Saskatoon Berry Amelanchier alnifolia Regulates Glucose Metabolism and Improves Cardiovascular and Liver Signs of Diet-Induced Metabolic Syndrome in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Rats and Diets
2.2. Phytochemical Characterisation of Saskatoon Berry Powder
2.3. Measurements on Live Rats
2.4. Measurements on Isolated Organs and Tissues
2.5. Statistical Analysis
3. Results
3.1. Composition of A. alnifolia Powder
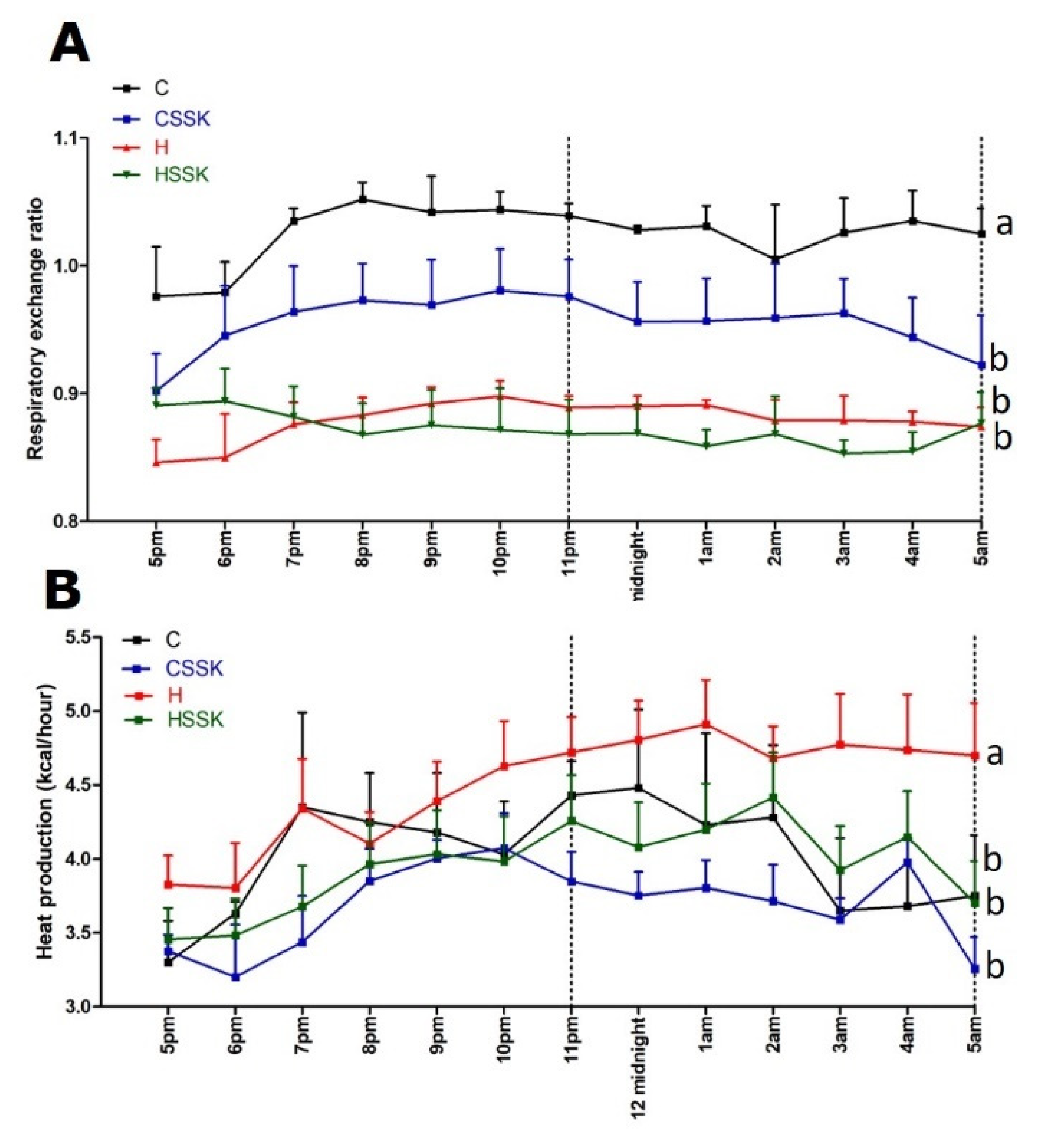
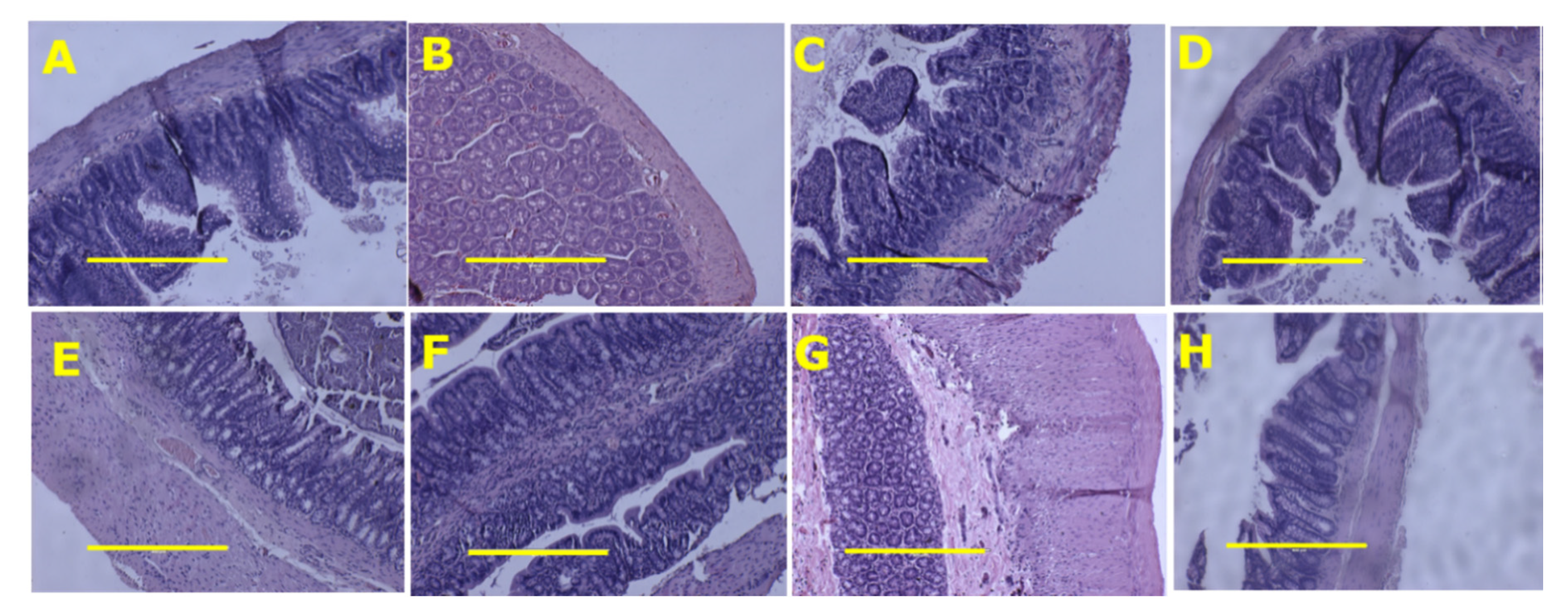
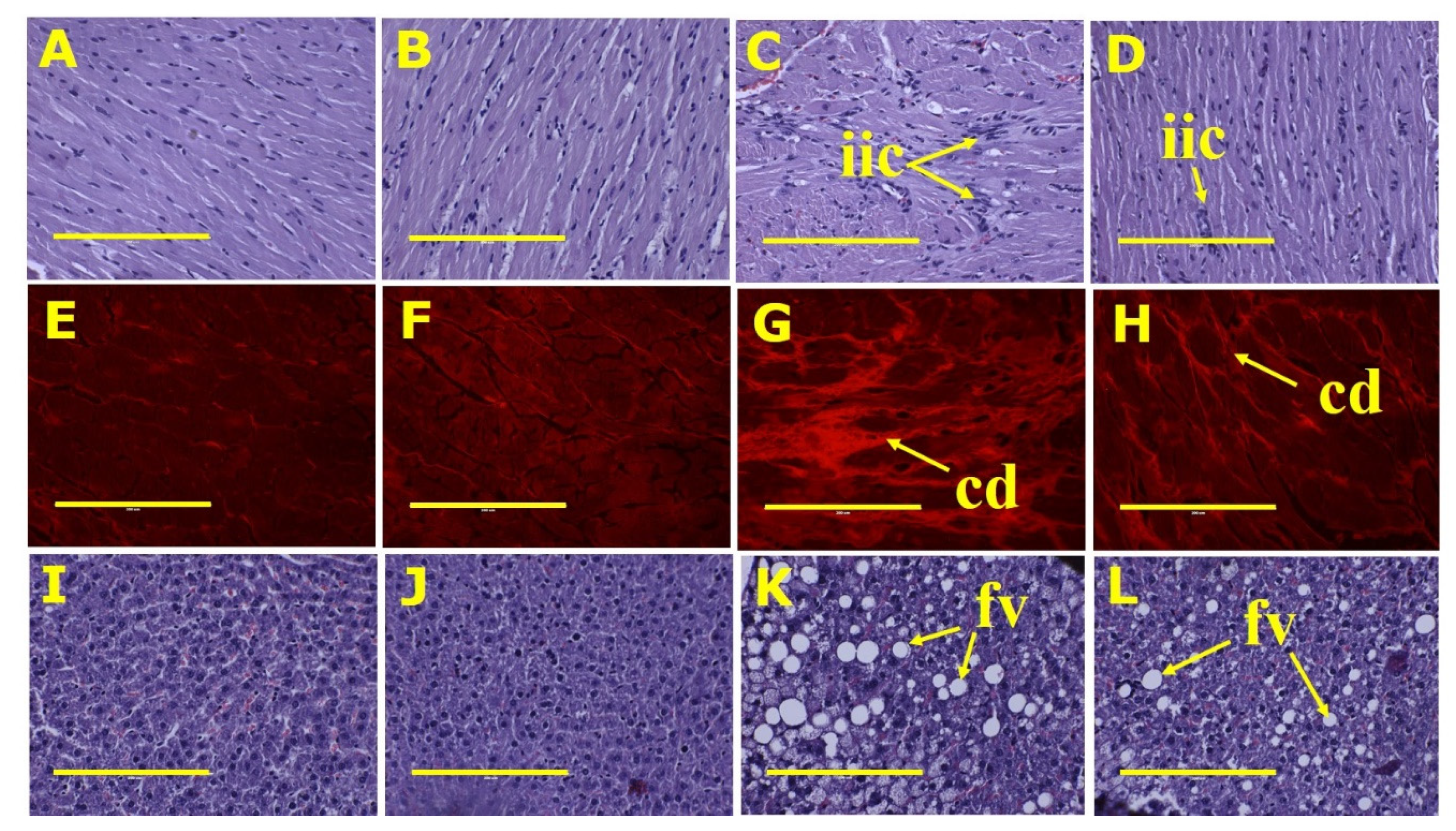
3.2. Metabolic, Cardiovascular, Liver and Gastrointestinal Tract Parameters
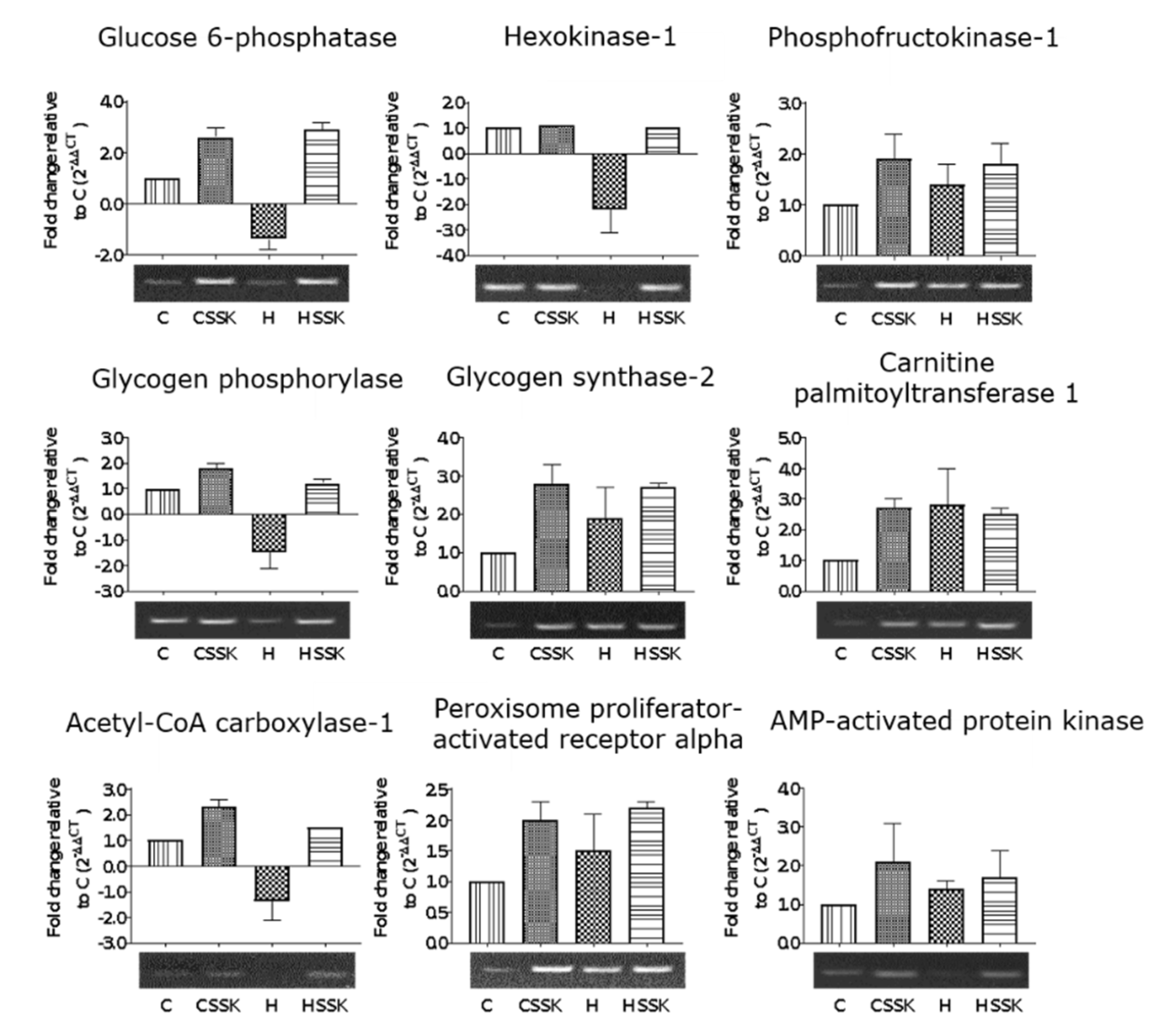
3.3. Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Seeram, N.P. Berry fruits: Compositional elements, biochemical activities, and the impact of their intake on human health, performance, and disease. J. Agric. Food Chem. 2008, 56, 627–629. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.; Ryland, D.; Isaak, C.K.; Prashar, S.; Siow, Y.L.; Taylor, C.G.; Aliani, M. Effect of vitamin D3 fortification and Saskatoon berry syrup addition on the flavor profile, acceptability, and antioxidant properties of rooibos tea (Aspalathus linearis). J. Food Sci. 2017, 82, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Lachowicz, S.; Oszmianski, J.; Pluta, S. The composition of bioactive compounds and antioxidant activity of Saskatoon berry (Amelanchier alnifolia Nutt.) genotypes grown in central Poland. Food Chem. 2017, 235, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef]
- Szajdek, A.; Borowska, E.J. Bioactive compounds and health-promoting properties of berry fruits: A review. Plant Foods Hum. Nutr. 2008, 63, 147–156. [Google Scholar] [CrossRef]
- Lachowicz, S.; Seliga, L.; Pluta, S. Distribution of phytochemicals and antioxidative potency in fruit peel, flesh, and seeds of Saskatoon berry. Food Chem. 2020, 305, 125430. [Google Scholar] [CrossRef]
- Juriková, T.; Balla, S.; Sochor, J.; Pohanka, M.; Mlcek, J.; Baron, M. Flavonoid profile of Saskatoon berries (Amelanchier alnifolia Nutt.) and their health promoting effects. Molecules 2013, 18, 12571–12586. [Google Scholar] [CrossRef]
- Meczarska, K.; Cyboran-Mikolajczyk, S.; Wloch, A.; Bonarska-Kujawa, D.; Oszmianski, J.; Kleszczynska, H. Polyphenol content and bioactivity of Saskatoon (Amelanchier alnifolia Nutt.) leaves and berries. Acta Pol. Pharm. 2017, 74, 660–669. [Google Scholar]
