Protective Mechanisms of Avocado Oil Extract Against Ototoxicity
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Cell Culture
2.3. Cell Viability Assay
2.4. mRNA Sequencing and Pathway Analysis
2.5. Real Time-qPCR
2.6. Cytoplasm and Nuclear Fractionation
2.7. Antioxidant Activity Assay
2.8. Western Immunoblot
2.9. Autophagy Assay
2.10. Statistical Analysis
3. Results
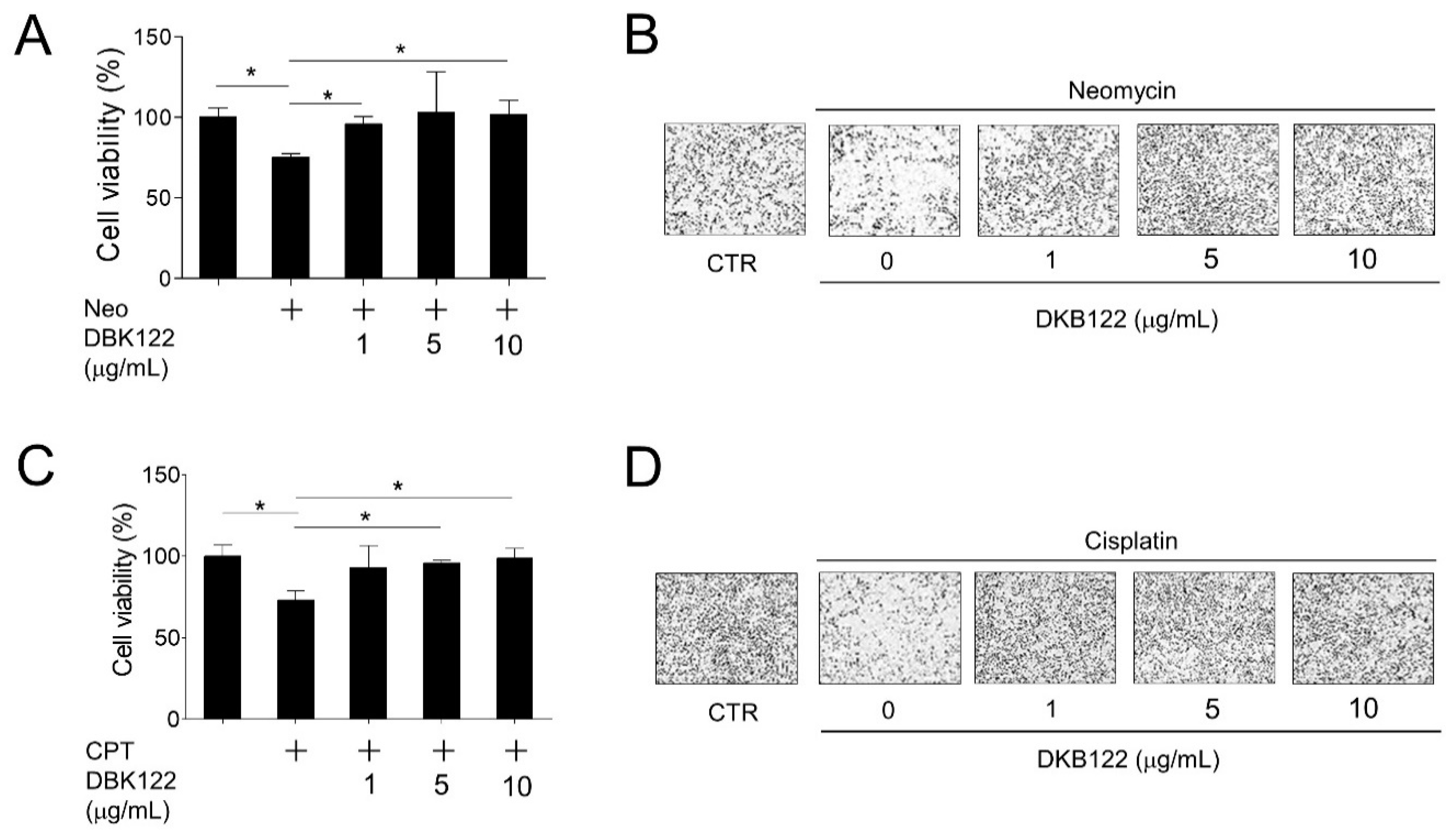
3.1. Protection of HEI-OC1 Cells by DKB122 From Chemical-Induced Cell Death
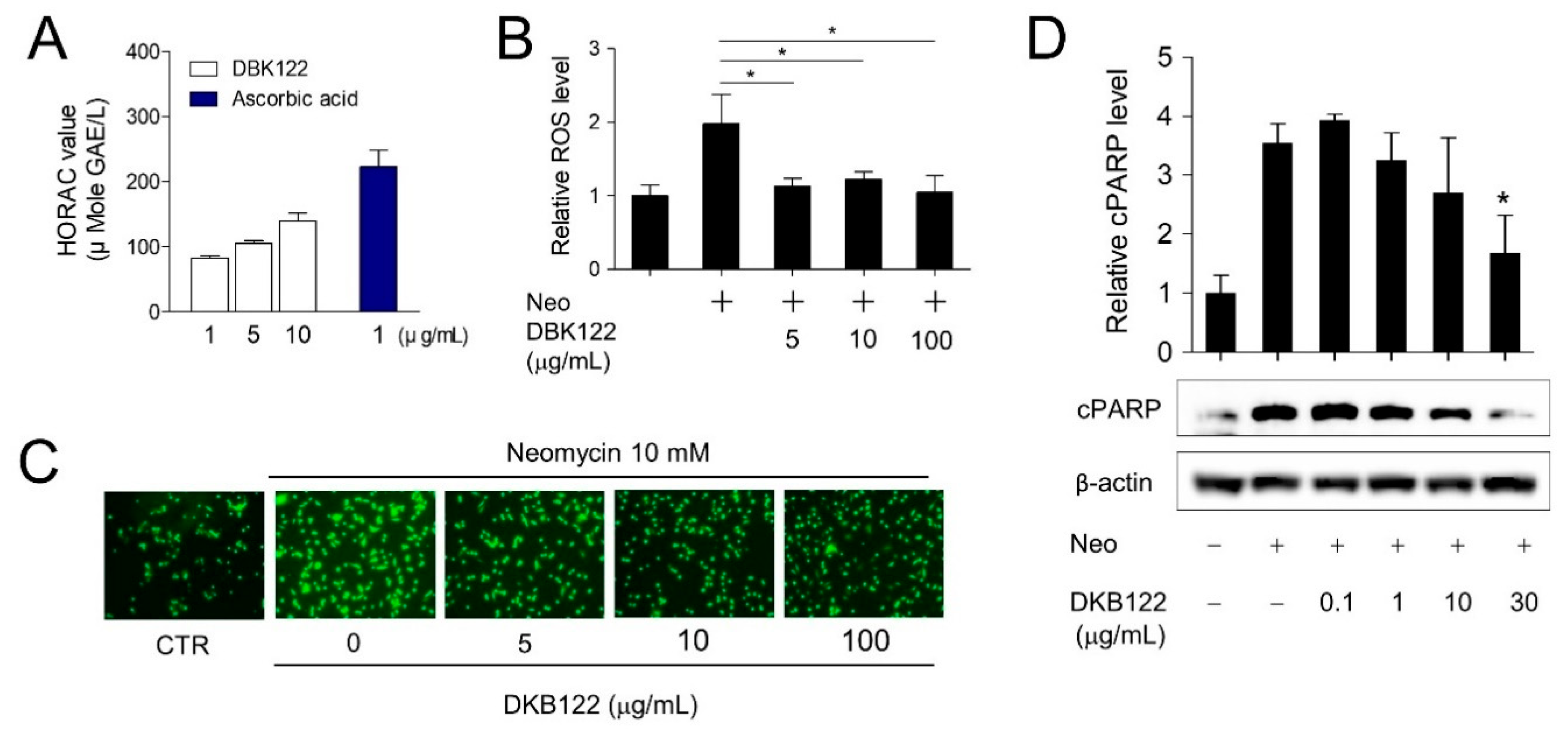
3.2. Activation Of Antioxidant Gene Expression By DKB122 In HEI-OC1 Cells
3.3. Antioxidant Effect of DKB122
3.4. Effects of DKB122 on the Expression of Genes Related to Noise-, Chemical-, and Age-Induced Hearing Loss
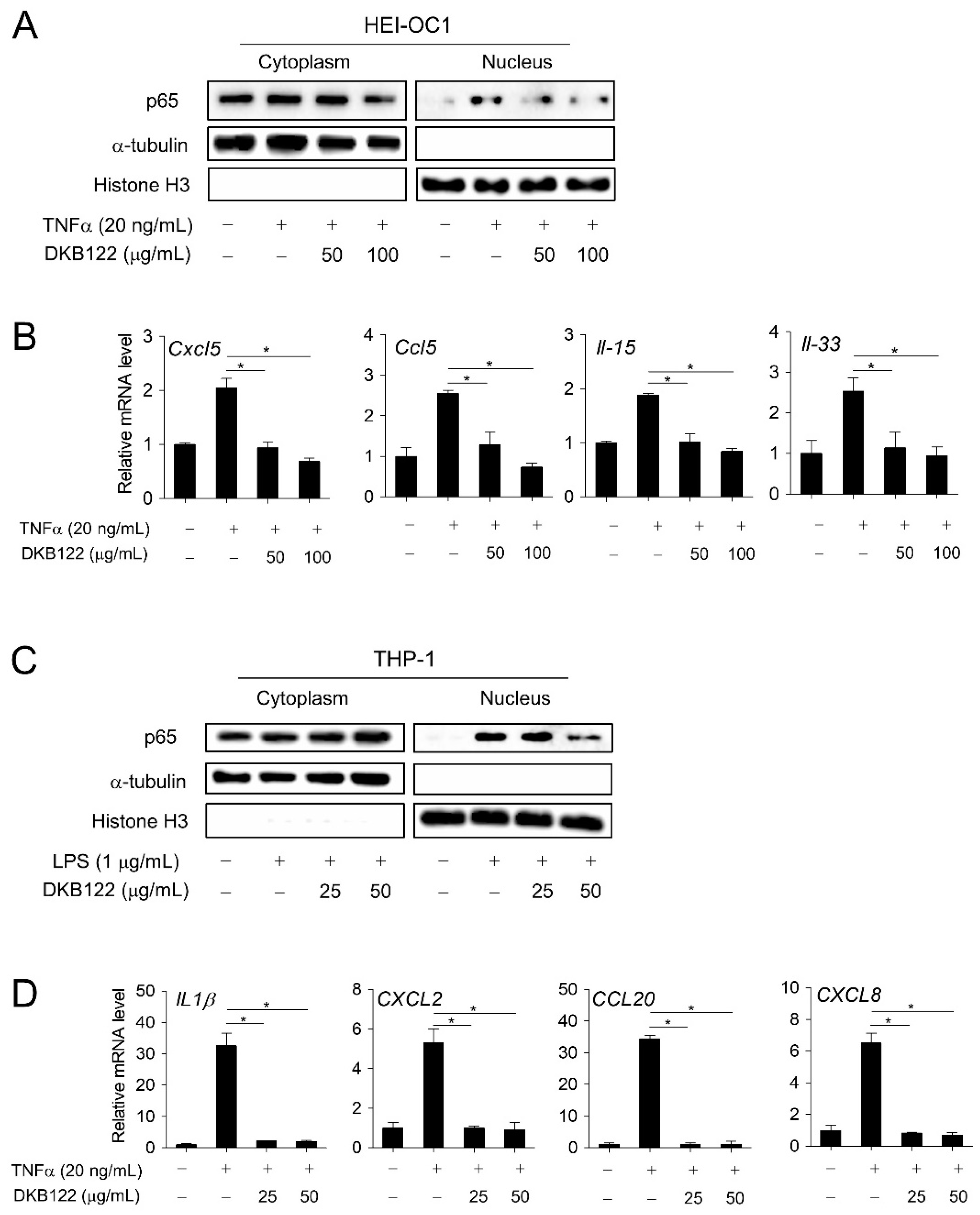
3.5. Inhibition of the NF-kB Pathway by DKB122
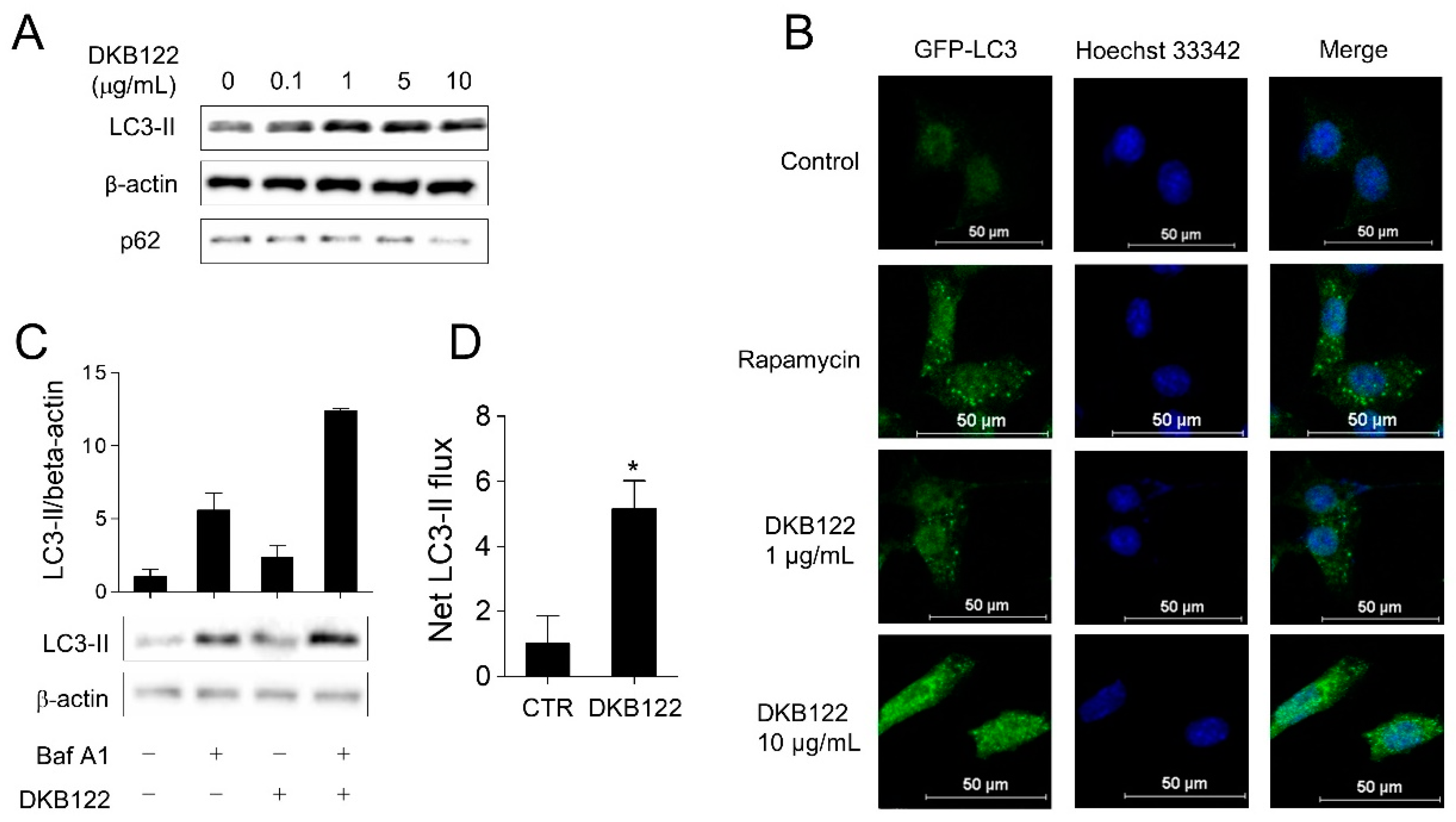
3.6. Activation of Autophagic Flux by DKB122
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Graydon, K.; Waterworth, C.; Miller, H.; Gunasekera, H. Global burden of hearing impairment and ear disease. J. Laryngol. Otol. 2019, 133, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Toubeau, G.; Laurent, G.; Carlier, M.; Abid, S.; Maldague, P.; Heuson-Stiennon, J.; Tulkens, P.M. Tissue repair in rat kidney cortex after short treatment with aminoglycosides at low doses. A comparative biochemical and morphometric study. Lab. Investig. J. Tech. Methods Pathol. 1986, 54, 385–393. [Google Scholar]
- Greenwood, G.J. Neomycin ototoxicity: Report of a case. AMA Arch. Otolaryngol. 1959, 69, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Forge, A.; Schacht, J. Aminoglycoside antibiotics. Audiol. Neurotol. 2000, 5, 3–22. [Google Scholar] [CrossRef]
- Lanvers-Kaminsky, C.; Zehnhoff-Dinnesen Parfitt, A.G.; Ciarimboli, G. Drug-induced ototoxicity: Mechanisms, Pharmacogenetics, and protective strategies. Clin. Pharmacol. Ther. 2017, 101, 491–500. [Google Scholar] [CrossRef]
