Exposure to the Danish Mandatory Vitamin D Fortification Policy in Prenatal Life and the Risk of Developing Coeliac Disease—The Importance of Season: A Semi Ecological Study
Abstract
1. Introduction
- (i)
- To investigate if season of birth and the concomitant production of vitamin D from sunlight was associated with the risk of developing CD
- (ii)
- To investigate if individuals with fetal exposure to extra vitamin D from the mandatory vitamin D food fortification policy had a decreased risk of developing CD later in life compared to individuals with no fetal exposure
- (iii)
- To examine if the risk of developing CD related to prenatal exposure to extra vitamin D from the fortification policy was dependent on season of birth and if risk reduction is potentially stronger for summer born compared to winter born children
2. Materials and Methods
2.1. Study Design
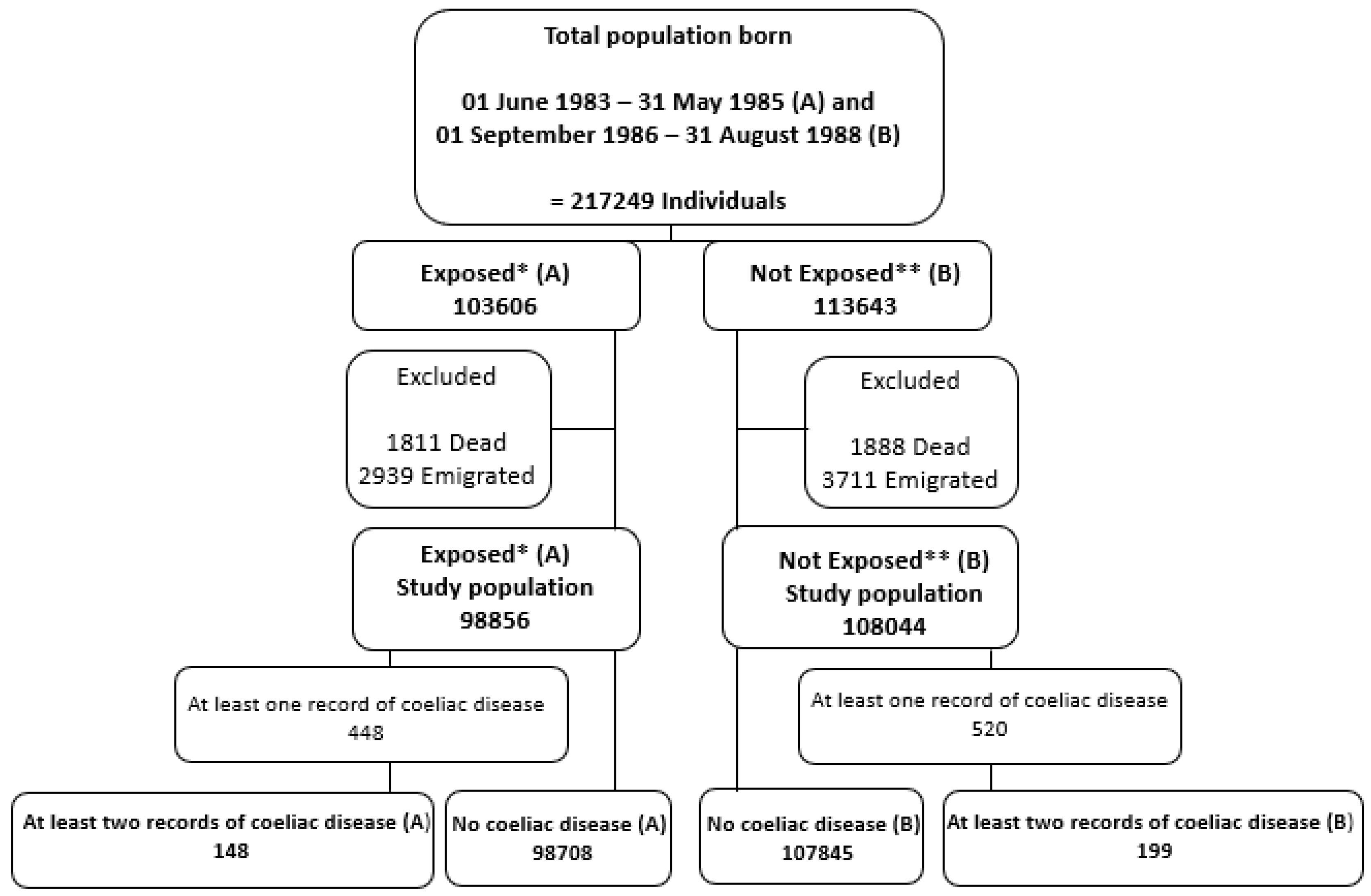
2.2. Study Population
2.3. Washout Period
2.4. Sources of Data
2.5. Exposure(s)
2.6. Outcome—Coeliac Disease
2.7. Statistical Analysis
2.8. Ethics
3. Results
4. Discussion
4.1. Season of Birth and Risk of Developing CD
4.2. Exposure to Extra Vitamin D from Fortification and The Development of CD
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lerner, A.; Jeremias, P.; Matthias, T. The world incidence and prevalence of autoimmune diseases is increasing. Int. J. Celiac Dis. 2016, 3, 151–155. [Google Scholar] [CrossRef]
- Grode, L.; Bech, B.H.; Jensen, T.M.; Humaidan, P.; Agerholm, I.E.; Plana-Ripoll, O.; Ramlau-Hansen, C.H. Prevalence, incidence, and autoimmune comorbidities of celiac disease: a nation-wide, population-based study in Denmark from 1977 to 2016. Eur. J. Gastroenterol. Hepatol. 2018, 30, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, A.; Skaaby, T.; Kårhus, L.L.; Schwarz, P.; Jørgensen, T.; Rumessen, J.J.; Linneberg, A. Screening for celiac disease in Danish adults. Scand. J. Gastroenterol. 2015, 50, 824–831. [Google Scholar] [CrossRef]
- Lebwohl, B.; Sanders, D.S.; Green, P.H.R. Coeliac disease. Lancet 2018, 391, 70–81. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Murray, J.A. Epidemiology of celiac disease. Gastroenterol. Clin. N. Am. 2019, 48, 1–18. [Google Scholar] [CrossRef] [PubMed]
- McAllister, B.P.; Williams, E.; Clarke, K. A comprehensive review of celiac disease/gluten-sensitive enteropathies. Clin. Rev. Allergy Immunol. 2018, 1–18. [Google Scholar] [CrossRef]
- Green, P.H.; Cellier, C. Celiac disease. N. Engl. J. Med. 2007, 357, 1731–1743. [Google Scholar] [CrossRef]
- Husby, S.; Koletzko, S.; Korponay-Szabo, I.R.; Mearin, M.L.; Phillips, A.; Shamir, R.; Troncone, R.; Giersiepen, K.; Branski, D.; Catassi, C.; et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 136–160. [Google Scholar] [CrossRef]
- Marild, K.; Tapia, G.; Midttun, O.; Ueland, P.M.; Magnus, M.C.; Rewers, M.; Stene, L.C.; Stordal, K. Smoking in pregnancy, cord blood cotinine and risk of celiac disease diagnosis in offspring. Eur. J. Epidemiol. 2019, 34, 637–649. [Google Scholar] [CrossRef]
