Neuroprotective Activity of Mentha Species on Hydrogen Peroxide-Induced Apoptosis in SH-SY5Y Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Plant Material and Preparation of Crude Extracts
2.2. Chemicals and Reagents
2.3. Primers and Antibodies
2.4. β-Secretase (BACE) Inhibition Activity
2.5. Aβ42-Aggregation Inhibition Activity
2.6. Cell Culture
2.7. Cell Viability
2.8. Caspase Assay
2.9. Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
2.10. Western Blot Analysis
2.11. Data Analysis
3. Results
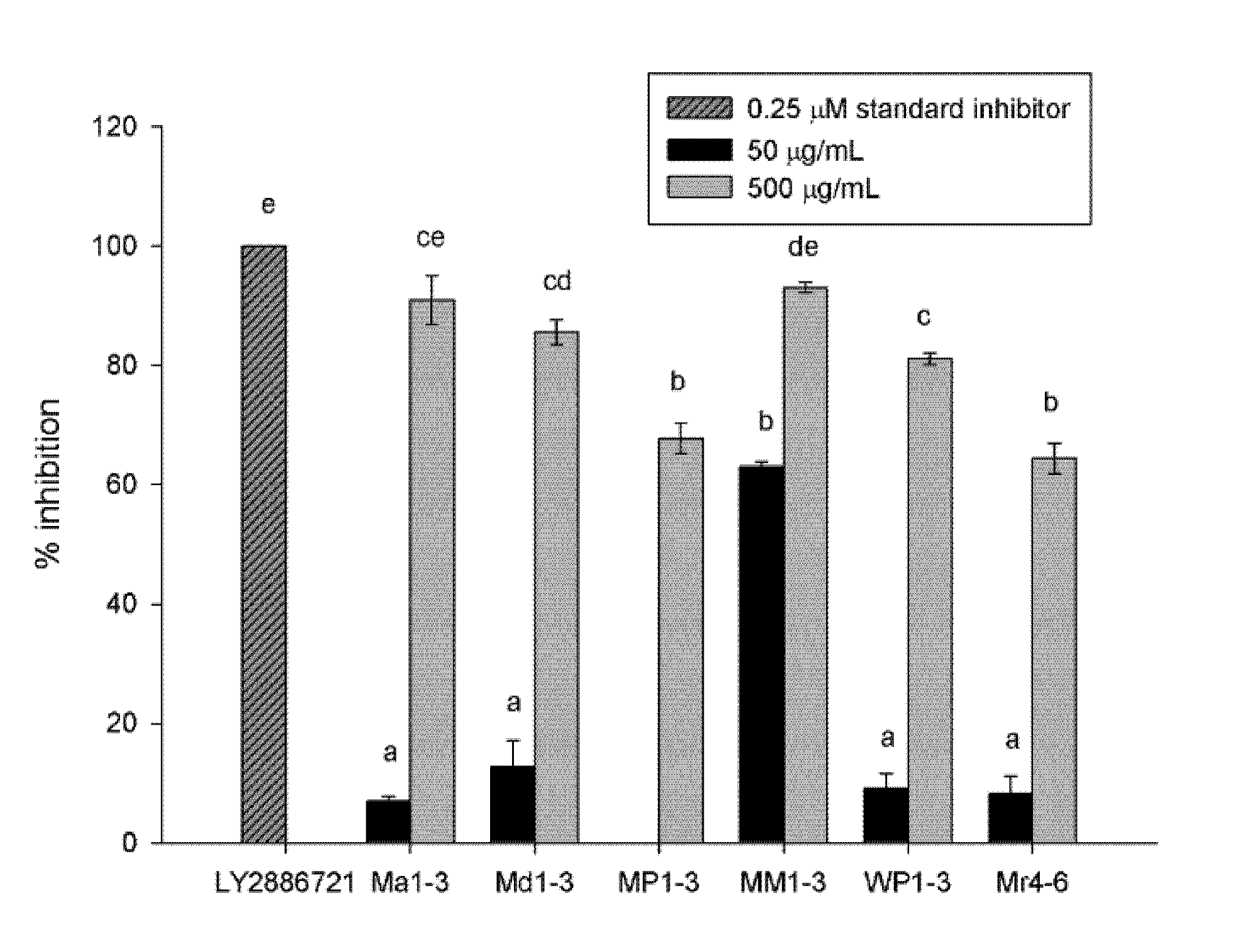
3.1. β-Secretase (BACE) Inhibition Activity
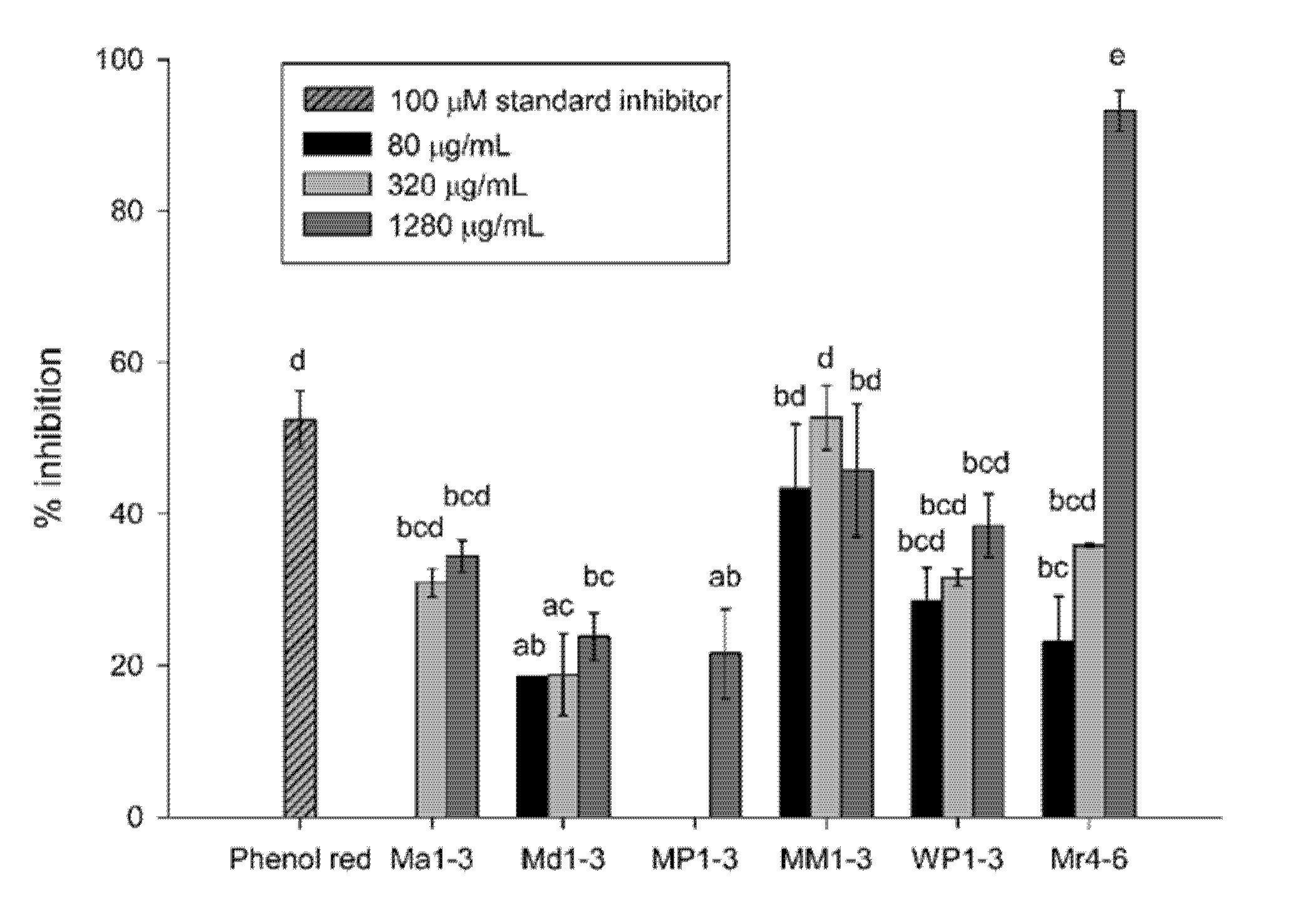
3.2. Aβ42-Aggregation Inhibition Activity
3.3. Cell Viability
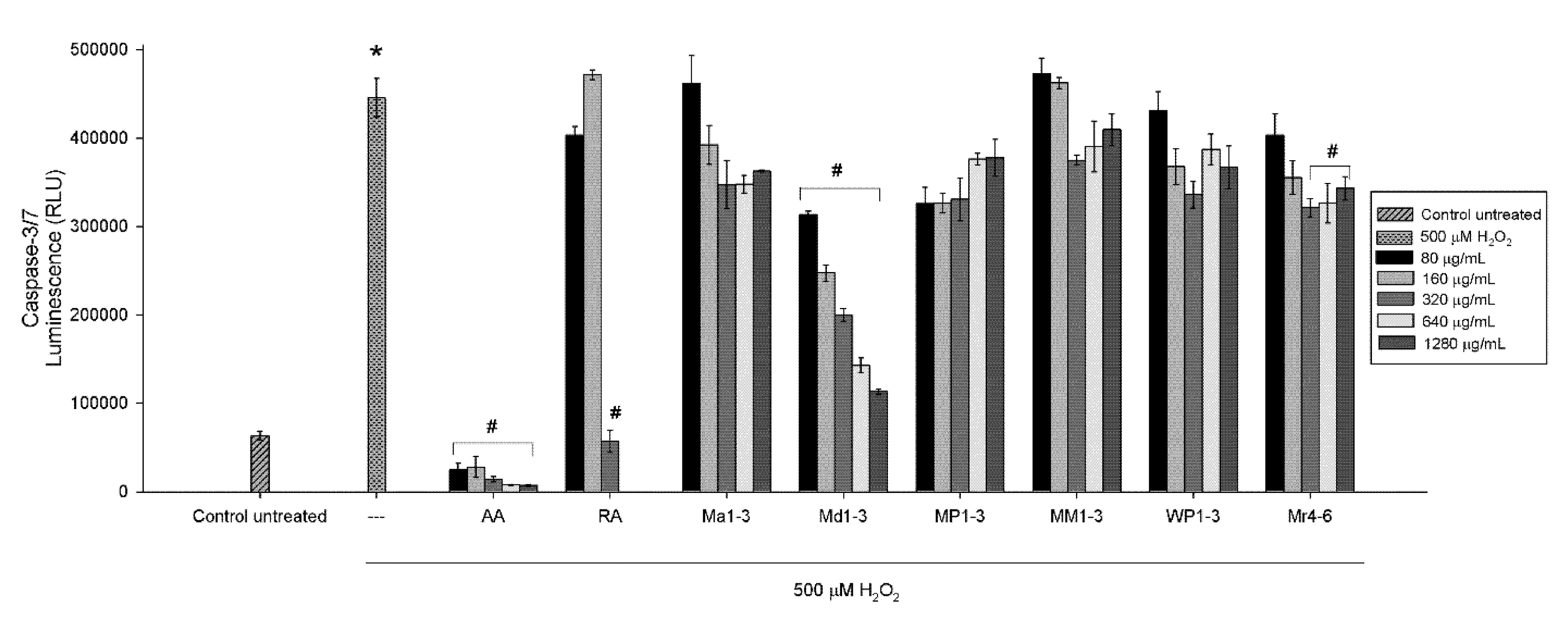
3.4. Effects of Mint Extracts on Caspase Activity
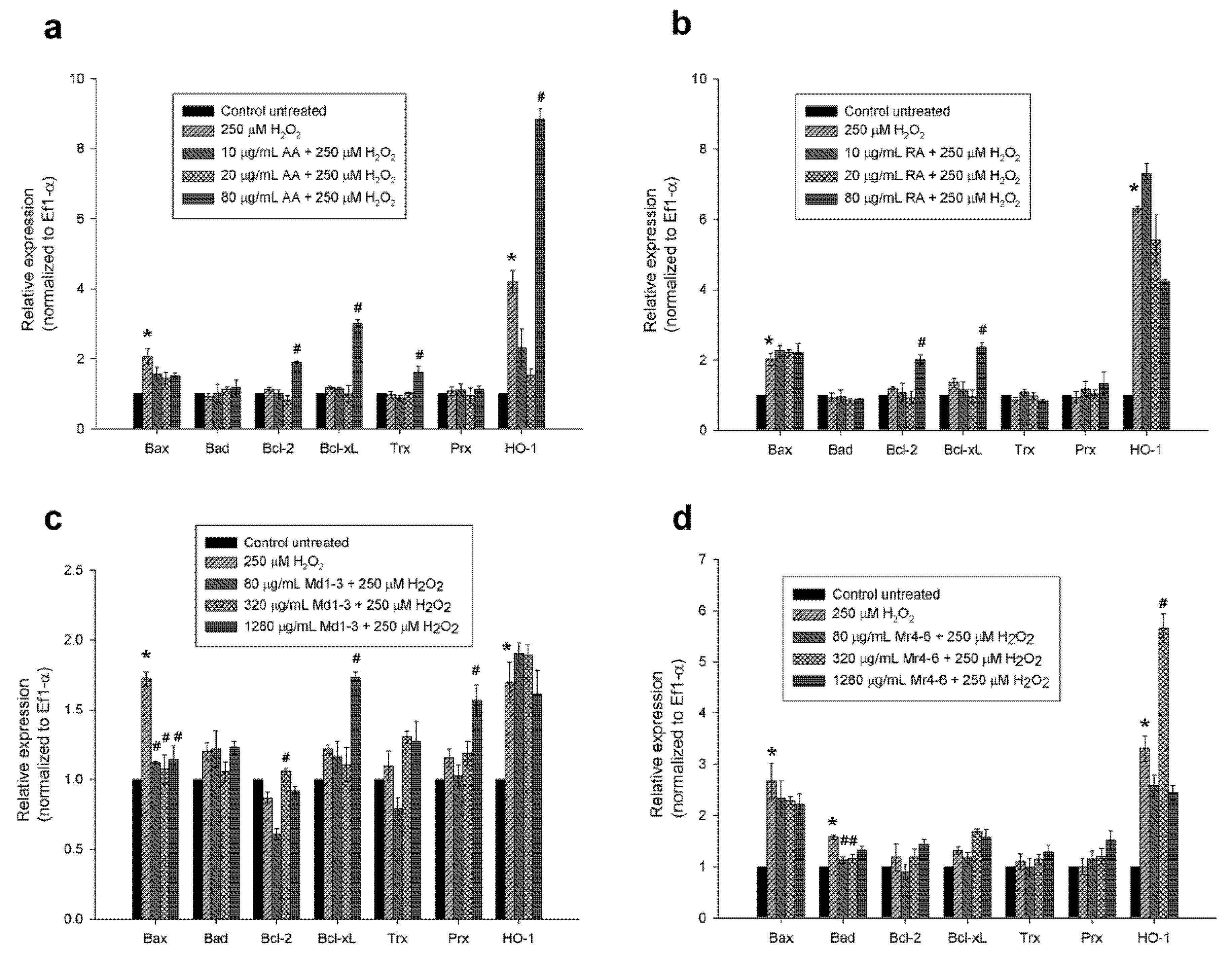
3.5. Effect of Mint Extracts on Transcriptomic Regulation of Apoptotic and Antioxidant Genes in SH-SY5Y Cells Exposed to H2O2
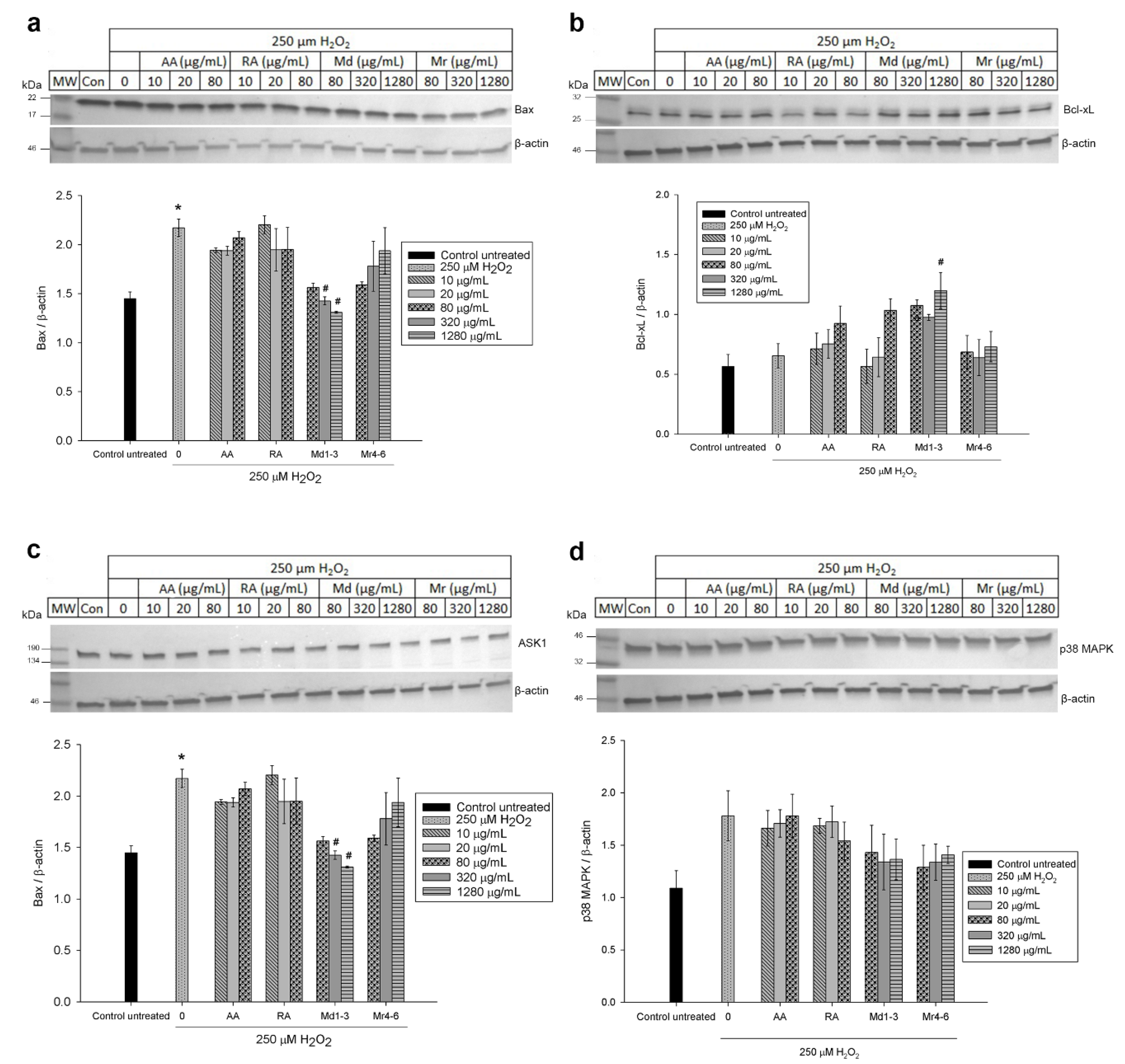
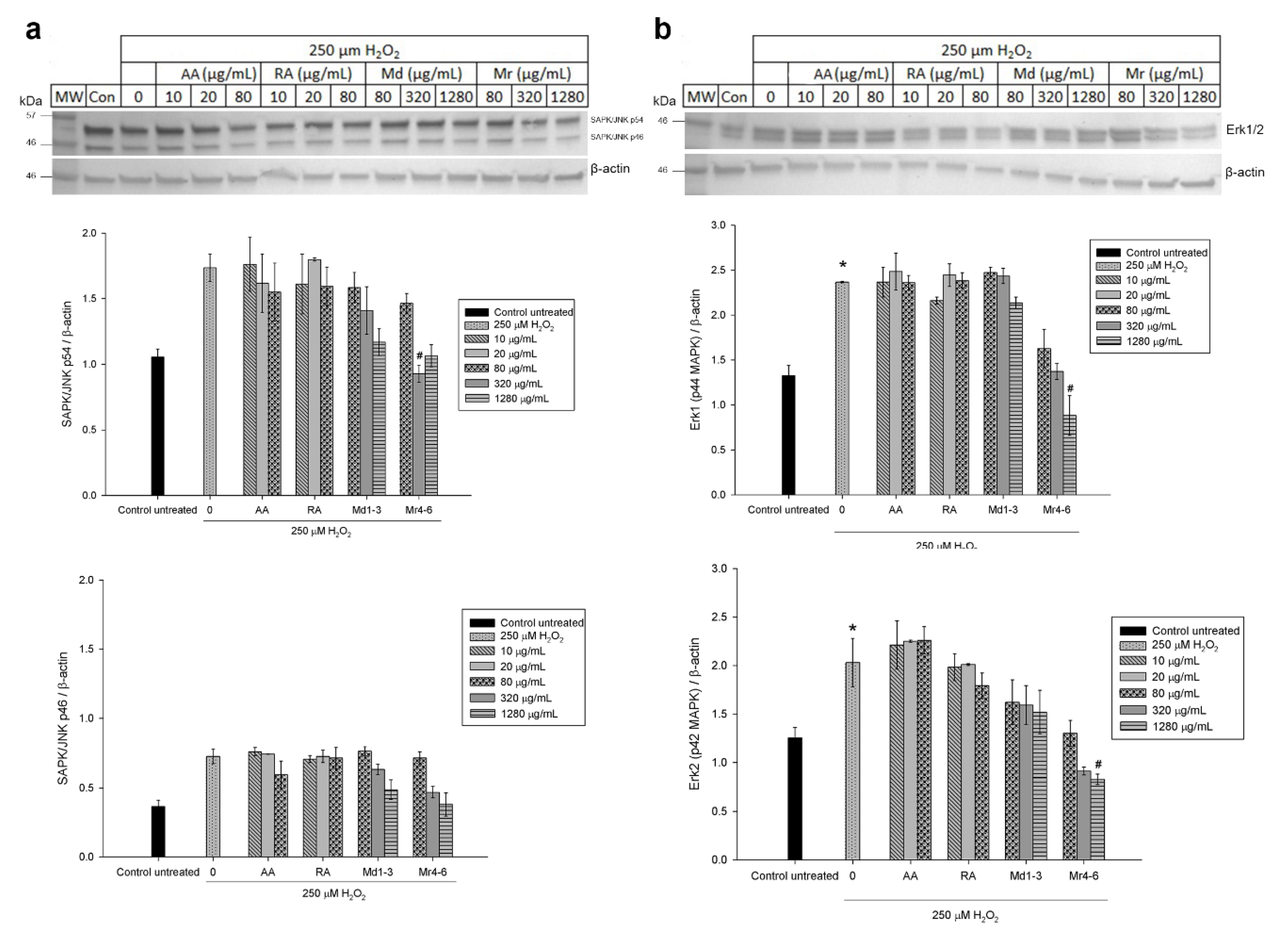
3.6. Effect of Mint Extracts on the Protein Expression in SH-SY5Y Cells Exposed to H2O2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Korolev, I.O. Alzheimer’s disease: A clinical and basic science review. Med. Stud. Res. J. 2014, 4, 24–33. [Google Scholar]
- Huang, L.-K.; Chao, S.-P.; Hu, C.-J. Clinical trials of new drugs for Alzheimer disease. J. Biomed. Sci. 2020, 27, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Panza, F.; Lozupone, M.; Logroscino, G.; Imbimbo, B.P. A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2019, 15, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Imbimbo, B.P.; Watling, M. Investigational BACE inhibitors for the treatment of Alzheimer’s disease. Expert Opin. Investig. Drugs 2019, 28, 967–975. [Google Scholar] [CrossRef] [PubMed]