- Karasawa, T.; Steyger, P.S. Intracellular mechanisms of aminoglycoside-induced cytotoxicity. Integr. Biol. 2011, 3, 879–886. [Google Scholar] [CrossRef]
- Kharkovets, T.; Dedek, K.; Maier, H.; Schweizer, M.; Khimich, D.; Nouvian, R.; Vardanyan, V.; Leuwer, R.; Moser, T.; Jentsch, T.J. Mice with altered KCNQ4 K+ channels implicate sensory outer hair cells in human progressive deafness. EMBO J. 2006, 25, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Huth, M.; Ricci, A.; Cheng, A. Mechanisms of aminoglycoside ototoxicity and targets of hair cell protection. Int. J. Otolaryngol. 2011, 2011, 937861. [Google Scholar] [CrossRef] [PubMed]
- Kalinec, G.M.; Webster, P.; Lim, D.J.; Kalinec, F. A cochlear cell line as an in vitro system for drug ototoxicity screening. Audiol. Neurotol. 2003, 8, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Billings, P.; Harris, J.P.; Keithley, E.M. Characterization of an experimentally induced inner ear immune response. Laryngoscope 2000, 110, 451–456. [Google Scholar] [CrossRef]
- Nam, Y.H.; Rodriguez, I.; Jeong, S.Y.; Pham, T.N.M.; Nuankaew, W.; Kim, Y.H.; Castañeda, R.; Jeong, S.Y.; Park, M.S.; Lee, K.W.; et al. Avocado Oil Extract Modulates Auditory Hair Cell Function through the Regulation of Amino Acid Biosynthesis Genes. Nutrients 2019, 11, 113. [Google Scholar] [CrossRef] [PubMed]
- Ashton, O.B.; Wong, M.; McGhie, T.K.; Vather, R.; Wang, Y.; Requejo-Jackman, C.; Ramankutty, P.; Woolf, A.B. Pigments in avocado tissue and oil. J. Agric. Food Chem. 2006, 54, 10151–10158. [Google Scholar] [CrossRef] [PubMed]
- Yanty, N.; Marikkar, J.; Long, K. Effect of varietal differences on composition and thermal characteristics of avocado oil. J. Am. Oil Chem. Soc. 2011, 88, 1997–2003. [Google Scholar] [CrossRef]
