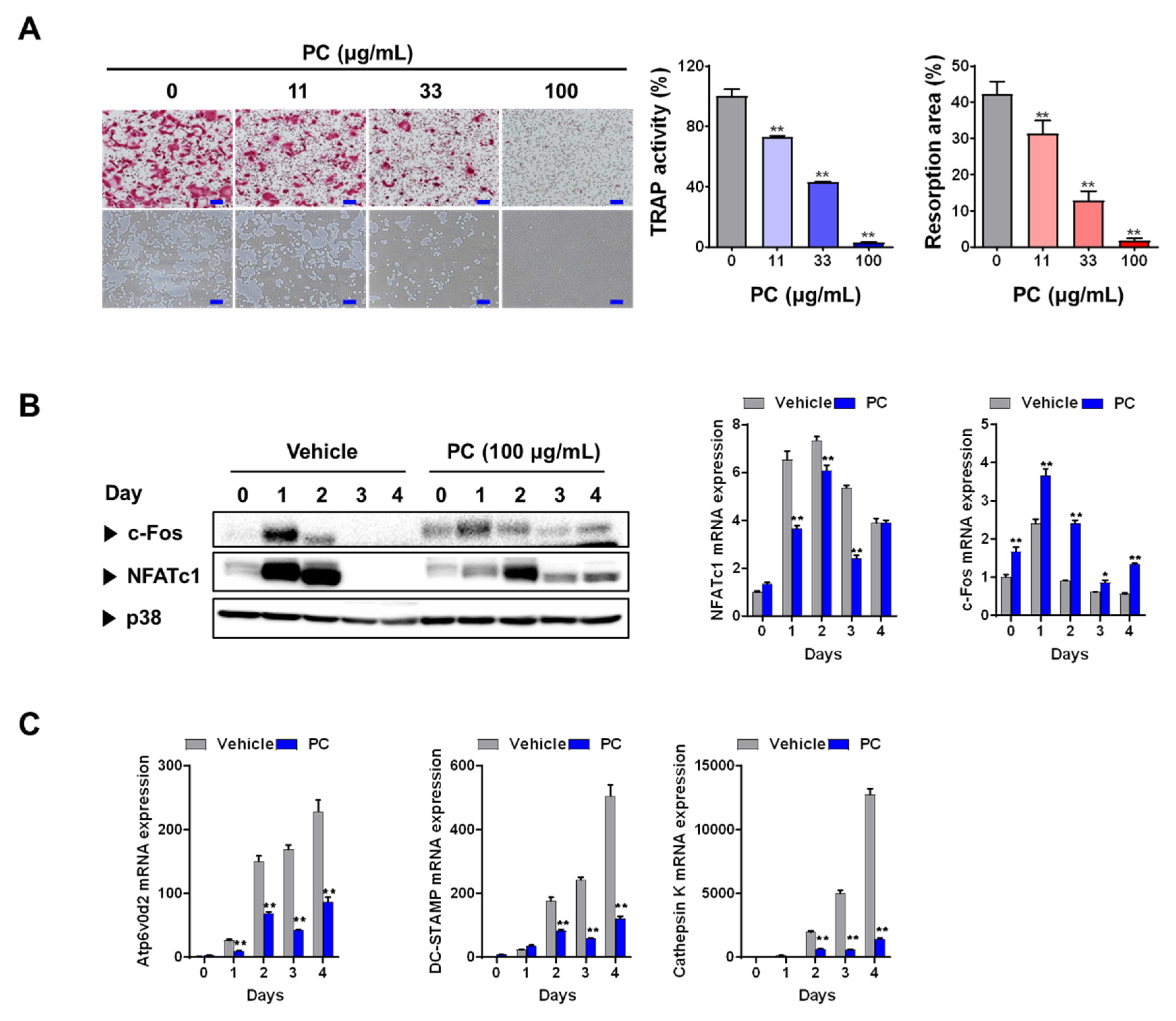
3.1. PC inhibits Osteoclastogenesis and Resorption Activity
3.2. PC Attenuates OVX-induced Bone Loss
3.3. Phytochemical Constituents of PC
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, B.; Wang, J.; Zhang, X.; Xie, Z.; Wu, H.; Liu, J.; Jie, Z.; Zhao, X.; Qin, A.; Fan, S.; et al. Administration of SB239063 ameliorates ovariectomy-induced bone loss via suppressing osteoclastogenesis in mice. Front. Pharmacol. 2019, 10, 900. [Google Scholar] [CrossRef] [PubMed]
- Riggs, B.L. The mechanisms of estrogen regulation of bone resorption. J. Clin. Investig. 2000, 106, 1203–1204. [Google Scholar] [CrossRef] [PubMed]
- Black, D.M.; Rosen, C.J. Clinical practice. Postmenopausal osteoporosis. N. Engl. J. Med. 2016, 374, 254–262. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Yang, H.; Zhang, Q.; Liu, C.; Zhao, J.; Zhang, L.; Chen, B. Natural products for treatment of osteoporosis: The effects and mechanisms on promoting osteoblast-mediated bone formation. Life Sci. 2016, 147, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Comhaire, F.H.; Depypere, H.T. Hormones, herbal preparations and nutriceuticals for a better life after the menopause: Part I. Climacteric 2015, 18, 358–363. [Google Scholar] [CrossRef] [PubMed]
- De Franciscis, P.; Colacurci, N.; Riemma, G.; Conte, A.; Pittana, E.; Guida, M.; Schiattarella, A. A nutraceutical approach to menopausal complaints. Medicina 2019, 55, 544. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Choi, E.; Yang, S.-M.; Ryoo, R.; Moon, E.; Kim, S.-H.; Kim, K.H. Bioactive compounds from sclerotia extract of Poria cocos that control adipocyte and osteoblast differentiation. Bioorg. Chem. 2018, 81, 27–34. [Google Scholar] [CrossRef]
- Zhu, L.-X.; Xu, J.; Wu, Y.; Su, L.-F.; Ching Lam, K.Y.; Qi, E.R.; Dong, X.-P.; Chen, H.-B.; Liu, Y.-D.; Zhao, Z.-Z. Comparative quality of the forms of decoction pieces evaluated by multidimensional chemical analysis and chemometrics: Poria cocos, a pilot study. J. Food Drug Anal. 2019, 27, 766–777. [Google Scholar] [CrossRef]
