Association between Polyphenol Intake and Breast Cancer Risk by Menopausal and Hormone Receptor Status
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Statistical Analysis
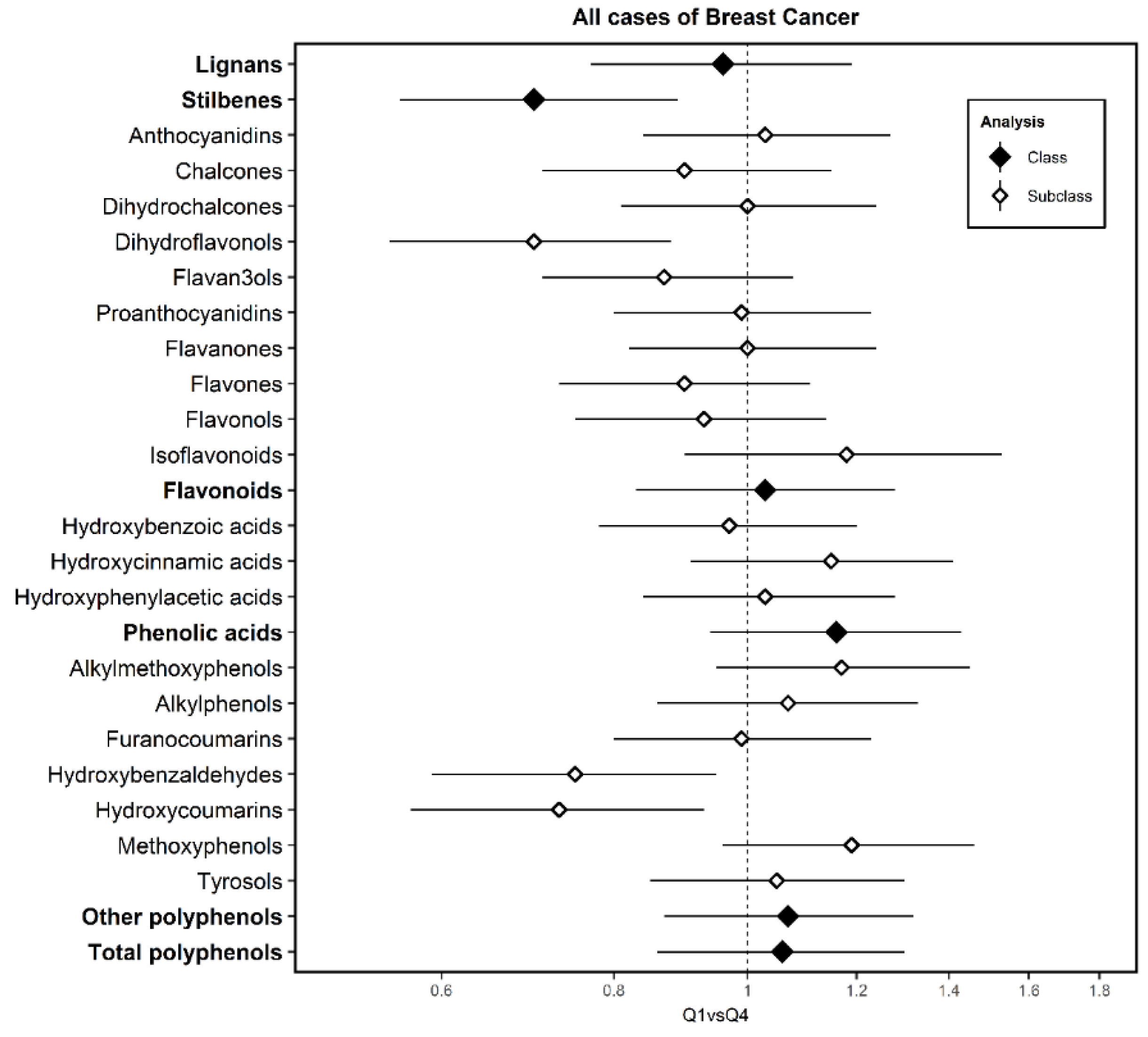
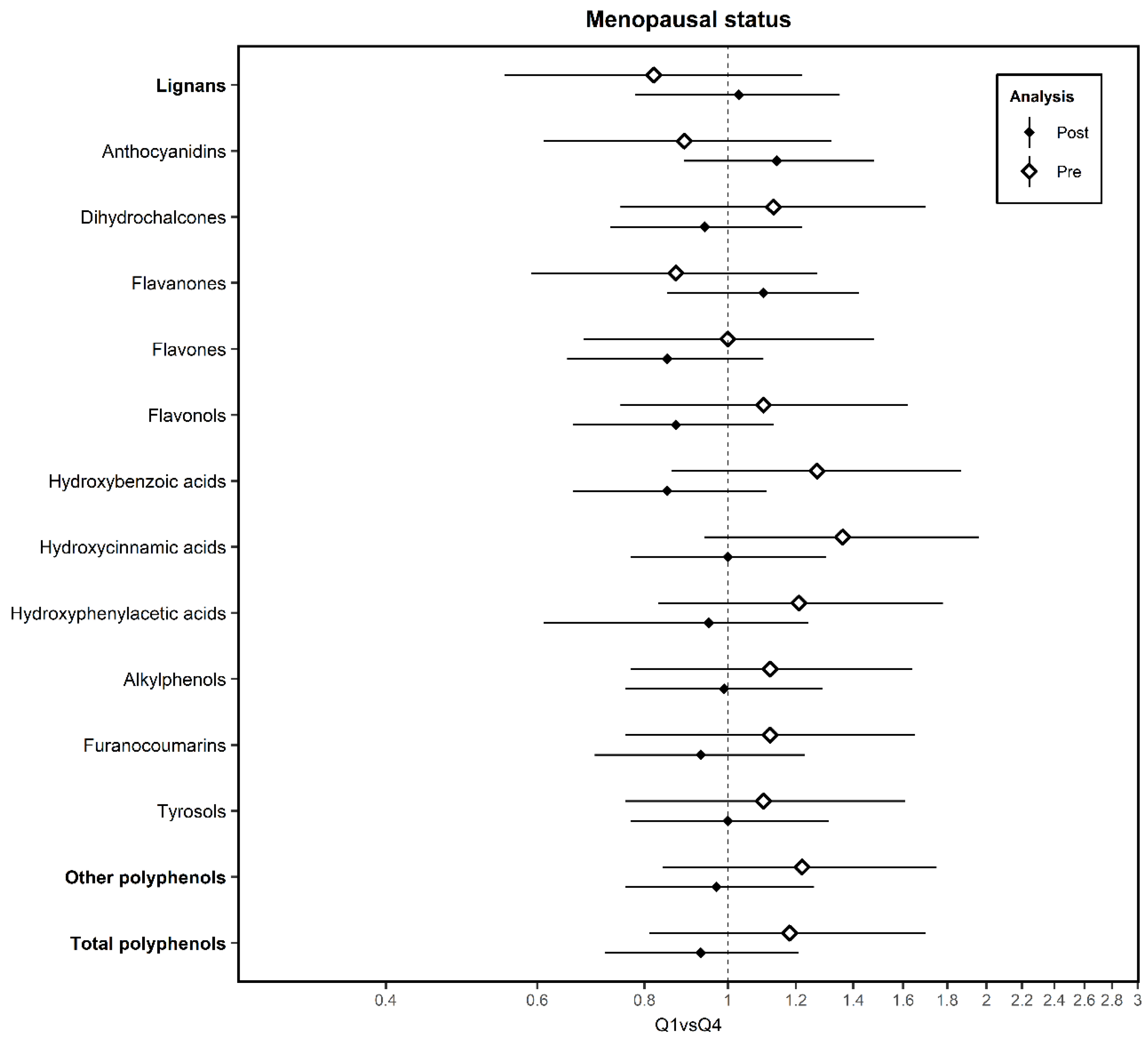
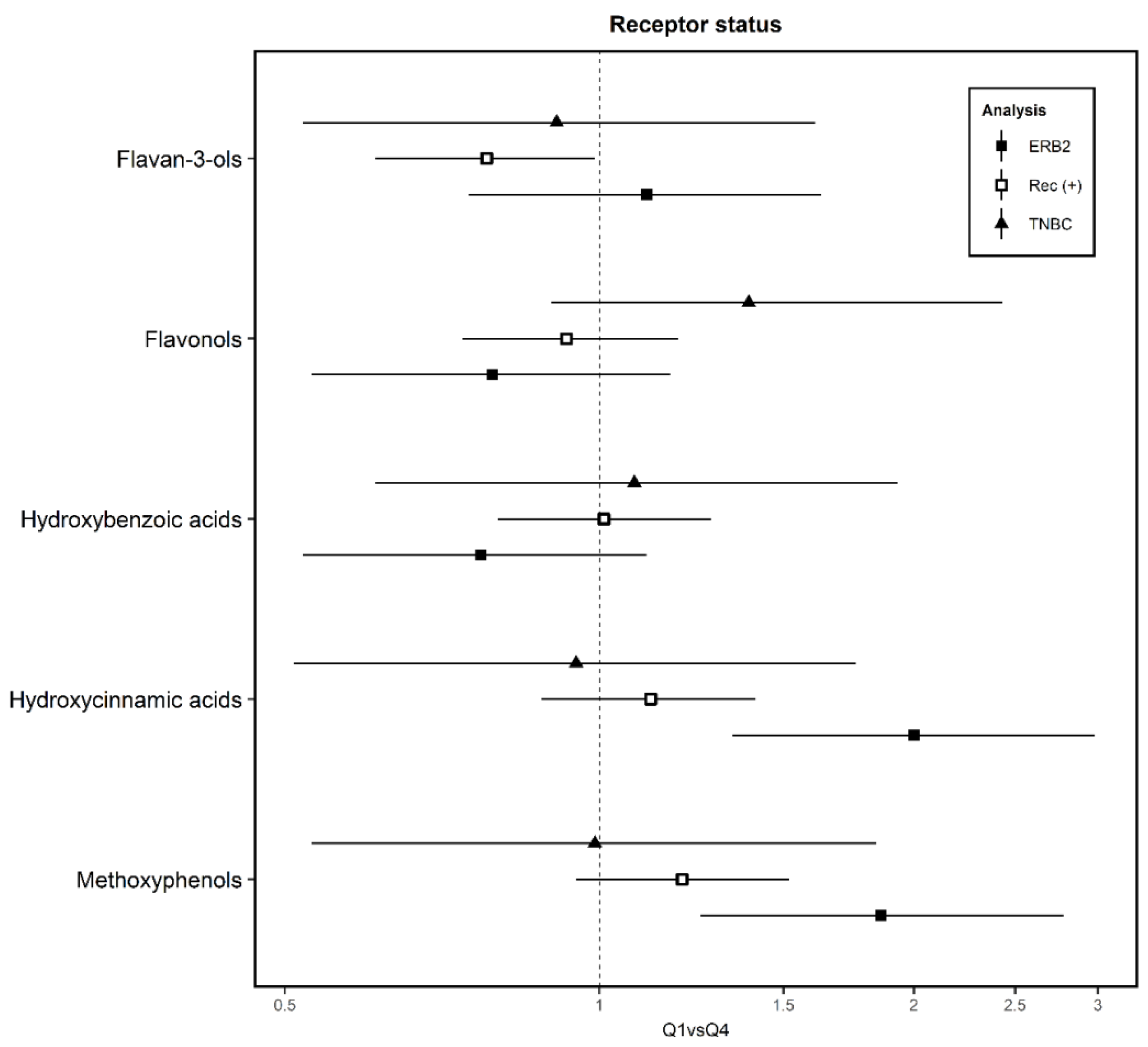
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hui, C.; Qi, X.; Qianyong, Z.; Xiaoli, P.; Jundong, Z.; Mantian, M. Flavonoids, flavonoid subclasses and breast cancer risk: A meta-analysis of epidemiologic studies. PLoS ONE 2013, 8, e54318. [Google Scholar] [CrossRef] [PubMed]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004, 134, 3479S–3485S. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Perez, C.; Ward, C.; Cook, G.; Mullen, P.; McPhail, D.; Harrison, D.J.; Langdon, S.P. Novel flavonoids as anti-cancer agents: Mechanisms of action and promise for their potential application in breast cancer. Biochem. Soc. Trans. 2014, 42, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Garcia, C.; Sanchez-Quesada, C.; Gaforio, J.J. Dietary Flavonoids as Cancer Chemopreventive Agents: An Updated Review of Human Studies. Antioxidants 2019, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Azqueta, A.; Collins, A. Polyphenols and DNA Damage: A Mixed Blessing. Nutrients 2016, 8, 785. [Google Scholar] [CrossRef]
- Ramos, S. Effects of dietary flavonoids on apoptotic pathways related to cancer chemoprevention. J. Nutr. Biochem. 2007, 18, 427–442. [Google Scholar] [CrossRef]