- Flores, M.; Saravia, C.; Vergara, C.E.; Avila, F.; Valdés, H.; Ortiz-Viedma, J. Avocado oil: Characteristics, properties, and applications. Molecules 2019, 24, 2172. [Google Scholar] [CrossRef]
- Krumreich, F.D.; Borges, C.D.; Mendonça, C.R.B.; Jansen-Alves, C.; Zambiazi, R.C. Bioactive compounds and quality parameters of avocado oil obtained by different processes. Food Chem. 2018, 257, 376–381. [Google Scholar] [CrossRef]
- Ranade, S.S.; Thiagarajan, P. A review on Persea americana Mill.(avocado)-its fruits and oil. Int. J. PharmTech Res. 2015, 8, 72–77. [Google Scholar]
- Del Toro-Equihua, M.; Velasco-Rodríguez, R.; López-Ascencio, R.; Vásquez, C. Effect of an avocado oil-enhanced diet (Persea americana) on sucrose-induced insulin resistance in Wistar rats. J. Food Drug Anal. 2016, 24, 350–357. [Google Scholar] [CrossRef]
- Nayak, B.; Raju, S.; Chalapathi Rao, A. Wound healing activity of Persea americana (avocado) fruit: A preclinical study on rats. J. Wound Care 2008, 17, 123–125. [Google Scholar] [CrossRef]
- Ortiz-Avila, O.; Esquivel-Martínez, M.; Olmos-Orizaba, B.E.; Saavedra-Molina, A.; Rodriguez-Orozco, A.R.; Cortés-Rojo, C. Avocado oil improves mitochondrial function and decreases oxidative stress in brain of diabetic rats. J. Diabetes Res. 2015, 2015, 485759. [Google Scholar] [CrossRef]
- Jin, M.L.; Kim, Y.W.; Jin, H.L.; Kang, H.; Lee, E.K.; Stallcup, M.R.; Jeong, K.W. Aberrant expression of SETD1A promotes survival and migration of estrogen receptor α-positive breast cancer cells. Int. J. Cancer 2018, 143, 2871–2883. [Google Scholar] [CrossRef]
- Chen, E.Y.; Tan, C.M.; Kou, Y.; Duan, Q.; Wang, Z.; Meirelles, G.V.; Clark, N.R.; Ma’ayan, A. Enrichr: Interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform. 2013, 14, 128. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; Gu, X.; Jeong, K.W. Photoactivation of N-retinylidene-N-retinylethanolamine compromises autophagy in retinal pigmented epithelial cells. Food Chem. Toxicol. 2019, 131, 110555. [Google Scholar] [CrossRef] [PubMed]