- May, P.C.; Willis, B.A.; Lowe, S.L.; Dean, R.A.; Monk, S.A.; Cocke, P.J.; Audia, J.E.; Boggs, L.N.; Borders, A.R.; Brier, R.A. The potent BACE1 inhibitor LY2886721 elicits robust central Aβ pharmacodynamic responses in mice, dogs, and humans. J. Neurosci. 2015, 35, 1199–1210. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Castegna, A.; Pocernich, C.B.; Drake, J.; Scapagnini, G.; Calabrese, V. Nutritional approaches to combat oxidative stress in Alzheimer’s disease. J. Nutr. Biochem. 2002, 13, 444–461. [Google Scholar] [CrossRef]
- Sonnen, J.A.; Breitner, J.C.; Lovell, M.A.; Markesbery, W.R.; Quinn, J.F.; Montine, T.J. Free radical-mediated damage to brain in Alzheimer’s disease and its transgenic mouse models. Free Radic. Biol. Med. 2008, 45, 219–230. [Google Scholar] [CrossRef]
- Nagai, H.; Noguchi, T.; Takeda, K.; Ichijo, H. Pathophysiological roles of ASK1-MAP kinase signaling pathways. J. Biochem. Mol. Biol. 2007, 40, 1–6. [Google Scholar] [CrossRef]
- Rang, H.P.; Ritter, J.M.; Flower, R.J.; Henderson, G. Rang & Dale’s Pharmacology; Elsevier: London, UK, 2015. [Google Scholar]
- Portt, L.; Norman, G.; Clapp, C.; Greenwood, M.; Greenwood, M.T. Anti-apoptosis and cell survival: A review. Biochim. Biophys. Acta Mol. Cell Res. 2011, 1813, 238–259. [Google Scholar] [CrossRef]
- Howells, C.C.; Baumann, W.T.; Samuels, D.C.; Finkielstein, C.V. The Bcl-2-associated death promoter (BAD) lowers the threshold at which the Bcl-2-interacting domain death agonist (BID) triggers mitochondria disintegration. J. Theor. Biol. 2011, 271, 114–123. [Google Scholar] [CrossRef]