- Oh, J.; Hlatky, L.; Jeong, Y.S.; Kim, D. Therapeutic Effectiveness of Anticancer Phytochemicals on Cancer Stem Cells. Toxins 2016, 8, 199. [Google Scholar] [CrossRef]
- Sznarkowska, A.; Kostecka, A.; Meller, K.; Bielawski, K.P. Inhibition of cancer antioxidant defense by natural compounds. Oncotarget 2017, 8, 15996–16016. [Google Scholar] [CrossRef]
- Khalid, E.B.; Ayman, E.E.; Rahman, H.; Abdelkarim, G.; Najda, A. Natural products against cancer angiogenesis. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2016, 37, 14513–14536. [Google Scholar] [CrossRef]
- Kandaswami, C.; Lee, L.T.; Lee, P.P.; Hwang, J.J.; Ke, F.C.; Huang, Y.T.; Lee, M.T. The antitumor activities of flavonoids. In Vivo 2005, 19, 895–909. [Google Scholar] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018. [Google Scholar] [CrossRef] [PubMed]
- World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. Continuous Update Project Expert Report. Available online: https://www.wcrf.org/dietandcancer (accessed on 24 January 2020).
- Ennour-Idrissi, K.; Maunsell, E.; Diorio, C. Effect of physical activity on sex hormones in women: A systematic review and meta-analysis of randomized controlled trials. Breast Cancer Res. BCR 2015, 17, 139. [Google Scholar] [CrossRef] [PubMed]
- Duncan, A.M. The role of nutrition in the prevention of breast cancer. AACN Clin. Issues 2004, 15, 119–135. [Google Scholar] [CrossRef]