- Sheth, S.; Mukherjea, D.; Rybak, L.P.; Ramkumar, V. Mechanisms of cisplatin-induced ototoxicity and otoprotection. Front. Cell. Neurosci. 2017, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; So, H.-S.; Lee, J.-H.; Lee, J.-H.; Park, C.; Park, S.-Y.; Kim, Y.H.; Youn, M.J.; Kim, S.J.; Chung, S.Y.; et al. Heme oxygenase-1 attenuates the cisplatin-induced apoptosis of auditory cells via down-regulation of reactive oxygen species generation. Free Radic. Biol. Med. 2006, 40, 1810–1819. [Google Scholar] [CrossRef]
- Park, H.-J.; Kim, M.-J.; Rothenberger, C.; Kumar, A.; Sampson, E.M.; Ding, D.; Han, C.; White, K.; Boyd, K.; Manohar, S.; et al. GSTA4 mediates reduction of cisplatin ototoxicity in female mice. Nat. Commun. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Maeda, A.; Crabb, J.W.; Palczewski, K. Microsomal glutathione S-transferase 1 in the retinal pigment epithelium: Protection against oxidative stress and a potential role in aging. Biochemistry 2005, 44, 480–489. [Google Scholar] [CrossRef]
- Sha, S.-H.; Zajic, G.; Epstein, C.J.; Schacht, J. Overexpression of copper/zinc-superoxide dismutase protects from kanamycin-induced hearing loss. Audiol. Neurotol. 2001, 6, 117–123. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, W.-G.; Zha, D.-J.; Qiu, J.-H.; Wang, J.-L.; Sha, S.-H.; Schacht, J. Aspirin attenuates gentamicin ototoxicity: From the laboratory to the clinic. Hear. Res. 2007, 226, 178–182. [Google Scholar] [CrossRef]
- Müller, U.; Barr-Gillespie, P.G. New treatment options for hearing loss. Nat. Rev. Drug Discov. 2015, 14, 346–365. [Google Scholar] [CrossRef]
- Zong, S.; Zeng, X.; Liu, T.; Wan, F.; Luo, P.; Xiao, H. Association of polymorphisms in heat shock protein 70 genes with the susceptibility to noise-induced hearing loss: A meta-analysis. PLoS ONE 2017, 12, e0188195. [Google Scholar] [CrossRef]
