Preoperative Exclusive Total Parental Nutrition is Associated with Clinical and Laboratory Remission in Severe Active Crohn’s Disease—A Pilot Study
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Scores
2.3. Total Parental Nutrition (TPN)
2.4. Statistics
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.; Chan, F.K.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2018, 390, 2769–2778. [Google Scholar] [CrossRef]
- Fichera, A.; Michelassi, F. Surgical treatment of Crohn’s disease. J. Gastrointest. Surg. 2007, 11, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Gionchetti, P.; Dignass, A.; Danese, S.; Magro Dias, F.J.; Rogler, G.; Lakatos, P.L.; Adamina, M.; Ardizzone, S.; Buskens, C.J.; Sebastian, S.; et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 2: Surgical Management and Special Situations. J. Crohn’s Colitis 2017, 11, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Frolkis, A.D.; Dykeman, J.; Negrón, M.E.; deBruyn, J.; Jette, N.; Fiest, K.M.; Frolkis, T.; Barkema, H.W.; Rioux, K.P.; Panaccione, R.; et al. Risk of surgery for inflammatory bowel diseases has decreased over time: A systematic review and meta-analysis of population-based studies. Gastroenterology 2013, 145, 996–1006. [Google Scholar] [CrossRef]
- Frolkis, A.D.; Lipton, D.S.; Fiest, K.M.; Negrón, M.E.; Dykeman, J.; Debruyn, J.; Jette, N.; Frolkis, T.; Rezaie, A.; Seow, C.H.; et al. Cumulative incidence of second intestinal resection in Crohn’s disease: A systematic review and meta-analysis of population-based studies. Am. J. Gastroenterol. 2014, 109, 1739–1748. [Google Scholar]
- Jones, D.W.; Finlayson, S.R. Trends in surgery for Crohn’s disease in the era of infliximab. Ann. Surg. 2010, 252, 307–312. [Google Scholar] [CrossRef]
- Forbes, A.; Escher, J.; Hébuterne, X.; Kłęk, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN guideline: Clinical nutrition in inflammatory bowel disease. Clin. Nutr. 2017, 36, 321–347. [Google Scholar] [CrossRef]
- Goh, J.; O’Morain, C.A. Review article: Nutrition and adult inflammatory bowel disease. Aliment Pharmacol. Ther. 2003, 17, 307–320. [Google Scholar] [CrossRef]
- Sungurtekin, H.; Sungurtekin, U.; Balci, C.; Zencir, M.; Erdem, E. The influence of nutritional status on complications after major intraabdominal surgery. J. Am. Coll. Nutr. 2004, 23, 227–232. [Google Scholar] [CrossRef]