- Qian, Q.; Zhou, N.; Qi, P.; Zhang, Y.; Mu, X.; Shi, X.; Wang, Q. A UHPLC-QTOF-MS/MS method for the simultaneous determination of eight triterpene compounds from Poria cocos (Schw.) Wolf extract in rat plasma: Application to a comparative pharmacokinetic study. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018, 1102–1103, 34–44. [Google Scholar] [CrossRef]
- Ríos, J.L. Chemical constituents and pharmacological properties of Poria cocos. Planta Med. 2011, 77, 681–691. [Google Scholar] [CrossRef]
- Li, X.; He, Y.; Zeng, P.; Liu, Y.; Zhang, M.; Hao, C.; Wang, H.; Lv, Z.; Zhang, L. Molecular basis for Poria cocos mushroom polysaccharide used as an antitumour drug in China. J. Cell. Mol. Med. 2019, 23, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Cao, Z.; Tickner, J.; Qiu, H.; Wang, C.; Chen, K.; Wang, Z.; Guo, C.; Dong, S.; Xu, J. Poria cocos polysaccharide attenuates RANKL-induced osteoclastogenesis by suppressing NFATc1 activity and phosphorylation of ERK and STAT3. Arch. Biochem. Biophys. 2018, 647, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.; Shim, K.S.; Kim, T.; An, H.; Lee, C.J.; Lee, K.J.; Ma, J.Y. Water extract of Acer tegmentosum reduces bone destruction by inhibiting osteoclast differentiation and function. Molecules 2014, 19, 3940–3954. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.-H.; Jang, S.-A.; Kim, T.; Ha, H. Anti-osteoporotic and anti-adipogenic effects of Rhus chinensis nutgalls in ovariectomized mice fed with a high-fat diet. Planta Med. 2019, 85, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.-H.; Jang, S.-A.; Kim, T.; Ha, H. Forsythia suspensa protects against bone loss in ovariectomized mice. Nutrients 2019, 11, 1831. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Zhou, R.; Xie, J.; Ye, H.; Liang, X.; Zhong, C.; Shen, B.; Qin, Y.; Zhang, S.; Huang, L. Insights into triterpene acids in fermented mycelia of edible fungus Poria cocos by a comparative study. Molecules 2019, 24, 1331. [Google Scholar] [CrossRef]