- Pussegoda, K.; Ross, C.; Visscher, H.; Yazdanpanah, M.; Brooks, B.; Rassekh, S.; Zada, Y.F.; Dubé, M.P.; Carleton, B.C.; Hayden, M.R.; et al. Replication of TPMT and ABCC3 genetic variants highly associated with cisplatin-induced hearing loss in children. Clin. Pharmacol. Ther. 2013, 94, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.H.; Zhang, C.; Frye, M.D. Immune cells and non-immune cells with immune function in mammalian cochleae. Hear. Res. 2018, 362, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Frye, M.D.; Ryan, A.F.; Kurabi, A. Inflammation associated with noise-induced hearing loss. J. Acoust. Soc. Am. 2019, 146, 4020–4032. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, M.; Okamoto, Y.; Shinden, S.; Okano, H.J.; Okano, H.; Ogawa, K.; Matsunaga, T. Pharmacological inhibition of cochlear mitochondrial respiratory chain induces secondary inflammation in the lateral wall: A potential therapeutic target for sensorineural hearing loss. PLoS ONE 2014, 9, e90089. [Google Scholar] [CrossRef] [PubMed]
- Pear Oofa. Extraction and characterization of oil from Avocado pear (Persea americana) and native pear (Dacryodes edulis) fruits. World 2011, 3, 27–34. [Google Scholar]
- Rueda, A.; Seiquer, I.; Olalla, M.; Giménez, R.; Lara, L.; Cabrera-Vique, C. Characterization of fatty acid profile of argan oil and other edible vegetable oils by gas chromatography and discriminant analysis. J. Chem. 2014, 2014, 843908. [Google Scholar] [CrossRef]
- Niso-Santano, M.; Malik, S.A.; Pietrocola, F.; Bravo-San Pedro, J.M.; Mariño, G.; Cianfanelli, V.; Ben-Younès, A.; Troncoso, R.; Markaki, M.; Sica, V.; et al. Unsaturated fatty acids induce non-canonical autophagy. EMBO J. 2015, 34, 1025–1041. [Google Scholar] [CrossRef]