- Altieri, B.; Muscogiuri, G.; Barrea, L.; Mathieu, C.; Vallone, C.V.; Mascitelli, L.; Bizzaro, G.; Altieri, V.M.; Tirabassi, G.; Balercia, G.; et al. Does vitamin D play a role in autoimmune endocrine disorders? A proof of concept. Rev. Endocr. Metab. Disord. 2017, 18, 335–346. [Google Scholar] [CrossRef]
- Palacios, C.; Kostiuk, L.K.; Pena-Rosas, J.P. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2019, 7, Cd008873. [Google Scholar] [CrossRef] [PubMed]
- Ivarsson, A.; Hernell, O.; Nystrom, L.; Persson, L.A. Children born in the summer have increased risk for coeliac disease. J. Epidemiol. Community Health 2003, 57, 36–39. [Google Scholar] [CrossRef][Green Version]
- Lewy, H.; Meirson, H.; Laron, Z. Seasonality of birth month of children with celiac disease differs from that in the general population and between sexes and is linked to family history and environmental factors. J. Pediatr. Gastroenterol. Nutr. 2009, 48, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, B.; Green, P.H.; Murray, J.A.; Ludvigsson, J.F. Season of birth in a nationwide cohort of coeliac disease patients. Arch. Dis. Child. 2013, 98, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Capriati, T.; Francavilla, R.; Castellaneta, S.; Ferretti, F.; Diamanti, A. Impact of the birth’s season on the development of celiac disease in Italy. Eur. J. Pediatr. 2015, 174, 1657–1663. [Google Scholar] [CrossRef]
- Namatovu, F.; Lindkvist, M.; Olsson, C.; Ivarsson, A.; Sandstrom, O. Season and region of birth as risk factors for coeliac disease a key to the aetiology? Arch. Dis. Child. 2016, 101, 1114–1118. [Google Scholar] [CrossRef]
- Unalp-Arida, A.; Ruhl, C.E.; Choung, R.S.; Brantner, T.L.; Murray, J.A. Lower prevalence of celiac disease and gluten-related disorders in persons living in southern vs northern latitudes of the United States. Gastroenterology 2017, 152, 1922–1932.e1922. [Google Scholar] [CrossRef] [PubMed]
- Tanpowpong, P.; Camargo, C.A. Early-life vitamin D deficiency and childhood-onset coeliac disease. Public Health Nutr. 2014, 17, 823–826. [Google Scholar] [CrossRef]
- Wei, R.; Christakos, S. Mechanisms underlying the regulation of innate and adaptive immunity by vitamin D. Nutrients 2015, 7, 8251–8260. [Google Scholar] [CrossRef] [PubMed]
- Hornsby, E.; Pfeffer, P.E.; Laranjo, N.; Cruikshank, W.; Tuzova, M.; Litonjua, A.A.; Weiss, S.T.; Carey, V.J.; O’Connor, G.; Hawrylowicz, C. Vitamin D supplementation during pregnancy: Effect on the neonatal immune system in a randomized controlled trial. J. Allergy Clin. Immunol. 2018, 141, 269–278.e261. [Google Scholar] [CrossRef] [PubMed]
- Marild, K.; Tapia, G.; Haugen, M.; Dahl, S.R.; Cohen, A.S.; Lundqvist, M.; Lie, B.A.; Stene, L.C.; Stordal, K. Maternal and neonatal vitamin D status, genotype and childhood celiac disease. PLoS ONE 2017, 12, e0179080. [Google Scholar] [CrossRef]
- Yang, J.; Tamura, R.N.; Aronsson, C.A.; Uusitalo, U.M.; Lernmark, A.; Rewers, M.; Hagopian, W.A.; She, J.X.; Toppari, J.; Ziegler, A.G.; et al. Maternal use of dietary supplements during pregnancy is not associated with coeliac disease in the offspring: The Environmental Determinants of Diabetes in the Young (TEDDY) study. Br. J. Nutr. 2017, 117, 466–472. [Google Scholar] [CrossRef]