- Li, J.; Li, W.; Jiang, Z.-G.; Ghanbari, H.A. Oxidative stress and neurodegenerative disorders. Int. J. Mol. Sci. 2013, 14, 24438–24475. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Park, K.A.; Lee, W.T.; Lee, J.E. Apoptosis signal regulating kinase 1 (ASK1): Potential as a therapeutic target for Alzheimer’s disease. Int. J. Mol. Sci. 2014, 15, 2119–2129. [Google Scholar] [CrossRef] [PubMed]
- Jivad, N.; Rabiei, Z. A review study on medicinal plants used in the treatment of learning and memory impairments. Asian Pac. J. Trop. Biomed. 2014, 4, 780–789. [Google Scholar] [CrossRef]
- Orhan, A.; Kartal, M.; Şener, B.; Başer, K.H.C. Inhibitory effect of Turkish Rosmarinus officinalis L. on acetylcholinesterase and butyrylcholinesterase enzymes. Food Chem. 2008, 108, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, D.M.; Prenzler, P.D.; Burrows, G.E.; Ryan, D.; Nielsen, S.; El Sawi, S.A.; El Alfy, T.S.; Abdelrahman, E.H.; Obied, H.K. Biophenols of mints: Antioxidant, acetylcholinesterase, butyrylcholinesterase and histone deacetylase inhibition activities targeting Alzheimer’s disease treatment. J. Funct. Foods 2017, 33, 345–362. [Google Scholar] [CrossRef]
- Joshi, H.; Bhadania, M. Evaluation of freeze dried extract of Mentha piperita in management of cognitive dysfunctions in mice. Alzheimer’s Dement. 2014, 10, 461. [Google Scholar] [CrossRef]
- Dheda, K.; Huggett, J.F.; Bustin, S.A.; Johnson, M.A.; Rook, G.; Zumla, A. Validation of housekeeping genes for normalizing RNA expression in real-time PCR. Biotechniques 2004, 37, 112–119. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, H.; Sun, Y.; Lin, X.; Chen, B.; Tan, C.; Cao, G.; Wang, Z. Protective effects of salidroside on hydrogen peroxide-induced apoptosis in SH-SY5Y human neuroblastoma cells. Eur. J. Pharmacol. 2007, 564, 18–25. [Google Scholar] [CrossRef]