- Tan, S.H.; Shui, G.; Zhou, J.; Li, J.J.E.; Bay, B.-H.; Wenk, M.R.; Shen, H.M. Induction of autophagy by palmitic acid via protein kinase C-mediated signaling pathway independent of mTOR (mammalian target of rapamycin). J. Biol. Chem. 2012, 287, 14364–14376. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, W.; He, Q.; Wu, Y.; Lu, Z.; Sun, J.; Liu, Z.; Shao, Y.; Wang, A. Oleic acid induces apoptosis and autophagy in the treatment of Tongue Squamous cell carcinomas. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, X.; Hill, K.; Chen, J.; Lemasters, J.; Yang, S.-M.; Sha, S.H. Autophagy attenuates noise-induced hearing loss by reducing oxidative stress. Antioxid. Redox Signal. 2015, 22, 1308–1324. [Google Scholar] [CrossRef]
- Mizushima, N.; Yoshimori, T.; Levine, B. Methods in mammalian autophagy research. Cell 2010, 140, 313–326. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Guo, L.; Shu, Y.; Fang, Q.; Zhou, H.; Liu, Y.; Liu, D.; Lu, L.; Zhang, X.; Ding, X.; et al. Autophagy protects auditory hair cells against neomycin-induced damage. Autophagy 2017, 13, 1884–1904. [Google Scholar] [CrossRef] [PubMed]
- Rubel, E.W.; Furrer, S.A.; Stone, J.S. A brief history of hair cell regeneration research and speculations on the future. Hear. Res. 2013, 297, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Choung, Y.; Taura, A.; Pak, K.; Choi, S.; Masuda, M.; Ryan, A. Generation of highly-reactive oxygen species is closely related to hair cell damage in rat organ of Corti treated with gentamicin. Neuroscience 2009, 161, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Kros, C.J.; Steyger, P.S. Aminoglycoside-and cisplatin-induced ototoxicity: Mechanisms and otoprotective strategies. Cold Spring Harb. Perspect. Med. 2019, 9, a033548. [Google Scholar] [CrossRef]
- Yang, C.-H.; Schrepfer, T.; Schacht, J. Age-related hearing impairment and the triad of acquired hearing loss. Front. Cell. Neurosci. 2015, 9, 276. [Google Scholar] [CrossRef]
- Fujioka, M.; Okano, H.; Ogawa, K. Inflammatory and immune responses in the cochlea: Potential therapeutic targets for sensorineural hearing loss. Front. Pharmacol. 2014, 5, 287. [Google Scholar] [CrossRef]