- Jacobsen, R.; Abrahamsen, B.; Bauerek, M.; Holst, C.; Jensen, C.B.; Knop, J.; Raymond, K.; Rasmussen, L.B.; Stougaard, M.; Sorensen, T.I.; et al. The influence of early exposure to vitamin D for development of diseases later in life. BMC Public Health 2013, 13, 515. [Google Scholar] [CrossRef]
- Morgenstern, H. Ecologic studies in epidemiology: Concepts, Principles, and Methods Annu. Rev. Public Health 1995, 16, 61–81. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, R.; Hypponen, E.; Sorensen, T.I.; Vaag, A.A.; Heitmann, B.L. Gestational and early infancy exposure to margarine fortified with vitamin D through a national Danish programme and the risk of type 1 diabetes: The D-Tect Study. PLoS ONE 2015, 10, e0128631. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Schmidt, S.A.; Sandegaard, J.L.; Ehrenstein, V.; Pedersen, L.; Sorensen, H.T. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin. Epidemiol. 2015, 7, 449–490. [Google Scholar] [CrossRef] [PubMed]
- Lynge, E.; Sandegaard, J.L.; Rebolj, M. The Danish National Patient Register. Scand. J. Public Health 2011, 39, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Mainz, J.; Hess, M.H.; Johnsen, S.P. The Danish unique personal identifier and the Danish Civil Registration System as a tool for research and quality improvement. Int. J. Qual. Health Care 2019. [Google Scholar] [CrossRef]
- Schmidt, M.; Pedersen, L.; Sorensen, H.T. Immunological role of vitamin D at the maternal-fetal interface. Eur. J. Epidemiol. 2014, 29, 541–549. [Google Scholar] [CrossRef]
- Hypponen, E.; Power, C. Hypovitaminosis D in British adults at age 45 y: nationwide cohort study of dietary and lifestyle predictors. Am. J. Clin. Nutr. 2007, 85, 860–868. [Google Scholar] [CrossRef]
- Dydensborg, S.; Størdal, K.; Plato Hansen, T.; Nybo Andersen, A.M.; Murray, J.A.; Lillevang, S.T.; Husby, S. Validation of celiac disease diagnoses recorded in the Danish National Patient Register using duodenal biopsies, celiac disease-specific antibodies, and human leukocyte-antigen genotypes. Clin. Epidemiol. 2016, 8, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Collin, P.; Salmi, T.T.; Hervonen, K.; Kaukinen, K.; Reunala, T. Dermatitis herpetiformis: a cutaneous manifestation of coeliac disease. Ann. Med. 2017, 49, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Ghareghani, M.; Reiter, R.J.; Zibara, K.; Farhadi, N. Latitude, vitamin D, melatonin, and gut microbiota act in concert to initiate multiple sclerosis: A new mechanistic pathway. Front. Immunol. 2018, 9, 2484. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.; Tjonneland, A.; Koster, B.; Brot, C.; Andersen, R.; Cohen, A.S.; Frederiksen, K.; Olsen, A. Vitamin D status and seasonal variation among Danish children and adults: A descriptive study. Nutrients 2018, 10, 1801. [Google Scholar] [CrossRef] [PubMed]