- Borhani, N.; Manoochehri, M.; Saleh Gargari, S.; Ghaffari Novin, M.; Mansouri, A.; Omrani, M.D. Decreased expression of proapoptotic genes caspase-8- and Bcl2-associated agonist of cell death (Bad) in ovarian cancer. Clin. Ovarian Other Gynecol. Cancer 2014, 7, 18–23. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Huang, H.-C.; Tang, D.; Xu, K.; Jiang, Z.-F. Curcumin attenuates amyloid-β-induced tau hyperphosphorylation in human neuroblastoma SH-SY5Y cells involving PTEN/Akt/GSK-3β signaling pathway. J. Recept. Signal Transduct. 2014, 34, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Chi, M.; Sun, X.; Wang, G.; Li, M.; Liu, L.; Li, X. Propofol-induced protection of SH-SY5Y cells against hydrogen peroxide is associated with the HO-1 via the ERK pathway. Int. J. Med. Sci. 2013, 10, 599–606. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roberds, S.L.; Anderson, J.; Basi, G.; Bienkowski, M.J.; Branstetter, D.G.; Chen, K.S.; Freedman, S.; Frigon, N.L.; Games, D.; Hu, K. BACE knockout mice are healthy despite lacking the primary β-secretase activity in brain: Implications for Alzheimer’s disease therapeutics. Hum. Mol. Genet. 2001, 10, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Menting, K.W.; Claassen, J.A. β-secretase inhibitor; a promising novel therapeutic drug in Alzheimer’s disease. Front. Aging Neurosci. 2014, 6, 165. [Google Scholar] [CrossRef] [PubMed]