- Matsumura, A.; Ghosh, A.; Pope, G.S.; Darbre, P.D. Comparative study of oestrogenic properties of eight phytoestrogens in MCF7 human breast cancer cells. J. Steroid Biochem. Mol. Biol. 2005, 94, 431–443. [Google Scholar] [CrossRef]
- Rice, S.; Whitehead, S.A. Phytoestrogens and breast cancer--promoters or protectors? Endocr. Relat. Cancer 2006, 13, 995–1015. [Google Scholar] [CrossRef]
- Kyro, C.; Zamora-Ros, R.; Scalbert, A.; Tjonneland, A.; Dossus, L.; Johansen, C.; Bidstrup, P.E.; Weiderpass, E.; Christensen, J.; Ward, H.; et al. Pre-diagnostic polyphenol intake and breast cancer survival: The European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Breast Cancer Res. Treat. 2015, 154, 389–401. [Google Scholar] [CrossRef]
- Touvier, M.; Druesne-Pecollo, N.; Kesse-Guyot, E.; Andreeva, V.A.; Fezeu, L.; Galan, P.; Hercberg, S.; Latino-Martel, P. Dual association between polyphenol intake and breast cancer risk according to alcohol consumption level: A prospective cohort study. Breast Cancer Res. Treat. 2013, 137, 225–236. [Google Scholar] [CrossRef]
- Sak, K. Epidemiological Evidences on Dietary Flavonoids and Breast Cancer Risk: A Narrative Review. Asian Pac. J. Cancer Prev. APJCP 2017, 18, 2309–2328. [Google Scholar] [CrossRef]
- Baena Ruiz, R.; Salinas Hernandez, P. Cancer chemoprevention by dietary phytochemicals: Epidemiological evidence. Maturitas 2016, 94, 13–19. [Google Scholar] [CrossRef]
- MCC-Spain, Estudio Multi-Caso Control Poblacional. Available online: http://www.mccspain.org/ (accessed on 15 January 2020).