- Asagiri, M.; Takayanagi, H. The molecular understanding of osteoclast differentiation. Bone 2007, 40, 251–264. [Google Scholar] [CrossRef]
- Baron, R.; Neff, L.; Louvard, D.; Courtoy, P.J. Cell-mediated extracellular acidification and bone resorption: Evidence for a low pH in resorbing lacunae and localization of a 100-kD lysosomal membrane protein at the osteoclast ruffled border. J. Cell Biol. 1985, 101, 2210–2222. [Google Scholar] [CrossRef]
- Drake, F.H.; Dodds, R.A.; James, I.E.; Connor, J.R.; Debouck, C.; Richardson, S.; Lee-Rykaczewski, E.; Coleman, L.; Rieman, D.; Barthlow, R.; et al. Cathepsin K, but not cathepsins B, L, or S, is abundantly expressed in human osteoclasts. J. Biol. Chem. 1996, 271, 12511–12516. [Google Scholar] [CrossRef]
- Takayanagi, H. The role of NFAT in osteoclast formation. Ann. N. Y. Acad. Sci. 2007, 1116, 227–237. [Google Scholar] [CrossRef]
- Matsuo, K.; Galson, D.L.; Zhao, C.; Peng, L.; Laplace, C.; Wang, K.Z.; Bachler, M.A.; Amano, H.; Aburatani, H.; Ishikawa, H.; et al. Nuclear factor of activated T-cells (NFAT) rescues osteoclastogenesis in precursors lacking c-Fos. J. Biol. Chem. 2004, 279, 26475–26480. [Google Scholar] [CrossRef] [PubMed]
- Soysa, N.S.; Alles, N. Osteoclast function and bone-resorbing activity: An overview. Biochem. Biophys. Res. Commun. 2016, 476, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, P.K.; Horowitz, M.C.; MacDougald, O.A.; Scheller, E.L.; Rodeheffer, M.S.; Rosen, C.J.; Klibanski, A. Marrow fat and bone--new perspectives. J. Clin. Endocrinol. Metab. 2013, 98, 935–945. [Google Scholar] [CrossRef]
- Ambrosi, T.H.; Scialdone, A.; Graja, A.; Gohlke, S.; Jank, A.M.; Bocian, C.; Woelk, L.; Fan, H.; Logan, D.W.; Schürmann, A.; et al. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell 2017, 20, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Marinozzi, F.; Marinozzi, A.; Bini, F.; Zuppante, F.; Pecci, R.; Bedini, R. Variability of morphometric parameters of human trabecular tissue from coxo-arthritis and osteoporotic samples. Ann. Ist. Super. Sanita. 