- Hosseinian, F.S.; Beta, T. Saskatoon and wild blueberries have higher anthocyanin contents than other Manitoba berries. J. Agric. Food Chem. 2007, 55, 10832–10838. [Google Scholar] [CrossRef]
- O’Neill, S.; O’Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015, 16, 1–12. [Google Scholar] [CrossRef]
- Brown, L.; Poudyal, H.; Panchal, S.K. Functional foods as potential therapeutic options for metabolic syndrome. Obes. Rev. 2015, 16, 914–941. [Google Scholar] [CrossRef] [PubMed]
- Panchal, S.K.; Poudyal, H.; Iyer, A.; Nazer, R.; Alam, A.; Diwan, V.; Kauter, K.; Sernia, C.; Campbell, F.; Ward, L.; et al. High-carbohydrate high-fat diet-induced metabolic syndrome and cardiovascular remodeling in rats. J. Cardiovasc. Pharmacol. 2011, 57, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Poudyal, H.; Panchal, S.; Brown, L. Comparison of purple carrot juice and β-carotene in a high-carbohydrate, high-fat diet-fed rat model of the metabolic syndrome. Br. J. Nutr. 2010, 104, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Bhaswant, M.; Fanning, K.; Netzel, M.; Mathai, M.L.; Panchal, S.K.; Brown, L. Cyanidin 3-glucoside improves diet-induced metabolic syndrome in rats. Pharmacol. Res. 2015, 102, 208–217. [Google Scholar] [CrossRef]
- Bhaswant, M.; Shafie, S.R.; Mathai, M.L.; Mouatt, P.; Brown, L. Anthocyanins in chokeberry and purple maize attenuate diet-induced metabolic syndrome in rats. Nutrition 2017, 41, 24–31. [Google Scholar] [CrossRef]
- Panchal, S.K.; Poudyal, H.; Arumugam, T.V.; Brown, L. Rutin attenuates metabolic changes, nonalcoholic steatohepatitis, and cardiovascular remodeling in high-carbohydrate, high-fat diet-fed rats. J. Nutr. 2011, 141, 1062–1069. [Google Scholar] [CrossRef]
- Panchal, S.K.; Poudyal, H.; Brown, L. Quercetin ameliorates cardiovascular, hepatic, and metabolic changes in diet-induced metabolic syndrome in rats. J. Nutr. 2012, 142, 1026–1032. [Google Scholar] [CrossRef]
- Bhandarkar, N.S.; Brown, L.; Panchal, S.K. Chlorogenic acid attenuates high-carbohydrate, high-fat diet-induced cardiovascular, liver, and metabolic changes in rats. Nutr. Res. 2019, 62, 78–88. [Google Scholar] [CrossRef]
- Panchal, S.K.; Brown, L. Cardioprotective and hepatoprotective effects of ellagitannins from European oak bark (Quercus petraea L.) extract in rats. Eur. J. Nutr. 2013, 52, 397–408. [Google Scholar] [CrossRef]
- Zhao, R.; Khafipour, E.; Sepehri, S.; Huang, F.; Beta, T.; Shen, G.X. Impact of Saskatoon berry powder on insulin resistance and relationship with intestinal microbiota in high fat-high sucrose diet-induced obese mice. J. Nutr. Biochem. 2019, 69, 130–138. [Google Scholar] [CrossRef]