- Castano-Vinyals, G.; Aragones, N.; Perez-Gomez, B.; Martin, V.; Llorca, J.; Moreno, V.; Altzibar, J.M.; Ardanaz, E.; de Sanjose, S.; Jimenez-Moleon, J.J.; et al. Population-based multicase-control study in common tumors in Spain (MCC-Spain): Rationale and study design. Gac. Sanit. 2015, 29, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Closas, R.; Garcia-Closas, M.; Kogevinas, M.; Malats, N.; Silverman, D.; Serra, C.; Tardon, A.; Carrato, A.; Castano-Vinyals, G.; Dosemeci, M.; et al. Food, nutrient and heterocyclic amine intake and the risk of bladder cancer. Eur. J. Cancer 2007, 43, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Calvert, C.; Cade, J.; Barrett, J.H.; Woodhouse, A. Using cross-check questions to address the problem of mis-reporting of specific food groups on Food Frequency Questionnaires. UKWCS Steering Group. United Kingdom Women’s Cohort Study Steering Group. Eur. J. Clin. Nutr. 1997, 51, 708–712. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remon, A.; M’Hiri, N.; Garcia-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database J. Biol. Databases Curation 2013, 2013, bat070. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture, Agricultural Research Service. USDA Food and Nutrient Database for Dietary Studies 2011–2012. Available online: https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/fndds/fndds_2011_2012.pdf (accessed on 24 January 2020).
- Zamora-Ros, R.; Ferrari, P.; Gonzalez, C.A.; Tjonneland, A.; Olsen, A.; Bredsdorff, L.; Overvad, K.; Touillaud, M.; Perquier, F.; Fagherazzi, G.; et al. Dietary flavonoid and lignan intake and breast cancer risk according to menopause and hormone receptor status in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Breast Cancer Res. Treat. 2013, 139, 163–176. [Google Scholar] [CrossRef]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S. [Google Scholar] [CrossRef]
- Zumel, N.; Mount, J. Practical Data Science with R.; Manning Publications Co.: 2014. Available online: https://www.manning.com/books/practical-data-science-with-r-second-edition (accessed on 24 January 2020).
- Wiseman, M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: A global perspective. Proc. Nutr. Soc. 2008, 67, 253–256. [Google Scholar] [CrossRef]
- StataCorp. Stata Statistical Software: Release 13; StataCorp LP: College Station, TX, USA, 2013. [Google Scholar]
- Python Software Foundation. Python Language Reference, 3.6.5. 2018. Available online: https://www.stata.com/ (accessed on 24 January 2020).
- R Development Core Team. R: A Language and Environment for Statistical Computing, 3.4.3; R Foundation for Statistical Computing: Vienna, Austria, 2010. [Google Scholar]
- Levi, F.; Pasche, C.; Lucchini, F.; Ghidoni, R.; Ferraroni, M.; La Vecchia, C. Resveratrol and breast cancer risk. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. 2005, 14, 139–142. [Google Scholar] [CrossRef]
- Feng, X.L.; Ho, S.C.; Mo, X.F.; Lin, F.Y.; Zhang, N.Q.; Luo, H.; Zhang, X.; Zhang, C.X. Association between flavonoids, flavonoid subclasses intake and breast cancer risk: A case-control study in China. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. 2019. [Google Scholar] [CrossRef]
- Gardeazabal, I.; Romanos-Nanclares, A.; Martinez-Gonzalez, M.A.; Sanchez-Bayona, R.; Vitelli-Storelli, F.; Gaforio, J.J.; Aramendia-Beitia, J.M.; Toledo, E. Total polyphenol intake and breast cancer risk in the SUN cohort. Br. J. Nutr. 2018. [Google Scholar] [CrossRef]