2012, 48, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.F.; Wang, K.F.; Mao, X.; Liang, W.Y.; Chen, W.J.; Li, S.; Qi, Q.; Cui, Y.-P.; Zhang, L.Z. Screening and analysis of the potential bioactive components of Poria cocos (schw.) wolf by HPLC and HPLC-MS(n) with the aid of chemometrics. Molecules 2016, 21, 227. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Li, S.; Liu, S.; Song, F.; Pi, Z.; Liu, Z. Targeted screening approach to systematically identify the absorbed effect substances of Poria cocos in vivo using ultrahigh performance liquid chromatography tandem mass spectrometry. J. Agric. Food Chem. 2018, 66, 8319–8327. [Google Scholar] [CrossRef]
- Kwon, J.; Lee, H.; Yoon, Y.D.; Hwang, B.Y.; Guo, Y.; Kang, J.S.; Kim, J.J.; Lee, D. Lanostane triterpenes isolated from Antrodia heteromorpha and their inhibitory effects on RANKL-induced osteoclastogenesis. J. Nat. Prod. 2016, 79, 1689–1693. [Google Scholar] [CrossRef]
- Lee, Y.H.; Lee, N.H.; Bhattarai, G.; Kim, G.E.; Lee, I.K.; Yun, B.S.; Hwang, P.H.; Yi, H.K. Anti-inflammatory effect of pachymic acid promotes odontoblastic differentiation via HO-1 in dental pulp cells. Oral Dis. 2013, 19, 193–199. [Google Scholar] [CrossRef]
- Wang, F.-Y.; Lv, W.-S.; Han, L. Determination and pharmacokinetic study of pachymic acid by LC-MS/MS. Biol. Pharm. Bull. 2015, 38, 1337–1344. [Google Scholar] [CrossRef]

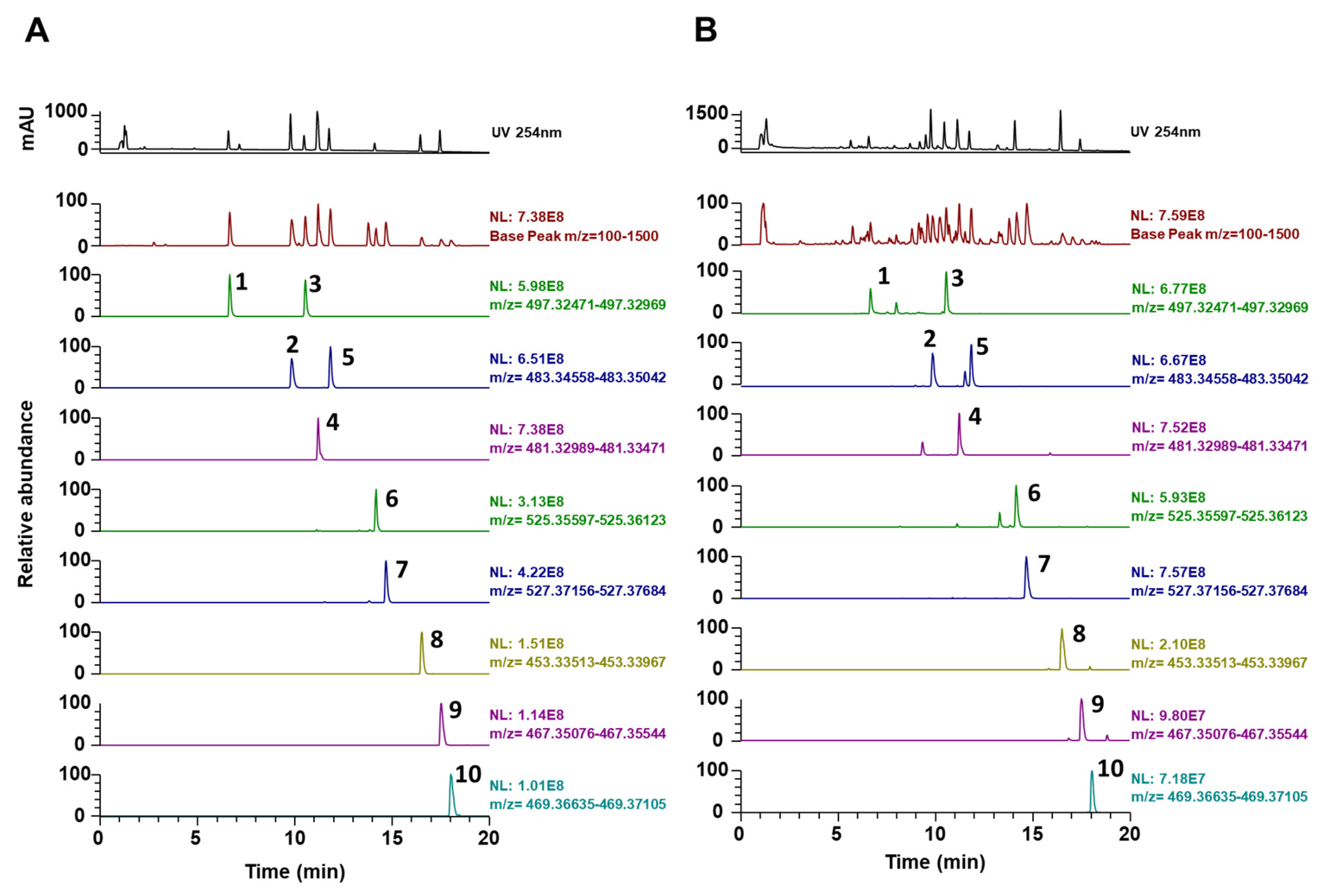
| No | Rt * (min) | Calculated (m/z) | Estimated (m/z) | Adducts | Error (ppm) | Formula | MS/MS Fragments (m/z) | Identifications [References] |
|---|---|---|---|---|---|---|---|---|
| 1 | 6.65 | 497.3272 | 497.3262 | [M-H]- | −2.045 | C31H46O5 | 419.2933, 405.2784, 403.2621 | 6α-Hydroxypolyporenic acid C [26] |
| 2 | 9.83 | 483.348 | 483.3469 | [M-H]- | −2.185 | C31H48O4 | 437.3413, 423.3274, 405.3148, 389.2856 | Dehydrotumulosic acid [27] |