- Riediger, N.D.; Lix, L.M.; Lukianchuk, V.; Bruce, S. Trends in diabetes and cardiometabolic conditions in a Canadian First Nation community, 2002–2003 to 2011–2012. Prev. Chronic Dis. 2014, 11, E198. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, P.; Mathangasinghe, Y.; Jayawardena, R.; Hills, A.P.; Misra, A. Prevalence and trends of metabolic syndrome among adults in the Asia-acific region: A systematic review. BMC Public Health 2017, 17, 101. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Sekar, S.; Shafie, S.R.; Prasadam, I.; Crawford, R.; Panchal, S.K.; Brown, L.; Xiao, Y. Saturated fatty acids induce development of both metabolic syndrome and osteoarthritis in rats. Sci. Rep. 2017, 7, 46457. [Google Scholar] [CrossRef] [PubMed]
- Morales-Luna, E.; Perez-Ramirez, I.F.; Salgado, L.M.; Castano-Tostado, E.; Gomez-Aldapa, C.A.; Reynoso-Camacho, R. The main beneficial effect of roselle (Hibiscus sabdariffa) on obesity is not only related to its anthocyanin content. J. Sci. Food Agric. 2019, 99, 596–605. [Google Scholar] [CrossRef]
- Krga, I.; Milenkovic, D. Anthocyanins: From sources and bioavailability to cardiovascular-health benefits and molecular mechanisms of action. J. Agric. Food Chem. 2019, 67, 1771–1783. [Google Scholar] [CrossRef]
- Naseri, R.; Farzaei, F.; Haratipour, P.; Nabavi, S.F.; Habtemariam, S.; Farzaei, M.H.; Khodarahmi, R.; Tewari, D.; Momtaz, S. Anthocyanins in the management of metabolic syndrome: A pharmacological and biopharmaceutical review. Front. Pharmacol. 2018, 9, 1310. [Google Scholar] [CrossRef]
- Lee, Y.M.; Yoon, Y.; Yoon, H.; Park, H.M.; Song, S.; Yeum, K.J. Dietary anthocyanins against obesity and inflammation. Nutrients 2017, 9, 1089. [Google Scholar] [CrossRef]
- Song, D.; Cheng, L.; Zhang, X.; Wu, Z.; Zheng, X. The modulatory effect and the mechanism of flavonoids on obesity. J. Food Biochem. 2019, 43, e12954. [Google Scholar] [CrossRef]
- Bondonno, C.P.; Croft, K.D.; Ward, N.; Considine, M.J.; Hodgson, J.M. Dietary flavonoids and nitrate: Effects on nitric oxide and vascular function. Nutr. Rev. 2015, 73, 216–235. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomas-Barberan, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef] [PubMed]