- Guo, Z.; Guo, D.; Gong, J.; Zhu, W.; Zuo, L.; Sun, J.; Li, N.; Li, J. Preoperative Nutritional Therapy Reduces the Risk of Anastomotic Leakage in Patients with Crohn’s Disease Requiring Resections. Gastroenterol. Res. Pract. 2016, 2016, 5017856. [Google Scholar] [CrossRef]
- Springer, J.E.; Doumouras, A.G.; Saleh, F.; Lee, J.; Amin, N.; Cadeddu, M.; Eskicioglu, C.; Hong, D. Drivers of Inpatient Costs after Colorectal Surgery Within a Publicly Funded Healthcare System. Dis. Colon Rectum 2019, 62, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Fumery, M.; Seksik, P.; Auzolle, C.; Munoz-Bongrand, N.; Gornet, J.M.; Boschetti, G.; Cotte, E.; Buisson, A.; Dubois, A.; Pariente, B.; et al. Postoperative Complications after Ileocecal Resection in Crohn’s Disease: A Prospective Study From the REMIND Group. Am. J. Gastroenterol. 2017, 112, 337–345. [Google Scholar] [CrossRef] [PubMed]
- El-Hussuna, A.; Iesalnieks, I.; Horesh, N.; Hadi, S.; Dreznik, Y.; Zmora, O. The effect of pre-operative optimization on post-operative outcome in Crohn’s disease resections. Int. J. Colorectal. Dis. 2017, 32, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Hegazi, R.A.; Hustead, D.S.; Evans, D.C. Preoperative standard oral nutrition supplements vs. immunonutrition: Results of a systematic review and meta-analysis. J. Am. Coll. Surg. 2014, 219, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Mazaki, T.; Ishii, Y.; Murai, I. Immunoenhancing enteral and parenteral nutrition for gastrointestinal surgery: A multiple-treatments meta-analysis. Ann. Surg. 2015, 261, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.F.; Panaccione, R.; Bossuyt, P.; Lukas, M.; Baert, F.; Vaňásek, T.; Danalioglu, A.; Novacek, G.; Armuzzi, A.; Hébuterne, X.; et al. Effect of tight control management on Crohn’s disease (CALM): A multicentre, randomised, controlled phase 3 trial. Lancet 2018, 390, 2779–2789. [Google Scholar] [CrossRef]
- Assa, A.; Matar, M.; Turner, D.; Broide, E.; Weiss, B.; Ledder, O.; Guz-Mark, A.; Rinawi, F.; Cohen, S.; Topf-Olivestone, C.; et al. Proactive Monitoring of Adalimumab Trough Concentration Associated with Increased Clinical Remission in Children with Crohn’s Disease Compared with Reactive Monitoring. Gastroenterology 2019, 157, 985–996.e2. [Google Scholar] [CrossRef]
- Papamichael, K.; Juncadella, A.; Wong, D.; Rakowsky, S.; Sattler, L.A.; Campbell, J.P.; Vaughn, B.P.; Cheifetz, A.S. Proactive Therapeutic Drug Monitoring of Adalimumab Is Associated with Better Long-term Outcomes Compared With Standard of Care in Patients with Inflammatory Bowel Disease. J. Crohns Colitis 2019, 13, 976–981. [Google Scholar] [CrossRef]