- So, H.; Kim, H.; Lee, J.-H.; Park, C.; Kim, Y.; Kim, E.; Kim, J.K.; Yun, K.J.; Lee, K.M.; Lee, H.Y.; et al. Cisplatin cytotoxicity of auditory cells requires secretions of proinflammatory cytokines via activation of ERK and NF-κB. J. Assoc. Res. Otolaryngol. 2007, 8, 338–355. [Google Scholar] [CrossRef]
- Kim, H.J.; So, H.S.; Lee, J.H.; Park, C.; Lee, J.B.; Youn, M.J.; Kim, S.J.; Yang, S.H.; Lee, K.M.; Kwon, K.B.; et al. Role of proinflammatory cytokines in cisplatin-induced vestibular hair cell damage. Head Neck J. Sci. Spec. Head Neck 2008, 30, 1445–1456. [Google Scholar] [CrossRef]
- Prasad, K. Involvement of Oxidative Stress, Inflammation, and Glutamate in Ototoxici-ty, and their Attenuation by Simultaneous Activation of Nrf2 and Elevation of Antioxidant Compounds. Int. J. Neurol. Neurother. 2019, 6, 83. [Google Scholar] [CrossRef]
- Medeiros-de-Moraes, I.M.; Gonçalves-de-Albuquerque, C.F.; Kurz, A.R.; Oliveira, F.M.D.J.; Abreu, V.H.P.D.; Torres, R.C.; Carvalho, V.F.; Estato, V.; Bozza, P.T.; Sperandio, M.; et al. Omega-9 oleic acid, the main compound of olive oil, mitigates inflammation during experimental sepsis. Oxidative Med. Cell. Longev. 2018, 2018, 6053492. [Google Scholar] [CrossRef] [PubMed]
- Vassiliou, E.K.; Gonzalez, A.; Garcia, C.; Tadros, J.H.; Chakraborty, G.; Toney, J.H. Oleic acid and peanut oil high in oleic acid reverse the inhibitory effect of insulin production of the inflammatory cytokine TNF-α both in vitro and in vivo systems. Lipids Health Dis. 2009, 8, 25. [Google Scholar] [CrossRef]
- Fujimoto, C.; Iwasaki, S.; Urata, S.; Morishita, H.; Sakamaki, Y.; Fujioka, M.; Kondo, K.; Mizushima, N.; Yamasoba, T. Autophagy is essential for hearing in mice. Cell Death Dis. 2017, 8, e2780. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Tian, C.; Kim, J.; Shin, B.; Choo, O.-S.; Kim, Y.-S.; Choung, Y.H. Autophagic flux, a possible mechanism for delayed gentamicin-induced ototoxicity. Sci. Rep. 2017, 7, 41356. [Google Scholar] [CrossRef] [PubMed]