- Volloch, V.; Rits, S. Results of beta secretase-inhibitor clinical trials support amyloid precursor protein-independent generation of beta amyloid in sporadic Alzheimer’s disease. Med. Sci. 2018, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Shimmyo, Y.; Kihara, T.; Akaike, A.; Niidome, T.; Sugimoto, H. Flavonols and flavones as BACE-1 inhibitors: Structure–activity relationship in cell-free, cell-based and in silico studies reveal novel pharmacophore features. Biochim. Biophys. Acta Gen. Subj. 2008, 1780, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Molecular pharmacology of rosmarinic and salvianolic acids: Potential seeds for Alzheimer’s and vascular dementia drugs. Int. J. Mol. Sci. 2018, 19, 458. [Google Scholar] [CrossRef]
- Phan, H.T.; Samarat, K.; Takamura, Y.; Azo-Oussou, A.F.; Nakazono, Y.; Vestergaard, M.D.C. Polyphenols Modulate Alzheimer’s Amyloid Beta Aggregation in a Structure-Dependent Manner. Nutrients 2019, 11, 756. [Google Scholar] [CrossRef]
- Hamaguchi, T.; Ono, K.; Murase, A.; Yamada, M. Phenolic compounds prevent Alzheimer’s pathology through different effects on the amyloid-β aggregation pathway. Am. J. Pathol. 2009, 175, 2557–2565. [Google Scholar] [CrossRef]
- Sabogal-Guáqueta, A.M.; Muñoz-Manco, J.I.; Ramírez-Pineda, J.R.; Lamprea-Rodriguez, M.; Osorio, E.; Cardona-Gómez, G.P. The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacology 2015, 93, 134–145. [Google Scholar] [CrossRef]
- Lou, H.; Fan, P.; Perez, R.G.; Lou, H. Neuroprotective effects of linarin through activation of the PI3K/Akt pathway in amyloid-β-induced neuronal cell death. Bioorg. Med. Chem. 2011, 19, 4021–4027. [Google Scholar] [CrossRef]
- Iuvone, T.; De Filippis, D.; Esposito, G.; D’Amico, A.; Izzo, A.A. The spice sage and its active ingredient rosmarinic acid protect PC12 cells from amyloid-β peptide-induced neurotoxicity. J. Pharmacol. Exp. Ther. 2006, 317, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Rong, H.; Liang, Y.; Niu, Y. Rosmarinic acid attenuates β-amyloid-induced oxidative stress via Akt/GSK-3β/Fyn-mediated Nrf2 activation in PC12 cells. Free Radic. Biol. Med. 2018, 120, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Cho, H.