- Carrera-Quintanar, L.; López Roa, R.I.; Quintero-Fabián, S.; Sánchez-Sánchez, M.A.; Vizmanos, B.; Ortuño-Sahagún, D. Phytochemicals that influence gut microbiota as prophylactics and for the treatment of obesity and inflammatory diseases. Mediat. Inflamm. 2018, 2018, 9734845. [Google Scholar] [CrossRef] [PubMed]
- de Souza, D.R.; Willems, J.L.; Low, N.H. Phenolic composition and antioxidant activities of Saskatoon berry fruit and pomace. Food Chem. 2019, 290, 168–177. [Google Scholar] [CrossRef]
- Zhao, R.; Xie, X.; Le, K.; Li, W.; Moghadasian, M.H.; Beta, T.; Shen, G.X. Endoplasmic reticulum stress in diabetic mouse or glycated LDL-treated endothelial cells: Protective effect of Saskatoon berry powder and cyanidin glycans. J. Nutr. Biochem. 2015, 26, 1248–1253. [Google Scholar] [CrossRef]
- Lachowicz, S.; Wisniewski, R.; Ochmian, I.; Drzymala, K.; Pluta, S. Anti-microbiological, anti-hyperglycemic and anti-obesity potency of natural antioxidants in fruit fractions of Saskatoon berry. Antioxidants 2019, 8, 397. [Google Scholar] [CrossRef]
- Fang, J. Bioavailability of anthocyanins. Drug Metab. Rev. 2014, 46, 508–520. [Google Scholar] [CrossRef]
- Fang, J. Some anthocyanins could be efficiently absorbed across the gastrointestinal mucosa: Extensive presystemic metabolism reduces apparent bioavailability. J. Agric. Food Chem. 2014, 62, 3904–3911. [Google Scholar] [CrossRef]
- Fang, J.; Huang, J. Accumulation of plasma levels of anthocyanins following multiple saskatoon berry supplements. Xenobiotica 2020, 50, 454–457. [Google Scholar] [CrossRef]
- Nicholson, K.M.; Anderson, N.G. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 2002, 14, 381–395. [Google Scholar] [CrossRef]
- Takata, M.; Ogawa, W.; Kitamura, T.; Hino, Y.; Kuroda, S.; Kotani, K.; Klip, A.; Gingras, A.C.; Sonenberg, N.; Kasuga, M. Requirement for Akt (protein kinase B) in insulin-induced activation of glycogen synthase and phosphorylation of 4E-BP1 (PHAS-1). J. Biol. Chem. 1999, 274, 20611–20618. [Google Scholar] [CrossRef] [PubMed]
- Minchenko, A.; Leshchinsky, I.; Opentanova, I.; Sang, N.; Srinivas, V.; Armstead, V.; Caro, J. Hypoxia-inducible factor-1-mediated expression of the 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 (PFKFB3) gene. Its possible role in the Warburg effect. J. Biol. Chem. 2002, 277, 6183–6187. [Google Scholar] [CrossRef] [PubMed]
- Hahn-Windgassen, A.; Nogueira, V.; Chen, C.C.; Skeen, J.E.; Sonenberg, N.; Hay, N. Akt activates the mammalian target of rapamycin by regulating cellular ATP level and AMPK activity. J. Biol. Chem. 2005, 280, 32081–32089. [Google Scholar] [CrossRef] [PubMed]
