Microbial Adaptation Due to Gastric Bypass Surgery: The Nutritional Impact
Abstract
1. Introduction
2. Materials and Methods
3. The Intestinal Microbiome in Obesity
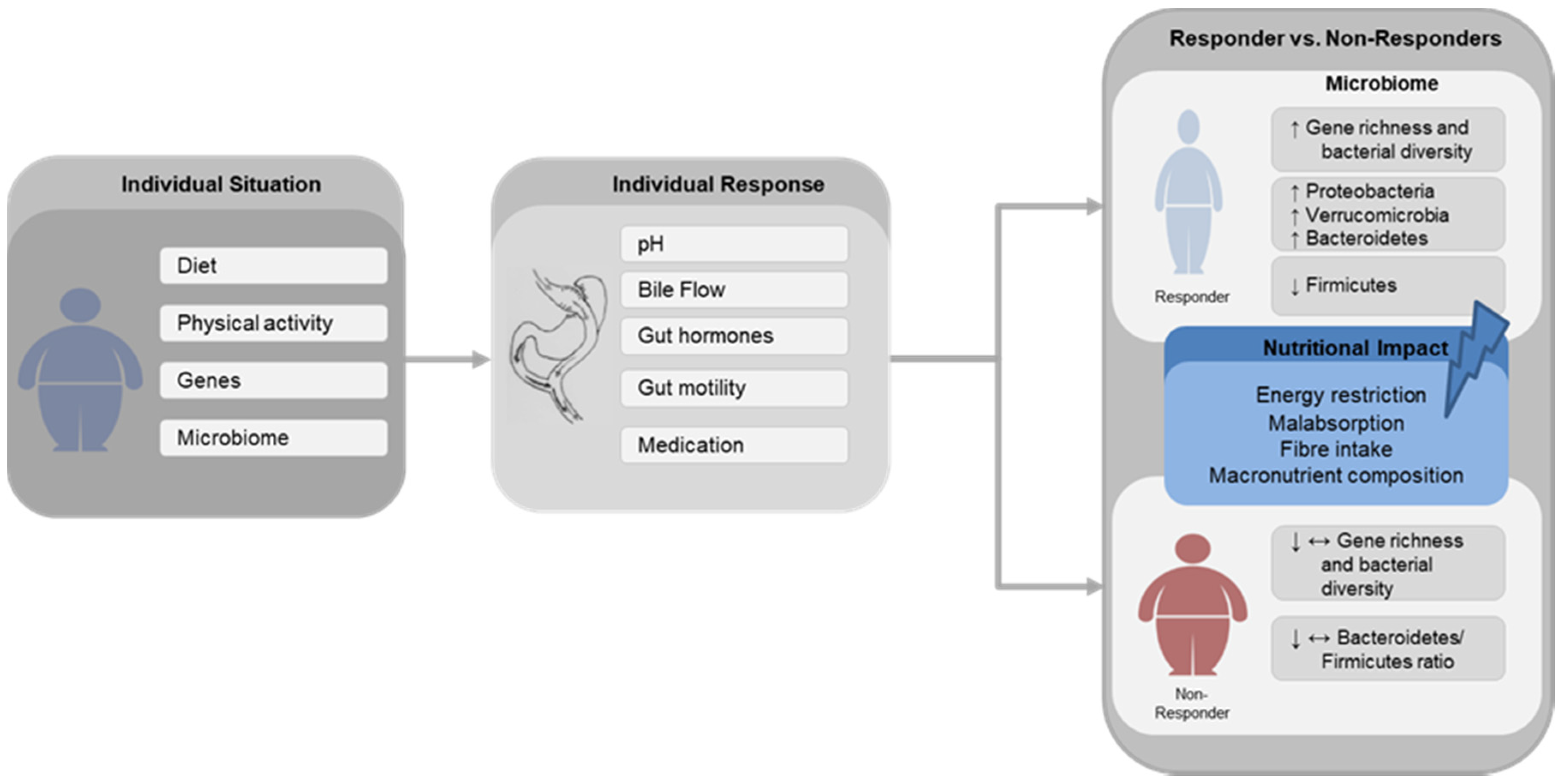
4. Impact of Gastric Bypass Surgery on Gut Microbiota
5. Diet and Microbiome
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO|Obesity. Available online: https://www.who.int/topics/obesity/en/ (accessed on 21 March 2020).