- Fink, B.N.; Steck, S.E.; Wolff, M.S.; Britton, J.A.; Kabat, G.C.; Schroeder, J.C.; Teitelbaum, S.L.; Neugut, A.I.; Gammon, M.D. Dietary flavonoid intake and breast cancer risk among women on Long Island. Am. J. Epidemiol. 2007, 165, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Torres-Sanchez, L.; Galvan-Portillo, M.; Wolff, M.S.; Lopez-Carrillo, L. Dietary consumption of phytochemicals and breast cancer risk in Mexican women. Public Health Nutr. 2009, 12, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Torres-Sánchez, L.; Galván-Portillo, M.; Lewis, S.; Gómez-Dantés, H.; López-Carrillo, L. Dieta y cáncer de mama en latinoamérica. Salud Pública De México 2009, 51, s181–s190. [Google Scholar]
- Peterson, J.; Lagiou, P.; Samoli, E.; Lagiou, A.; Katsouyanni, K.; La Vecchia, C.; Dwyer, J.; Trichopoulos, D. Flavonoid intake and breast cancer risk: A case—Control study in Greece. Br. J. Cancer 2003, 89, 1255–1259. [Google Scholar] [CrossRef]
- Bosetti, C.; Spertini, L.; Parpinel, M.; Gnagnarella, P.; Lagiou, P.; Negri, E.; Franceschi, S.; Montella, M.; Peterson, J.; Dwyer, J.; et al. Flavonoids and breast cancer risk in Italy. Cancer Epidemiol. Biomark. Prev. A Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2005, 14, 805–808. [Google Scholar] [CrossRef]
- Qin, L.Q.; Xu, J.Y.; Wang, P.Y.; Hoshi, K. Soyfood intake in the prevention of breast cancer risk in women: A meta-analysis of observational epidemiological studies. J. Nutr. Sci. Vitaminol. 2006, 52, 428–436. [Google Scholar] [CrossRef][Green Version]
- Trock, B.J.; Hilakivi-Clarke, L.; Clarke, R. Meta-analysis of soy intake and breast cancer risk. J. Natl. Cancer Inst. 2006, 98, 459–471. [Google Scholar] [CrossRef]
- Dong, J.Y.; Qin, L.Q. Soy isoflavones consumption and risk of breast cancer incidence or recurrence: A meta-analysis of prospective studies. Breast Cancer Res. Treat. 2011, 125, 315–323. [Google Scholar] [CrossRef]
- Puranik, N.V.; Srivastava, P.; Bhatt, G.; John Mary, D.J.S.; Limaye, A.M.; Sivaraman, J. Determination and analysis of agonist and antagonist potential of naturally occurring flavonoids for estrogen receptor (ERalpha) by various parameters and molecular modelling approach. Sci. Rep. 2019, 9, 7450. [Google Scholar] [CrossRef]
- Hsieh, R.W.; Rajan, S.S.; Sharma, S.K.; Guo, Y.; DeSombre, E.R.; Mrksich, M.; Greene, G.L. Identification of ligands with bicyclic scaffolds provides insights into mechanisms of estrogen receptor subtype selectivity. J. Biol. Chem. 2006, 281, 17909–17919. [Google Scholar] [CrossRef]
- Kuiper, G.G.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; van der Saag, P.T.; van der Burg, B.; Gustafsson, J.A. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef] [PubMed]
- Basu, P.; Maier, C. Phytoestrogens and breast cancer: In vitro anticancer activities of isoflavones, lignans, coumestans, stilbenes and their analogs and derivatives. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 107, 1648–1666. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gapstur, S.M.; Gaudet, M.M.; Peterson, J.J.; Dwyer, J.T.; McCullough, M.L. Evidence for an association of dietary flavonoid intake with breast cancer risk by estrogen receptor status is limited. J. Nutr. 2014, 144, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Damianaki, A.; Bakogeorgou, E.; Kampa, M.; Notas, G.; Hatzoglou, A.; Panagiotou, S.; Gemetzi, C.; Kouroumalis, E.; Martin, P.M.; Castanas, E. Potent inhibitory action of red wine polyphenols on human breast cancer cells. J. Cell. Biochem. 2000, 78, 429–441. [Google Scholar] [CrossRef]
- Knott, C.S.; Coombs, N.; Stamatakis, E.; Biddulph, J.P. All cause mortality and the case for age specific alcohol consumption guidelines: Pooled analyses of up to 10 population based cohorts. BMJ 2015, 350, h384. [Google Scholar] [CrossRef]