- Massa, M.L.; Gagliardino, J.J.; Francini, F. Liver glucokinase: An overview on the regulatory mechanisms of its activity. IUBMB Life 2011, 63, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, J.C.; Favre, C.; Gomis, R.R.; Fernandez-Novell, J.M.; Garcia-Rocha, M.; de la Iglesia, N.; Cid, E.; Guinovart, J.J. Control of glycogen deposition. FEBS Lett. 2003, 546, 127–132. [Google Scholar] [CrossRef]
- Stoffel, M.; Froguel, P.; Takeda, J.; Zouali, H.; Vionnet, N.; Nishi, S.; Weber, I.T.; Harrison, R.W.; Pilkis, S.J.; Lesage, S.; et al. Human glucokinase gene: Isolation, characterization, and identification of two missense mutations linked to early-onset non-insulin-dependent (type 2) diabetes mellitus. Proc. Natl. Acad. Sci. USA 1992, 89, 7698–7702. [Google Scholar] [CrossRef]
- Bell, G.I.; Kayano, T.; Buse, J.B.; Burant, C.F.; Takeda, J.; Lin, D.; Fukumoto, H.; Seino, S. Molecular biology of mammalian glucose transporters. Diabetes Care 1990, 13, 198–208. [Google Scholar] [CrossRef]
- Han, H.S.; Kang, G.; Kim, J.S.; Choi, B.H.; Koo, S.H. Regulation of glucose metabolism from a liver-centric perspective. Exp. Mol. Med. 2016, 48, e218. [Google Scholar] [CrossRef]
- Newsholme, E.A.; Brand, K.; Lang, J.; Stanley, J.C.; Williams, T. The maximum activities of enzymes that are involved in substrate cycles in liver and muscle of obese mice. Biochem. J. 1979, 182, 621–624. [Google Scholar] [CrossRef]
- Newsholme, E.A.; Parry-Billings, M. Some evidence for the existence of substrate cycles and their utility in vivo. Biochem. J. 1992, 285, 340–341. [Google Scholar] [CrossRef]
- Newsholme, E.A.; Stanley, J.C. Substrate cycles: Their role in control of metabolism with specific references to the liver. Diabetes Metab. Rev. 1987, 3, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Parry-Billings, M.; Newsholme, E.A. The possible role of glutamine substrate cycles in skeletal muscle. Biochem. J. 1991, 279, 327–328. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.G.; Filsell, O.H.; Topping, D.L. Effects of fructose concentration on carbohydrate metabolism, heat production and substrate cycling in isolated rat hepatocytes. Biochem. J. 1979, 184, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Geidl-Flueck, B.; Gerber, P.A. Insights into the hexose liver metabolism-glucose versus fructose. Nutrients 2017, 9, 1026. [Google Scholar] [CrossRef]