- Ovesen, L.; Andersen, R.; Jakobsen, J. Geographical differences in vitamin D status, with particular reference to European countries. Proc. Nutr. Soc. 2003, 62, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D: A millenium perspective. J. Cell. Biochem. 2003, 88, 296–307. [Google Scholar] [CrossRef]
- Tanpowpong, P.; Obuch, J.C.; Jiang, H.; McCarty, C.E.; Katz, A.J.; Leffler, D.A.; Kelly, C.P.; Weir, D.C.; Leichtner, A.M.; Camargo, C.A., Jr. Multicenter study on season of birth and celiac disease: evidence for a new theoretical model of pathogenesis. J. Pediatr. 2013, 162, 501–504. [Google Scholar] [CrossRef]
- Assa, A.; Waisbourd-Zinman, O.; Daher, S.; Shamir, R. Birth month as a risk factor for the diagnosis of celiac disease later in life: A population-based study. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 367–370. [Google Scholar] [CrossRef]
- Kahrs, C.R.; Chuda, K.; Tapia, G.; Stene, L.C.; Marild, K.; Rasmussen, T.; Ronningen, K.S.; Lundin, K.E.A.; Kramna, L.; Cinek, O.; et al. Enterovirus as trigger of coeliac disease: nested case-control study within prospective birth cohort. BMJ 2019, 364, l231. [Google Scholar] [CrossRef]
- Bouziat, R.; Hinterleitner, R.; Brown, J.J.; Stencel-Baerenwald, J.E.; Ikizler, M.; Mayassi, T.; Meisel, M.; Kim, S.M.; Discepolo, V.; Pruijssers, A.J.; et al. Reovirus infection triggers inflammatory responses to dietary antigens and development of celiac disease. Science 2017, 356, 44–50. [Google Scholar] [CrossRef]
- Haraldsdottir, J.; Thaarup, S. Tilsætning af vitaminer og milderaler til levnedsmidler; Nordisk Ministerråd: Copenhagen, Denmark, 1989. [Google Scholar]
- Caio, G.; Volta, U.; Sapone, A.; Leffler, D.A.; De Giorgio, R.; Catassi, C.; Fasano, A. Celiac disease: a comprehensive current review. BMC Med. 2019, 17, 142. [Google Scholar] [CrossRef] [PubMed]
- McGovern, N.; Shin, A.; Low, G.; Low, D.; Duan, K.; Yao, L.J.; Msallam, R.; Low, I.; Shadan, N.B.; Sumatoh, H.R.; et al. Human fetal dendritic cells promote prenatal T-cell immune suppression through arginase-2. Nature 2017, 546, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.L.; Hollis, B.W. The implications of vitamin D status during pregnancy on mother and her developing child. Front. Endocrinol. (Lausanne) 2018, 9, 500. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.; Angquist, L.; Jacobsen, R.; Vaag, A.; Heitmann, B.L. A retrospective analysis of a societal experiment among the Danish population suggests that exposure to extra doses of vitamin A during fetal development may lower type 2 diabetes mellitus (T2DM) risk later in life. Br. J. Nutr. 2017, 117, 731–736. [Google Scholar] [CrossRef]
- Stougaard, M.; Damm, P.; Frederiksen, P.; Jacobsen, R.; Heitmann, B.L. Exposure to vitamin D from fortified margarine during fetal life and later risk of pre-eclampsia: the D-tect Study. Public Health Nutr. 2018, 21, 721–731. [Google Scholar] [CrossRef]
- Jacobsen, R.; Moldovan, M.; Vaag, A.A.; Hypponen, E.; Heitmann, B.L. Vitamin D fortification and seasonality of birth in type 1 diabetic cases: D-tect study. J. Dev. Orig. Health Dis. 2016, 7, 114–119. [Google Scholar] [CrossRef]