- Grosso, G.; Bella, F.; Godos, J.; Sciacca, S.; Del Rio, D.; Ray, S.; Galvano, F.; Giovannucci, E.L. Possible role of diet in cancer: Systematic review and multiple meta-analyses of dietary patterns, lifestyle factors, and cancer risk. Nutr. Rev. 2017, 75, 405–419. [Google Scholar] [CrossRef]
- Wang, Y.; Stevens, V.L.; Shah, R.; Peterson, J.J.; Dwyer, J.T.; Gapstur, S.M.; McCullough, M.L. Dietary flavonoid and proanthocyanidin intakes and prostate cancer risk in a prospective cohort of US men. Am. J. Epidemiol. 2014, 179, 974–986. [Google Scholar] [CrossRef]
- Bo, Y.; Sun, J.; Wang, M.; Ding, J.; Lu, Q.; Yuan, L. Dietary flavonoid intake and the risk of digestive tract cancers: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 24836. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Knaze, V.; Rothwell, J.A.; Hemon, B.; Moskal, A.; Overvad, K.; Tjonneland, A.; Kyro, C.; Fagherazzi, G.; Boutron-Ruault, M.C.; et al. Dietary polyphenol intake in Europe: The European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur. J. Nutr. 2016, 55, 1359–1375. [Google Scholar] [CrossRef]
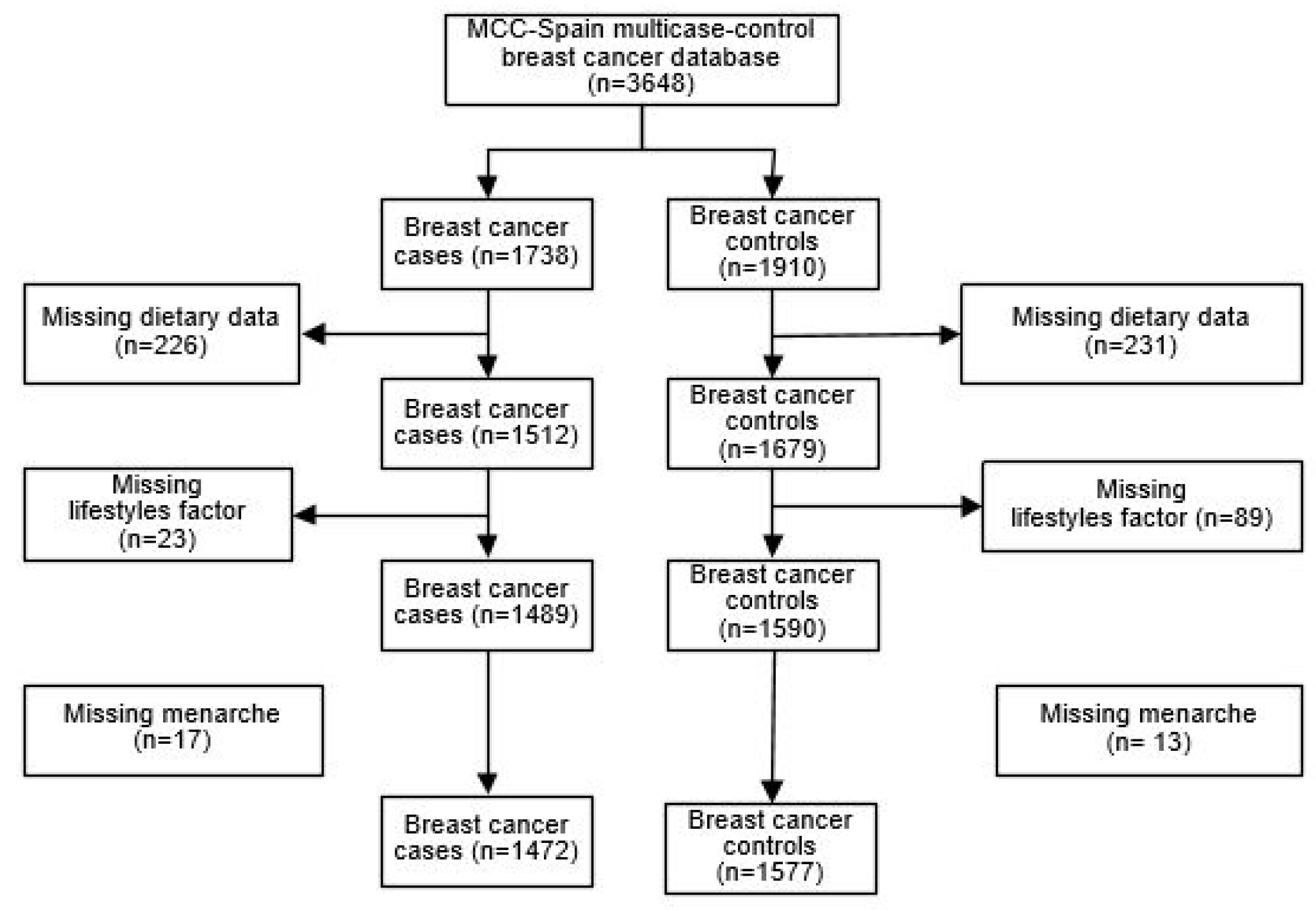
| Variables | Controls (N = 1577) | Breast Cancer Cases (N = 1472) | Premenopausal (N = 1006) | Postmenopausal (N = 2043) | |||
|---|---|---|---|---|---|---|---|
| Control (N = 471) | Cases (N = 535) | Control (N = 1106) | Cases (N = 937) | ||||
| Socioeconomic status | High (%) | 281 (17.78) | 238 (16.19) | 143 (30.36) | 129 (24.11) | 138 (12.48) | 109 (11.63) |
| Medium (%) | 8170 (51.90) | 786 (53.39) | 265 (56.26) | 335 (62.62) | 552 (49.51) | 451 (48.13) | |
| Low (%) | 479 (30.32) | 448 (30.42) | 63 (13.38) | 71 (13.27) | 416 (37.61) | 377 (40.23) | |
| Smoking status (%) | Yes | 639 (40.51) | 660 (44.92) | 261 (55.41) | 328 (61.31) | 378 (34.18) | 332 (35.43) |
| No | 938 (59.49) | 812 (55.08) | 210 (44.59) | 207 (38.69) | 728 (65.82) | 605 (64.57) | |
| Family history of breast cancer (%) | Yes | 145 (9.24) | 212 (14.36) | 25 (5.31) | 77 (14.39) | 120 (10.85) | 135 (14.41) |
| No | 1432 (90.76) | 1260 (85.64) | 446 (94.69) | 458 (85.61) | 986 (89.15) | 802 (85.59) | |
| NSAID (%) | Yes | 807 (51.14) | 656 (44.58) | 240 (50.96) | 235 (43.93) | 567 (51.27) | 421 (44.93) |
| No | 770 (48.86) | 816 (55.42) | 231 (49.04) | 300 (56.07) | 539 (48.73) | 516 (55.07) | |
| BMI (kg/m2) | <30 kg/m2 | 1313 (83.29) | 1214 (82.38) | 417 (88.54) | 491 (91.78) | 896 (81.01) | 723 (77.16) |
| ≥30 kg/m2 | 264 (16.71) | 268 (17.62) | 54 (11.46) | 44 (8.22) | 210 (18.99) | 214 (22.84) | |
| Alcohol consumption (g/day) | 0 g/day | 401 (25.57) | 357 (24.46) | 97 (20.59) | 97 (18.13) | 304 (27.49) | 260 (27.75) |
| 0–12 g/day | 965 (61.08) | 887 (60.09) | 320 (67.94) | 367 (69.60) | 645 (58.32) | 520 (55.50) | |
| 12–47 g/day | 192 (12.15) | 202 (13.69) | 50 (10.62) | 62 (11.59) | 142 (12.82) | 140 (14.94) | |
| >47 g/day | 19 (1.2) | 26 (1.76) | 4 (0.85) | 9 (1.68) | 15 (1.36) | 17 (1.81) | |
| Physical activity | 0 METS*h/week | 592 (37.59) | 629 (42.62) | 207 (43.95) | 242 (45.23) | 385 (34.81) | 387 (41.30) |
| 0–8 METS*h/week | 257 (16.33) | 229 (15.51) | 86 (18.26) | 101 (18.88) | 171 (14.46) | 128 (13.66) | |
| 8–16 METS*h/week | 227 (14.37) | 190 (12.94) | 66 (14.01) | 71 (13.27) | 161 (14.56) | 119 (12.70) | |
| >16 METS*h/week | 501 (31.71) | 424 (28.93) | 112 (23.78) | 121 (22.62) | 389 (35.17) | 303 (32.34) | |
| Oral contraceptive consumption | never | 792 (50.19) | 763 (51.83) | 139 (29.51) | 178 (33.27) | 653 (59.04) | 585 (62.43) |
| ever | 785 (49.81) | 709 (48.17) | 332 (70.49) | 357 (66.73) | 453 (40.96) | 352 (37.57) | |
| Hormone replace therapy | never | 1403 (88.99) | 1335 (90.65) | - | - | 933 (84.36) | 801 (85.49) |
| ever | 121 (7.66) | 104 (7.05) | 470 (99.79) | 534 (99.81) | 120 (10.85) | 103 (10.99) | |