- Youn, C.K.; Kim, J.; Park, J.-H.; Do, N.Y.; Cho, S.I. Role of autophagy in cisplatin-induced ototoxicity. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 1814–1819. [Google Scholar] [CrossRef] [PubMed]
- Hailey, D.W.; Esterberg, R.; Linbo, T.H.; Rubel, E.W.; Raible, D.W. Fluorescent aminoglycosides reveal intracellular trafficking routes in mechanosensory hair cells. J. Clin. Investig. 2017, 127, 472–486. [Google Scholar] [CrossRef]
- Hashino, E.; Shero, M.; Salvi, R.J. Lysosomal targeting and accumulation of aminoglycoside antibiotics in sensory hair cells. Brain Res. 1997, 777, 75–85. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, T.N.M.; Jeong, S.Y.; Kim, D.H.; Park, Y.H.; Lee, J.S.; Lee, K.W.; Moon, I.S.; Choung, S.Y.; Kim, S.H.; Kang, T.H.; et al. Protective Mechanisms of Avocado Oil Extract Against Ototoxicity. Nutrients 2020, 12, 947. https://doi.org/10.3390/nu12040947
Pham TNM, Jeong SY, Kim DH, Park YH, Lee JS, Lee KW, Moon IS, Choung SY, Kim SH, Kang TH, et al. Protective Mechanisms of Avocado Oil Extract Against Ototoxicity. Nutrients. 2020; 12(4):947. https://doi.org/10.3390/nu12040947
Chicago/Turabian StylePham, Thu Nguyen Minh, Seo Yeon Jeong, Do Hoon Kim, Yu Hwa Park, Jung Suk Lee, Kye Wan Lee, In Seok Moon, Se Young Choung, Seung Hyun Kim, Tong Ho Kang, and et al. 2020. "Protective Mechanisms of Avocado Oil Extract Against Ototoxicity" Nutrients 12, no. 4: 947. https://doi.org/10.3390/nu12040947
APA StylePham, T. N. M., Jeong, S. Y., Kim, D. H., Park, Y. H., Lee, J. S., Lee, K. W., Moon, I. S., Choung, S. Y., Kim, S. H., Kang, T. H., & Jeong, K. W. (2020). Protective Mechanisms of Avocado Oil Extract Against Ototoxicity. Nutrients, 12(4), 947. https://doi.org/10.3390/nu12040947