- Valery, P.C.; Moloney, A.; Cotterill, A.; Harris, M.; Sinha, A.K.; Green, A.C. Prevalence of obesity and metabolic syndrome in Indigenous Australian youths. Obes. Rev. 2009, 10, 255–261. [Google Scholar] [CrossRef]
- Juonala, M.; Singh, G.R.; Davison, B.; van Schilfgaarde, K.; Skilton, M.R.; Sabin, M.A.; Cheung, M.; Sayers, S.; Burgner, D.P. Childhood metabolic syndrome, inflammation and carotid intima-media thickness. The Aboriginal Birth Cohort Study. Int. J. Cardiol. 2016, 203, 32–36. [Google Scholar] [CrossRef]
- Batal, M.; Decelles, S. A scoping review of obesity among Indigenous peoples in Canada. J. Obes. 2019, 2019, 9741090. [Google Scholar] [CrossRef]
- Kelly, L.; Matsumoto, C.L.; Schreiber, Y.; Gordon, J.; Willms, H.; Olivier, C.; Madden, S.; Hopko, J.; Tobe, S.W. Prevalence of chronic kidney disease and cardiovascular comorbidities in adults in First Nations communities in northwest Ontario: A retrospective observational study. CMAJ Open 2019, 7, E568–E572. [Google Scholar] [CrossRef]
- de Schweinitz, P.A.; Wojcicki, J.M. First Nations approaches to childhood obesity: Healthy lifestyles in Canada compared with alternatives for Alaska native communities. Children 2017, 4, 38. [Google Scholar] [CrossRef]
| Physiological Variables | C | CSSK | H | HSSK | p Value | ||
|---|---|---|---|---|---|---|---|
| Diet | Treatment | Interaction | |||||
| 8 week body weight, g | 361 ± 4 b | 369 ± 5 b | 457 ± 14 a | 437 ± 6 a | <0.0001 | 0.43 | 0.07 |
| 16 week body weight, g | 390 ± 9 c | 396 ± 6 c | 577 ± 20 a | 500 ± 11 b | 0.007 | 0.0001 | 0.002 |
| 9–16 week body weight gain, % | 7.4 ± 1.8 c | 7.1 ± 1.0 c | 18.2 ± 3.1 a | 14.5 ± 1.6 b | <0.0001 | 0.33 | 0.41 |
| 16 week systolic blood pressure, mmHg | 111.9 ± 1.8 bc | 122.5 ± 0.7 b | 141.0 ± 2.6 a | 126.4 ± 3.1 b | <0.0001 | 0.38 | <0.0001 |
| Diastolic stiffness constant (κ) | 18.2 ± 1.3 bc | 21.3 ± 1.0 b | 26.9 ± 1.0 a | 21.6 ± 0.8 b | 0.0001 | 0.30 | 0.0003 |
| Left ventricle + septum, mg/mm | 20.8 ± 0.6 c | 21.0 ± 0.6 c | 25.3 ± 0.8 a | 22.6 ± 0.7 bc | 0.0001 | 0.08 | 0.042 |
| Right ventricle, mg/mm | 4.0 ± 0.2 | 4.2 ± 0.6 | 4.6 ± 0.4 | 4.6 ± 0.5 | 0.28 | 0.83 | 0.83 |
| Water intake 0–8 weeks, mL/day | 38.8 ± 2.5 b | 44.4 ± 3.6 a | 32.8 ± 1.4 c | 34.7 ± 1.0 c | 0.002 | 0.12 | 0.44 |
| Water intake 9–16 weeks, mL/day | 30.8 ± 2.6 | 34.5 ± 3.0 | 33.0 ± 2.0 | 32.0 ± 1.4 | 0.95 | 0.57 | 0.32 |
| Food intake 0–8 weeks, g/day | 47.7 ± 1.3 a | 48.2 ± 1.2 a | 33.6 ± 0.9 | 32.7 ± 1.4 | <0.0001 | 0.87 | 0.57 |
| Food intake 9–16 weeks, g/day | 43.7 ± 1.1 a | 40.5 ± 1.2 a | 32.8 ± 1.0 b | 27.7 ± 1.3 bc | <0.0001 | 0.0008 | 0.42 |