- Jacobsen, R.; Moldovan, M.; Vaag, A.A.; Hypponen, E.; Heitmann, B.L. Vitamin D fortification and seasonality of birth in type 1 diabetic cases: D-tect study - ERRATUM. J. Dev. Orig. Health Dis. 2016, 7, 429. [Google Scholar] [CrossRef]
- Thorsteinsdottir, F.; Maslova, E.; Jacobsen, R.; Frederiksen, P.; Keller, A.; Backer, V.; Heitmann, B.L. Exposure to Vitamin D Fortification Policy in Prenatal Life and the Risk of Childhood Asthma: Results From the D-Tect Study. Nutrients 2019, 11, 924. [Google Scholar] [CrossRef]
- Aarkrog, A. Studies of chernobyl debris in Denmark. Environ. Int. 1988, 14, 149–155. [Google Scholar] [CrossRef]
| CD (n = 347) | No CD (n = 206,553) | |||||
|---|---|---|---|---|---|---|
| n | % | n | % | p–Value 1 | ||
| Gender | <0.001 | |||||
| Women | 221 | 63.7 | 100,801 | 48.8 | ||
| Men | 126 | 36.3 | 105,752 | 51.2 | ||
| Season of birth | 0.02 | |||||
| Nov–Jan (winter) | 58 | 16.7 | 46,696 | 22.6 | ||
| Feb–Apr (spring) | 83 | 23.9 | 52,745 | 25.5 | ||
| May–Jul (summer) | 103 | 29.7 | 55,064 | 26.7 | ||
| Aug–Oct (autumn) | 103 | 29.7 | 52,048 | 25.2 | ||
| Age at diagnosis 2 | <0.001 | |||||
| <2 years | 100 | 28.8 | ||||
| 2–14 years | 54 | 15.6 | ||||
| 15+ years | 193 | 55.6 | ||||
| Odds Ratio | 95% CI 1 | p–Value | ||
|---|---|---|---|---|
| Vitamin D fortification policy 2 | 0.054 | |||
| Not exposed (ref) | 1 | |||
| exposed | 0.81 | 0.66; 1.00 | ||
| Gender | <0.001 | |||
| Women (ref) | 1 | |||
| Men | 0.54 | 0.44; 0.68 | ||
| Season of birth 3 | 0.02 | |||
| Nov–Jan (winter)(ref) | 1 | |||
| Feb–Apr (spring) | 1.28 | 0.91; 1.78 | 0.16 | |
| May–Jul (summer) | 1.51 | 1.10; 2.09 | 0.01 | |
| Aug–Oct (autumn) | 1.60 | 1.16; 2.21 | 0.004 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moos, C.; Duus, K.S.; Frederiksen, P.; Heitmann, B.L.; Andersen, V. Exposure to the Danish Mandatory Vitamin D Fortification Policy in Prenatal Life and the Risk of Developing Coeliac Disease—The Importance of Season: A Semi Ecological Study. Nutrients 2020, 12, 1243. https://doi.org/10.3390/nu12051243
Moos C, Duus KS, Frederiksen P, Heitmann BL, Andersen V. Exposure to the Danish Mandatory Vitamin D Fortification Policy in Prenatal Life and the Risk of Developing Coeliac Disease—The Importance of Season: A Semi Ecological Study. Nutrients. 2020; 12(5):1243. https://doi.org/10.3390/nu12051243
Chicago/Turabian StyleMoos, Caroline, Katrine S. Duus, Peder Frederiksen, Berit L. Heitmann, and Vibeke Andersen. 2020. "Exposure to the Danish Mandatory Vitamin D Fortification Policy in Prenatal Life and the Risk of Developing Coeliac Disease—The Importance of Season: A Semi Ecological Study" Nutrients 12, no. 5: 1243. https://doi.org/10.3390/nu12051243
APA StyleMoos, C., Duus, K. S., Frederiksen, P., Heitmann, B. L., & Andersen, V. (2020). Exposure to the Danish Mandatory Vitamin D Fortification Policy in Prenatal Life and the Risk of Developing Coeliac Disease—The Importance of Season: A Semi Ecological Study. Nutrients, 12(5), 1243. https://doi.org/10.3390/nu12051243