-S.; Park, E.; Kim, S.; Lee, S.-Y.; Kim, C.-S.; Kim, D.K.; Kim, S.-J.; Chun, H.S. Rosmarinic acid protects human dopaminergic neuronal cells against hydrogen peroxide-induced apoptosis. Toxicology 2008, 250, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.; Ismail, M.; Farhana Fathy, S.; Asma Musa, S.N.; Umar Imam, M.; Foo, J.B.; Iqbal, S. Neuroprotective effects of germinated brown rice against hydrogen peroxide induced cell death in human SH-SY5Y cells. Int. J. Mol. Sci. 2012, 13, 9692–9708. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Min, K.; Seo, H.; Lee, K.; Kim, M.; Jeong, H.; Park, N.; Shin, Y.; Ko, S. Anti-oxidative and anti-inflammatory property of ethanol extracts of Chungyul medicines in human neuroblastoma cells, SH-SY5Y. Orient. Pharm. Exp. Med. 2013, 13, 239–245. [Google Scholar] [CrossRef]
- Désiré, L.; Bourdin, J.; Loiseau, N.; Peillon, H.; Picard, V.; De Oliveira, C.; Bachelot, F.; Leblond, B.; Taverne, T.; Beausoleil, E. RAC1 inhibition targets amyloid precursor protein processing by γ-secretase and decreases Aβ production in vitro and in vivo. J. Biol. Chem. 2005, 280, 37516–37525. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; May, J.M. Ascorbic acid protects SH-SY5Y neuroblastoma cells from apoptosis and death induced by β-amyloid. Brain Res. 2006, 1097, 52–58. [Google Scholar] [CrossRef]
- Shimohama, S. Apoptosis in Alzheimer’s disease-an update. Apoptosis 2000, 5, 9–16. [Google Scholar] [CrossRef]