| Saskatoon berry powder intake, g/day | — | 1.3 ± 0.04 | — | 0.9 ± 0.04 | |||
| Cyanidin 3-glucoside intake, mg/kg/day | — | 7.43 ± 0.21 | — | 5.18 ± 0.22 | |||
| Quercetin intake, mg/kg/day | — | 5.61 ± 0.12 | — | 3.90 ± 0.10 | |||
| Rutin intake, mg/kg/day | — | 2.18 ± 0.05 | — | 1.52 ± 0.06 | |||
| Chlorogenic acid intake, mg/kg/day | — | 2.41 ± 0.06 | — | 1.68 ± 0.05 | |||
| Energy intake 0–8 weeks, kJ/day | 534 ± 15 b | 540 ± 13 b | 728 ± 16 a | 701 ± 26 a | <0.0001 | 0.57 | 0.37 |
| Energy intake 9–16 weeks, kJ/day | 493 ± 13 c | 453 ± 13 d | 712 ± 19 a | 606 ± 22 b | <0.0001 | 0.0001 | 0.06 |
| Feed efficiency 0–8 weeks, g/kJ | 0.05 ± 0.01 c | 0.06 ± 0.01 c | 0.19 ± 0.01 a | 0.15 ± 0.01 b | <0.0001 | 0.14 | 0.016 |
| Feed efficiency 9–16 weeks, g/kJ | 0.05 ± 0.01 b | 0.06 ± 0.01 b | 0.12 ± 0.02 a | 0.10 ± 0.01 a | <0.0001 | 0.62 | 0.14 |
| Metabolic Variables | C | CSSK | H | HSSK | p Value | ||
|---|---|---|---|---|---|---|---|
| Diet | Treatment | Interaction | |||||
| 16 week bone mineral content, g | 12.3 ± 0.4 b | 11.8 ± 0.3 b | 17.6 ± 0.9 a | 17.0 ± 0.5 a | 0.0001 | 0.34 | 0.93 |
| 16 week bone mineral density, g/cm2 | 0.182 ± 0.004 b | 0.184 ± 0.002 b | 0.190 ± 0.003 a | 0.194 ± 0.004 a | 0.008 | 0.29 | 0.86 |
| 16 week lean mass, g | 307.3 ± 5.9 | 324.3 ± 10.6 | 321.8 ± 7.3 | 304.3 ± 6.6 | 0.73 | 0.97 | 0.033 |
| 16 week fat mass, g | 65.4 ± 10.0 b | 67.4 ± 8.9 b | 226.8 ± 25.7 a | 188.0 ± 15.7 a | <0.0001 | 0.27 | 0.22 |
| 8 week abdominal circumference, cm | 16.6 ± 0.5 b | 18.3 ± 0.1 ab | 19.7 ± 0.5 a | 20.7 ± 0.1 a | <0.0001 | 0.0005 | 0.34 |
| 16 week abdominal circumference, cm | 17.7 ± 0.4 c | 18.8 ± 0.2 b | 23.5 ± 0.7 a | 21.6 ± 0.2 ab | <0.0001 | 0.35 | 0.001 |
| Visceral adiposity, % | 5.6 ± 0.5 c | 5.3 ± 0.2 c | 11.3 ± 0.5 a | 8.8 ± 0.4 b | <0.0001 | 0.002 | 0.012 |
| Retroperitoneal fat, mg/mm | 218 ± 23 c | 233 ± 13 c | 706 ± 67 a | 469 ± 33 b | <0.0001 | 0.008 | 0.003 |
| Epididymal fat, mg/mm | 116 ± 10 c | 74 ± 7 d | 324 ± 20 a | 191 ± 14 b | <0.0001 | <0.0001 | 0.005 |
| Omental fat, mg/mm | 116 ± 17 c | 127 ± 9 c | 315 ± 19 a | 220 ± 19 b | <0.0001 | 0.015 | 0.003 |
| Total abdominal fat, mg/mm | 451 ± 43 c | 433 ± 22 c | 1345 ± 97 a | 880 ± 57 b | <0.0001 | 0.0003 | 0.0007 |
| Brown fat, mg/mm | 21.8 ± 2.6 c | 33.9 ± 1.9 a | 31.4 ± 1.8 ab | 29.7 ± 2.1 b | 0.21 | 0.018 | 0.002 |
| 8 week 0 min [blood glucose], mmol/L | 2.8 ± 0.2 b | 2.9 ± 0.1 b | 3.4 ± 0.1 a | 3.0 ± 0.1 a | 0.011 | 0.26 | 0.07 |