| 3 | 10.53 | 497.3272 | 497.3263 | [M-H]- | −1.922 | C31H46O5 | 423.2895, 379.2977, 211.1488 | Poricoic acid A [16] |
| 4 | 11.19 | 481.3323 | 481.3312 | [M-H]- | −2.269 | C31H46O4 | 435.3270, 421.3116, 311.2012, 97.0639 | Polyporenic acid C [16] |
| 5 | 11.83 | 483.348 | 483.347 | [M-H]- | −2.058 | C31H48O4 | 437.3439, 423.3260, 405.3159, 337.2531 | 3-Epidehydrotumulosic acid [27] |
| 6 | 14.16 | 525.3586 | 525.3573 | [M-H]- | −2.379 | C33H50O5 | 465.3364, 355.2273, | Dehydropachymic acid [16] |
| 7 | 14.67 | 527.3742 | 527.3732 | [M-H]- | −1.955 | C33H52O5 | 527.3356, 405.3150, 221.1897 | Pachymic acid [16] |
| 8 | 16.5 | 453.3374 | 453.3364 | [M-H]- | −2.327 | C30H46O3 | 453.3361, 435.3230, 371.2557, 337.2522 | Dehydrotrametenolic acid [9] |
| 9 | 17.53 | 467.3531 | 467.3519 | [M-H]- | −2.442 | C31H48O3 | 467.3520, 371.2567, 352.2839, 337.2527 | Dehydroeburicoic acid [16] |
| 10 | 18.04 | 469.3687 | 469.3677 | [M-H]- | −2.095 | C31H50O3 | 469.3675, 373.2722, 339.2684 | Eburicoic acid [16] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, Y.-H.; Jang, S.-A.; Lee, A.; Kim, T.; Ha, H. Poria Cocos Ameliorates Bone Loss in Ovariectomized Mice and Inhibits Osteoclastogenesis In Vitro. Nutrients 2020, 12, 1383. https://doi.org/10.3390/nu12051383
Hwang Y-H, Jang S-A, Lee A, Kim T, Ha H. Poria Cocos Ameliorates Bone Loss in Ovariectomized Mice and Inhibits Osteoclastogenesis In Vitro. Nutrients. 2020; 12(5):1383. https://doi.org/10.3390/nu12051383
Chicago/Turabian StyleHwang, Youn-Hwan, Seon-A Jang, Ami Lee, Taesoo Kim, and Hyunil Ha. 2020. "Poria Cocos Ameliorates Bone Loss in Ovariectomized Mice and Inhibits Osteoclastogenesis In Vitro" Nutrients 12, no. 5: 1383. https://doi.org/10.3390/nu12051383
APA StyleHwang, Y.-H., Jang, S.-A., Lee, A., Kim, T., & Ha, H. (2020). Poria Cocos Ameliorates Bone Loss in Ovariectomized Mice and Inhibits Osteoclastogenesis In Vitro. Nutrients, 12(5), 1383. https://doi.org/10.3390/nu12051383