- Dennery, P.A. Signaling function of heme oxygenase proteins. Antioxid. Redox Signal. 2014, 20, 1743–1753. [Google Scholar] [CrossRef]
- Pae, H.-O.; Kim, E.-C.; Chung, H.-T. Integrative survival response evoked by heme oxygenase-1 and heme metabolites. J. Clin. Biochem. Nutr. 2008, 42, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Kamata, H.; Hirata, H. Redox regulation of cellular signalling. Cell. Signal. 1999, 11, 1–14. [Google Scholar] [CrossRef]
- Lu, Z.; Xu, S. ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life 2006, 58, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, D.N.; Reddy, E.P. JNK signaling in apoptosis. Oncogene 2008, 27, 6245–6251. [Google Scholar] [CrossRef]
- Latimer, H.R.; Veal, E.A. Peroxiredoxins in regulation of MAPK signalling pathways; sensors and barriers to signal transduction. Mol. Cells 2016, 39, 40–45. [Google Scholar] [CrossRef]
- Kwon, S.-H.; Kim, J.-A.; Hong, S.-I.; Jung, Y.-H.; Kim, H.-C.; Lee, S.-Y.; Jang, C.-G. Loganin protects against hydrogen peroxide-induced apoptosis by inhibiting phosphorylation of JNK, p38, and ERK1/2 MAPKs in SH-SY5Y cells. Neurochem. Int. 2011, 58, 533–541. [Google Scholar] [CrossRef]
- Son, Y.; Cheong, Y.-K.; Kim, N.-H.; Chung, H.-T.; Kang, D.G.; Pae, H.-O. Mitogen-activated protein kinases and reactive oxygen species: How can ROS activate MAPK pathways? J. Signal Transduct. 2011, 2011, 792639. [Google Scholar] [CrossRef]

| Gene Name | Primer Sequences | Reference |
|---|---|---|
| Elongation factor 1-alpha (EF1α) | F: 5′-CTGAACCATCCAGGCCAAAT-3′ R: 5′-GCCGTGTGGCAATCCAAT-3′ | [18] |
| Bcl-2-associated × protein (Bax) | F: 5′-GGGGACGAACTGGACAGTAA-3′ R: 5′-CAGTTGAAGTTGCCGTCAGA-3′ | [19] |