| not known | 53 (3.35) | 33 (2.3) | 1 (0.21) | 2 (0.20) | 53 (4.79) | 33 (3.52) | |
| Number of children | 0 | 303 (19.18) | 309 (20.93) | 132 (28.03) | 137 (25.61) | 171 (15.46) | 171 (18.25) |
| 1 | 251 (15.95) | 278 (18.83) | 114 (24.20) | 136 (25.42) | 137 (12.39) | 142 (15.15) | |
| 2 | 629 (39.94) | 592 (40.31) | 183 (38.85) | 215 (40.19) | 446 (40.33) | 377 (40.23) | |
| >2 | 394 (24.94) | 294 (19.92) | 42 (8.92) | 47 (8.79) | 352 (31.83) | 247 (26.36) | |
| Menarche | <11 years old | 81 (5.25) | 94 (6.37) | 23 (4.88) | 31 (5.79) | 58 (5.24) | 63 (6.72) |
| 12–14 years old | 1305 (82.66) | 1211 (82.25) | 413 (87.69) | 460 (85.98) | 892 (80.65) | 751 (80.15) | |
| >14 years old | 191 (12.09) | 167 (11.38) | 35 (7.43) | 44 (8.22) | 156 (14.10) | 123 (13.13) | |
| Polyphenol Class | Subclass | Compound | Food Sources * | Mean Intake (g/d) |
|---|---|---|---|---|
| Lignans | 1-Acetoxypinoresinol, Pinoresinol, 7-Hydroxymatairesinol, 7-Oxomatairesinol, Conidendrin, Cyclolariciresinol, Isolariciresinol, Lariciresinol, Lariciresinol-sesquilignan, Matairesinol, Medioresinol, Pinoresinol, Secoisolariciresinol, Secoisolariciresinol-sesquilignan, Syringaresinol | Olive oil (94.8%), Gazpacho (5.2%) | 2.92 | |
| Stilbene | d-Viniferin, Pallidol, Piceatannol, Resveratrol | Red wine (76.1%), Strawberry (7.7%), Rosé/White wine (7.5%), Grapes (5.8%), Lentils (1.1%), Chocolate (1.1%) | 0.85 | |
| Flavonoids | 143.38 | |||
| Anthocyanins | Cyanidin, Delphinidin, Malvidin, Pelargonidin, Peonidin, Petunidin, Pinotin A, Vitisin A | Sweet cherry (39.6%), Strawberry (21.0%), Plum (11.3%), Grapes (10.6%), Olives (9.6%), Red wine (6.5%) | 19.42 | |
| Chalcones | Xanthumol | Beer Ale (95%), Beer alcohol free (5%) | 0.002 | |
| Dihydrochalcones | Phloretin, 3-Hydroxyphloretin | Apple (73.4%), Nonorange juice (26.6%) | 1.05 | |
| Dyhydroflavonols | Dihydroquercetin | Red wine (95%), Rosé/White wine (5%) | 0.83 | |
| Flavanols | (-)-Epicatechin, (-)-Epigallocatechin, (+)-Catechin, (+)-Epicatechin-(2a-7)(4a-8)-epicatechin, (+)-Gallocatechin, Cinnamtannin A2 | Cocoa powder (58.1%), Chocolate (13.1%), Broad bean seed (5.6%), Plum (5.3%), Red Wine (5.3%), Apple (3.5%), Sweet cherry (1.7%), Persimmon/Custard apple (1.5%), Strawberry (1.0%), Grapes (1.0%) | 23.10 | |
| Flavanones | 6-Prenylnaringenin, 8-Prenylnaringenin, Eriodictyol, Hesperetin, Isosakuranetin, Isoxanthohumol, Naringenin | Orange pure juice (72.2%), Non-orange pure juice (24.1%), Red wine (1.5%) | 43.33 | |
| flavones | Apigenin, Chrysoeriol, Diosmetin, Luteolin, Nobiletin, Sinensetin, Tangeretin, Tetramethylscutellarein | Globe artichoke (62.9%), Celery (18.1%), Olives (11.7%), Orange pure juice (2.0%), Vegetable soup (1.3%), Sweet pepper green (1.1%), Lettuce (1.1%) | 4.00 | |
| Flavonols | 3,7-Dimethylquercetin, 3-Methoxynobiletin, 5,3’,4’-Trihydroxy-3-methoxy-6:7-methylenedioxyflavone, 5,4’-Dihydroxy-3,3’-dimethoxy-6:7-methylenedioxyflavone, 6,8-Dihydroxykaempferol, Ferulic acid, Isorhamnetin, Jaceidin, Kaempferol, Morin, Myricetin, Patuletin, Quercetin, Spinacetin | Swiss chard (23.2%), Common beans (18.9%), Endive (8.0%), Olives (7.9%), Chocolate (7.8%), Asparagus (7.2%), Chickpea/Common beans (5.8%), Lettuce (3.7%), Red wine (3.2%), Plum (2.2%), Green bean (2.0%), Onion (1.8%), Apple (1.4%), Grapes (1.1%) | 23.10 | |
| Isoflavonoids | Biochanin A, Daidzein, Genistein, Glycitein, Formononetin | Soy milk (93.9%), Common Beans (4.3%), Chickpea/Common beans (1.3%) | 2.26 | |
| Phenolic acids | 163.85 | |||