- Zittan, E.; Kelly, O.B.; Kirsch, R.; Milgrom, R.; Burns, J.; Nguyen, G.C.; Croitoru, K.; Van Assche, G.; Silverberg, M.S.; Steinhart, A.H. Low Fecal Calprotectin Correlates with Histological Remission and Mucosal Healing in Ulcerative Colitis and Colonic Crohn’s Disease. Inflamm. Bowel Dis. 2016, 22, 623–630. [Google Scholar] [CrossRef]
- Zittan, E.; Kelly, O.B.; Gralnek, I.M.; Silverberg, M.S.; Hillary Steinhart, A. Fecal calprotectin correlates with active colonic inflammatory bowel disease but not with small intestinal Crohn’s disease activity. JGH Open 2018, 2, 201–206. [Google Scholar] [CrossRef]
- Seo, M.; Okada, M.; Yao, T.; Furukawa, H.; Matake, H. The role of total parenteral nutrition in the management of patients with acute attacks of inflammatory bowel disease. J. Clin. Gastroenterol. 1999, 29, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Turkot, M.; Sobocki, J. Results of home parenteral nutrition in patients with severe inflammatory bowel disease-an alternative for surgery of malnourished patients. Pol. Prz. Chir. 2017, 89, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.P.; Steinhart, A.H.; Cohen, Z.; McLeod, R.S. Home total parenteral nutrition: An alternative to early surgery for complicated inflammatory bowel disease. J. Gastrointest. Surg. 2003, 7, 562–566. [Google Scholar] [CrossRef]
- Ostro, M.J.; Greenberg, G.R.; Jeejeebhoy, K.N. Total parenteral nutrition and complete bowel rest in the management of Crohn’s disease. JPEN J. Parenter. Enteral. Nutr. 1985, 9, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Varayil, J.E.; Hurt, R.T.; Enders, F.; Kelly, D.G. Mo2120 Infection Rate in Inflammatory Bowel Disease (IBD) Patients on Home Parenteral Nutrition (HPN). Gastroenterology 2013, 144, S748. [Google Scholar] [CrossRef]
- Galandiuk, S.; O’Neill, M.; McDonald, P.; Fazio, V.W.; Steiger, E. A century of home parenteral nutrition for Crohn’s disease. Am. J. Surg. 1990, 159, 540–544. [Google Scholar] [CrossRef]
- Scolapio, J.S.; Fleming, C.R.; Kelly, D.G.; Wick, D.M.; Zinsmeister, A.R. Survival of Home Parenteral Nutrition-Treated Patients: 20 Years of Experience at the Mayo Clinic. Mayo Clin. Proc. 1999, 74, 217–222. [Google Scholar] [CrossRef]