| Bcl-2-associated death promoter (Bad) | F: 5′-CCCAGAGTTTGAGCCGAGTG-3′ R: 5′-CCCATCCCTTCGTCGTCCT-3′ | [20] |
| B-cell lymphoma 2 (Bcl-2) | F: 5′-CGACTTCGCCGAGATGTCCAGCCAG-3′ R: 5′-ACTTGTGGCCCAGATAGGCACCCAG-3′ | [19] |
| B-cell lymphoma-extra large (Bcl-xL) | F: 5′-TTCAGTGACCTGACATCCCA-3′ R: 5′-TCCACAAAAGTATCCCAGCC-3′ | [19] |
| Thioredoxin (Trx) | F: 5′-TGCTTTTCAGGAAGCCTTG-3′ R: 5′-TGTTGGCATGCATTTGACTT-3′ | [19] |
| Peroxiredoxin-I (Prx) | F: 5′-TGCCAGATGGTCAGTTTAAA-3′ R: 5′-CAGCTGGGCACACTTCCCCA-3′ | [19] |
| Heme oxygenase-1 (HO-1) | F: 5′-CACGCCTACACCCGCTACCT-3′ R: 5′-TCTGTCACCCTGTGCTTGAC-3′ | [19] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanafy, D.M.; Prenzler, P.D.; Burrows, G.E.; Gurusinghe, S.; Thejer, B.M.; Obied, H.K.; Hill, R.A. Neuroprotective Activity of Mentha Species on Hydrogen Peroxide-Induced Apoptosis in SH-SY5Y Cells. Nutrients 2020, 12, 1366. https://doi.org/10.3390/nu12051366
Hanafy DM, Prenzler PD, Burrows GE, Gurusinghe S, Thejer BM, Obied HK, Hill RA. Neuroprotective Activity of Mentha Species on Hydrogen Peroxide-Induced Apoptosis in SH-SY5Y Cells. Nutrients. 2020; 12(5):1366. https://doi.org/10.3390/nu12051366
Chicago/Turabian StyleHanafy, Doaa M., Paul D. Prenzler, Geoffrey E. Burrows, Saliya Gurusinghe, Bashar M. Thejer, Hassan K. Obied, and Rodney A. Hill. 2020. "Neuroprotective Activity of Mentha Species on Hydrogen Peroxide-Induced Apoptosis in SH-SY5Y Cells" Nutrients 12, no. 5: 1366. https://doi.org/10.3390/nu12051366
APA StyleHanafy, D. M., Prenzler, P. D., Burrows, G. E., Gurusinghe, S., Thejer, B. M., Obied, H. K., & Hill, R. A. (2020). Neuroprotective Activity of Mentha Species on Hydrogen Peroxide-Induced Apoptosis in SH-SY5Y Cells. Nutrients, 12(5), 1366. https://doi.org/10.3390/nu12051366