| Hydroxybenzoic acids | Valoneic acid dilactone, 2,3-Dihydroxybenzoic acid, 2,4-Dihydroxybenzoic acid, 2,6-Dihydroxybenzoic acid, 2-Hydroxybenzoic acid, 3,5-Dihydroxybenzoic acid, 3-Hydroxybenzoic acid, 4-Hydroxybenzoic acid, Benzoic acid, Ellagic acid, Gallagic acid, Gallic acid, Gentisic acid, Protocatechuic acid, Syringic acid, Vanillic acid | Olives (44.1%), Red wine (19.4%), Non-orange pure juice (11.8%), Strawberry (6.4%), Nuts (5.7%), Rosé/White wine (2.1%), Beer Ale (1.9%), Banana (1.6%), Lentils (1.7%) | 14.47 | |
| Hydroxycinnamic acids | Caffeic acid, Caffeoyl aspartic acid, Cinnamic acid, Ferulic acid, Hydroxycaffeic acid, m-Coumaric acid, o-Coumaric acid, p-Coumaric acid, Sinapic acid | Coffee (36.3%), Globe artichoke (16.4%), Olives (11.1%), Plum (7.2%), Sweet cherry (7.0%), cocoa powder (5.9%), Red wine (2.1%), Apple (2.0%), chocolate (1.9%), Peach/Apricot (1.5%), Carrot (1.5%), Potato (1.2%), Grapes (1.0%) | 149.37 | |
| Hydroxyphenylacetic acids | 3,4-Dihydroxyphenylacetic acid, 4-Hydroxyphenylacetic acid, Homovanillic acid, Homoveratric acid, Methoxyphenylacetic acid | Olives (96.3%), Red wine (2.3%) | 0.55 | |
| Other polyphenols | 13.12 | |||
| Alkylmethoxyphenols | 4-Vinylguaiacol | Coffee (96.4%), Beer Ale (3.6%) | 0.72 | |
| Alkylphenols | 3-Methylcatechol, 4-Ethylcatechol, 4-Methylcatechol, 3-Methylcatechol, 4-Vinylphenol | Coffee (83.4%), Cocoa powder (14.8%), Beer (1.8%) | 0.1 | |
| furanocoumarins | Bergapten, Isopimpinellin, Psoralen, Xanthotoxin | Celery (91.6%), Non-orange pure juice (8.4%) | 0.03 | |
| Hydroxybenzaldehydes | Protocatechuic aldehyde, Syringaldehyde, Vanillin | Red wine (67.2%), Cocoa powder (9.5%), Cognac/Rum/Whisky (7.8%), Olives (4.7%), Rosé/White wine (4.7%), Sherry (3.3%), Cider/Champagne (1.1%) | 0.17 | |
| Hydroxycoumarins | 4-Hydroxycoumarin, Esculetin, Mellein, Scopoletin, Umbelliferone | Rosé/White wine (58.8%), Beer Ale (22.8%), Cocoa powder (11.4%), Sherry (7.0%) | 0.04 | |
| Methoxyphenols | Guaiacol | Coffee (100%) | 0.10 | |
| Tyrosol | Hydroxytyrosol acetate (4-DHPEA-AC), Hydroxytyrosol, Oleoside 11-methylester, Tyrosol acetate (p-HPEA-AC), Tyrosol | Olives (83.2%), Olive oil (11.9%), Red wine (2.4%), Cider/Champagne (1.0%) | 11.98 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitelli-Storelli, F.; Zamora-Ros, R.; Molina, A.J.; Fernández-Villa, T.; Castelló, A.; Barrio, J.P.; Amiano, P.; Ardanaz, E.; Obón-Santacana, M.; Gómez-Acebo, I.; et al. Association between Polyphenol Intake and Breast Cancer Risk by Menopausal and Hormone Receptor Status. Nutrients 2020, 12, 994. https://doi.org/10.3390/nu12040994
Vitelli-Storelli F, Zamora-Ros R, Molina AJ, Fernández-Villa T, Castelló A, Barrio JP, Amiano P, Ardanaz E, Obón-Santacana M, Gómez-Acebo I, et al. Association between Polyphenol Intake and Breast Cancer Risk by Menopausal and Hormone Receptor Status. Nutrients. 2020; 12(4):994. https://doi.org/10.3390/nu12040994
Chicago/Turabian StyleVitelli-Storelli, Facundo, Raul Zamora-Ros, Antonio J. Molina, Tania Fernández-Villa, Adela Castelló, Juan Pablo Barrio, Pilar Amiano, Eva Ardanaz, Mireia Obón-Santacana, Inés Gómez-Acebo, and et al. 2020. "Association between Polyphenol Intake and Breast Cancer Risk by Menopausal and Hormone Receptor Status" Nutrients 12, no. 4: 994. https://doi.org/10.3390/nu12040994
APA StyleVitelli-Storelli, F., Zamora-Ros, R., Molina, A. J., Fernández-Villa, T., Castelló, A., Barrio, J. P., Amiano, P., Ardanaz, E., Obón-Santacana, M., Gómez-Acebo, I., Fernández-Tardón, G., Molina-Barceló, A., Alguacil, J., Marcos-Gragera, R., Ruiz-Moreno, E., Pedraza, M., Gil, L., Guevara, M., Castaño-Vinyals, G., ... Martín, V. (2020). Association between Polyphenol Intake and Breast Cancer Risk by Menopausal and Hormone Receptor Status. Nutrients, 12(4), 994. https://doi.org/10.3390/nu12040994