- Howard, L.; Ament, M.; Fleming, C.R.; Shike, M.; Steiger, E. Current use and clinical outcome of home parenteral and enteral nutrition therapies in the United States. Gastroenterology 1995, 109, 355–365. [Google Scholar] [CrossRef]
| Age (years) | 30.8 ± 11.6 |
| Male | 13 (65%) |
| Female | 7 (35%) |
| Disease Duration (years) | 8 (IQR 2.5–11) |
| BMI (kg/m2) | 19.2 (IQR 17.7–21) |
| Albumin (g/dL) | 2.7 (IQR 2.5–3.4) |
| CRP (mg/L) | 57.2 (IQR 30.4–110) |
| Fecal Calprotectin (μg/g) | 679 (IQR 332–924) |
| Disease Location: | |
| L1 | 7 (35%) |
| L2 | 1 (5%) |
| L3 | 6 (30%) |
| L1, L4b | 5 (25%) |
| L3, L4b | 1 (5%) |
| Disease Behavior: | |
| B2 | 9 (45%) |
| B2, B3 | 3 (15%) |
| B1, B2 | 4 (20%) |
| B2, P | 1 (5%) |
| B3, P | 1 (5%) |
| B1, B2, P | 1 (5%) |
| B2, B3, P | 1 (5%) |
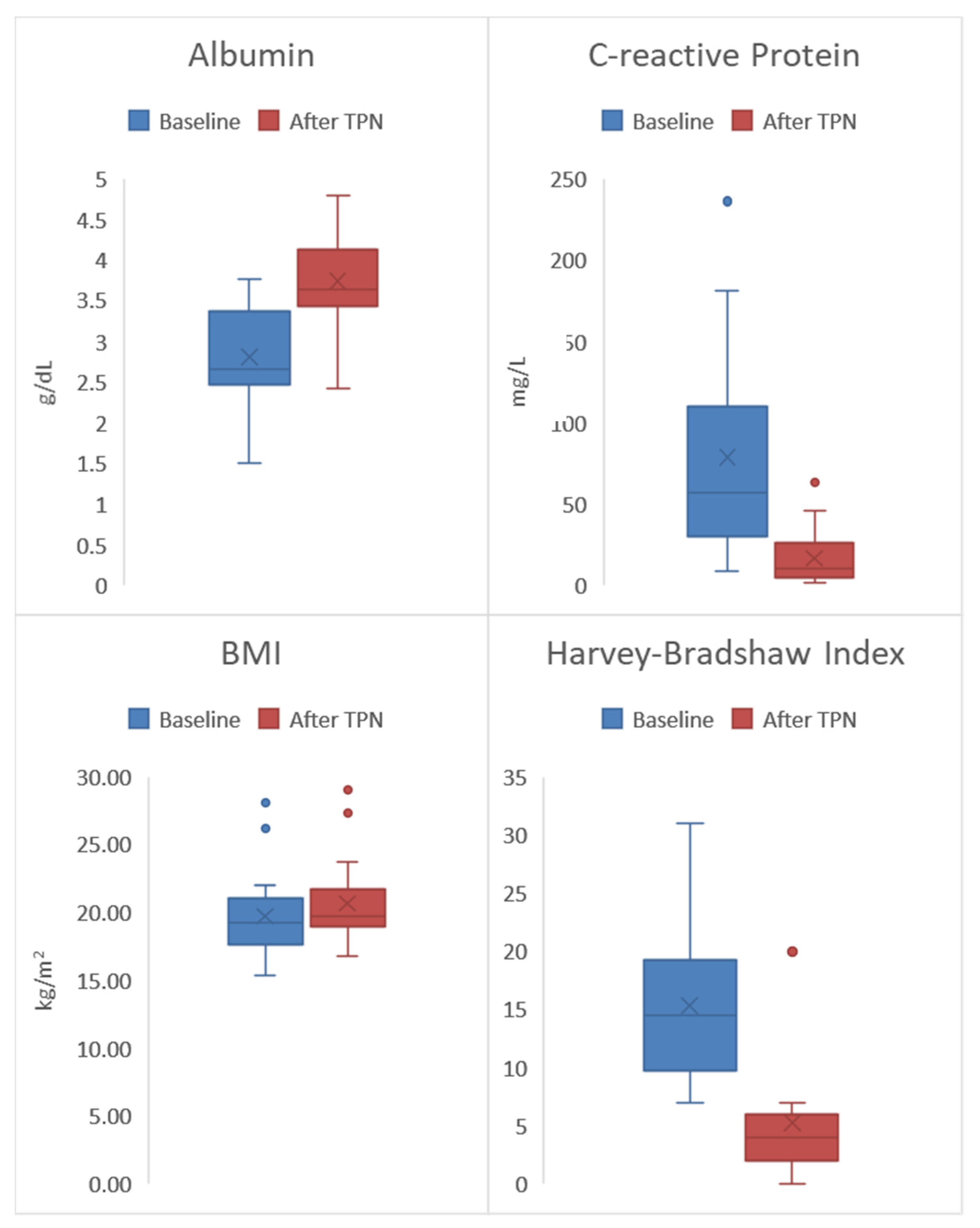
| Assessment | Baseline | After TPN Treatment | p-Value |
|---|---|---|---|
| Albumin (g/dL) | 2.7 (IQR 2.5–3.4) | 3.6 (IQR 3.4–4.1) | 0.001 |
| CRP (mg/L) | 57.2 (IQR 30.4–110) | 10.3 (IQR 5–26.7) | 0.001 |
| Fecal Calprotectin (μg/g) | 679 (IQR 332–924) | 200 (IQR 127–278) | 0.001 |
| BMI (kg/m2) | 19.2 (IQR 17.7–21) | 19.7 (IQR 18.9–21.8) | 0.017 |
| HBI | 14.5 (IQR 9.7–19.2) | 4 (IQR 2–6) | 0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zittan, E.; Gralnek, I.M.; Hatoum, O.A.; Sakran, N.; Kolonimos, N. Preoperative Exclusive Total Parental Nutrition is Associated with Clinical and Laboratory Remission in Severe Active Crohn’s Disease—A Pilot Study. Nutrients 2020, 12, 1244. https://doi.org/10.3390/nu12051244
Zittan E, Gralnek IM, Hatoum OA, Sakran N, Kolonimos N. Preoperative Exclusive Total Parental Nutrition is Associated with Clinical and Laboratory Remission in Severe Active Crohn’s Disease—A Pilot Study. Nutrients. 2020; 12(5):1244. https://doi.org/10.3390/nu12051244
Chicago/Turabian StyleZittan, Eran, Ian M. Gralnek, Ossama A. Hatoum, Nasser Sakran, and Nitzan Kolonimos. 2020. "Preoperative Exclusive Total Parental Nutrition is Associated with Clinical and Laboratory Remission in Severe Active Crohn’s Disease—A Pilot Study" Nutrients 12, no. 5: 1244. https://doi.org/10.3390/nu12051244
APA StyleZittan, E., Gralnek, I. M., Hatoum, O. A., Sakran, N., & Kolonimos, N. (2020). Preoperative Exclusive Total Parental Nutrition is Associated with Clinical and Laboratory Remission in Severe Active Crohn’s Disease—A Pilot Study. Nutrients, 12(5), 1244. https://doi.org/10.3390/nu12051244