| 16 week 0 min [blood glucose], mmol/L | 3.4 ± 0.2 ab | 3.4 ± 0.1 ab | 4.2 ± 0.2 a | 3.8 ± 0.1 a | 0.0004 | 0.21 | 0.21 |
| 8 week OGTT-AUC, mmol/L × min | 647 ± 25 d | 703 ± 21 c | 820 ± 21 a | 756 ± 12 b | <0.0001 | 0.85 | 0.005 |
| 16 week OGTT-AUC, mmol/L × min | 599 ± 10 c | 547 ± 18 d | 712 ± 34 a | 590 ± 11 b | 0.0005 | 0.0001 | 0.10 |
| Liver, mg/mm | 230 ± 15 c | 233 ± 5 c | 399 ± 23 a | 326 ± 12 b | <0.0001 | 0.026 | 0.016 |
| Liver fat vacuoles area, fat vacuoles/200 µm2 | 8.6 ± 1.0 c | 9.2 ± 1.3 c | 98.1 ± 8.8 a | 74.3 ± 5.9 b | <0.0001 | 0.051 | 0.042 |
| Plasma aspartate transaminase, U/L | 108.5 ± 10.6 b | 118.1 ± 8.4 b | 169.7 ± 22.2 a | 137.3 ± 14.3 ab | 0.022 | 0.50 | 0.22 |
| Plasma alanine transaminase, U/L | 39.8 ± 4.6 b | 45.3 ± 4.6 a | 54.8 ± 5.4 a | 38.7 ± 5.2 b | 0.42 | 0.32 | 0.044 |
| Plasma triglycerides, mmol/L | 0.57 ± 0.07 b | 0.65 ± 0.10 b | 1.86 ± 0.22 a | 2.25 ± 0.27 a | <0.0001 | 0.35 | 0.54 |
| Plasma total cholesterol, mmol/L | 1.43 ± 0.08 c | 1.42 ± 0.07 c | 1.98 ± 0.10 a | 1.64 ± 0.07 ab | <0.0001 | 0.039 | 0.051 |
| Plasma non-esterified fatty acids, mmol/L | 1.72 ± 0.24 b | 1.59 ± 0.20 bc | 3.44 ± 0.40 a | 3.28 ± 0.45 a | <0.0001 | 0.70 | 0.97 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
du Preez, R.; Wanyonyi, S.; Mouatt, P.; Panchal, S.K.; Brown, L. Saskatoon Berry Amelanchier alnifolia Regulates Glucose Metabolism and Improves Cardiovascular and Liver Signs of Diet-Induced Metabolic Syndrome in Rats. Nutrients 2020, 12, 931. https://doi.org/10.3390/nu12040931
du Preez R, Wanyonyi S, Mouatt P, Panchal SK, Brown L. Saskatoon Berry Amelanchier alnifolia Regulates Glucose Metabolism and Improves Cardiovascular and Liver Signs of Diet-Induced Metabolic Syndrome in Rats. Nutrients. 2020; 12(4):931. https://doi.org/10.3390/nu12040931
Chicago/Turabian Styledu Preez, Ryan, Stephen Wanyonyi, Peter Mouatt, Sunil K. Panchal, and Lindsay Brown. 2020. "Saskatoon Berry Amelanchier alnifolia Regulates Glucose Metabolism and Improves Cardiovascular and Liver Signs of Diet-Induced Metabolic Syndrome in Rats" Nutrients 12, no. 4: 931. https://doi.org/10.3390/nu12040931
APA Styledu Preez, R., Wanyonyi, S., Mouatt, P., Panchal, S. K., & Brown, L. (2020). Saskatoon Berry Amelanchier alnifolia Regulates Glucose Metabolism and Improves Cardiovascular and Liver Signs of Diet-Induced Metabolic Syndrome in Rats. Nutrients, 12(4), 931. https://doi.org/10.3390/nu12040931