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Delzenne, N.M. The role of the gut microbiota in energy metabolism and metabolic disease. Curr. Pharm. Des. 2009, 15, 1546–1558. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Zuo, H.-J.; Xie, Z.-M.; Zhang, W.-W.; Li, Y.-R.; Wang, W.; Ding, X.-B.; Pei, X.-F. Gut bacteria alteration in obese people and its relationship with gene polymorphism. World J. Gastroenterol. 2011, 17, 1076–1081. [Google Scholar] [CrossRef]
- Oliveira, S.C.; Neves, J.S.; Souteiro, P.; Pedro, J.; Magalhães, D.; Guerreiro, V.; Bettencourt-Silva, R.; Costa, M.M.; Varela, A.; Barroso, I.; et al. Impact of Bariatric Surgery on Long-term Cardiovascular Risk: Comparative Effectiveness of Different Surgical Procedures. Obes. Surg. 2020, 30, 673–680. [Google Scholar] [CrossRef]
- Courcoulas, A.P.; Gallagher, J.W.; Neiberg, R.H.; Eagleton, E.B.; DeLany, J.P.; Lang, W.; Punchai, S.; Gourash, W.; Jakicic, J.M. Bariatric Surgery vs Lifestyle Intervention for Diabetes Treatment: 5-Year Outcomes From a Randomized Trial. J. Clin. Endocrinol. Metab. 2020, 105. [Google Scholar] [CrossRef]
- Singh, P.; Subramanian, A.; Adderley, N.; Gokhale, K.; Singhal, R.; Bellary, S.; Nirantharakumar, K.; Tahrani, A.A. Impact of bariatric surgery on cardiovascular outcomes and mortality: A population-based cohort study. Br. J. Surg. 2020, 107, 432–442. [Google Scholar] [CrossRef]
- Khorgami, Z.; Shoar, S.; Saber, A.A.; Howard, C.A.; Danaei, G.; Sclabas, G.M. Outcomes of Bariatric Surgery Versus Medical Management for Type 2 Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trials. Obes. Surg. 2019, 29, 964–974. [Google Scholar] [CrossRef]
- Schauer, P.R.; Bhatt, D.L.; Kirwan, J.P.; Wolski, K.; Aminian, A.; Brethauer, S.A.; Navaneethan, S.D.; Singh, R.P.; Pothier, C.E.; Nissen, S.E.; et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes—5-Year Outcomes. N. Engl. J. Med. 2017, 376, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, H.; Estok, R.; Fahrbach, K.; Banel, D.; Jensen, M.D.; Pories, W.J.; Bantle, J.P.; Sledge, I. Weight and type 2 diabetes after bariatric surgery: Systematic review and meta-analysis. Am. J. Med. 2009, 122, 248–256.e5. [Google Scholar] [CrossRef] [PubMed]
- Bouter, K.E.; van Raalte, D.H.; Groen, A.K.; Nieuwdorp, M. Role of the Gut Microbiome in the Pathogenesis of Obesity and Obesity-Related Metabolic Dysfunction. Gastroenterology 2017, 152, 1671–1678. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef]
- Alang, N.; Kelly, C.R. Weight gain after fecal microbiota transplantation. Open Forum Infect. Dis. 2015, 2, ofv004. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Mocanu, V.; Cai, C.; Dang, J.; Slater, L.; Deehan, E.C.; Walter, J.; Madsen, K.L. Impact of Fecal Microbiota Transplantation on Obesity and Metabolic Syndrome—A Systematic Review. Nutrients 2019, 11, 2291. [Google Scholar] [CrossRef] [PubMed]
- Falony, G.; Joossens, M.; Vieira-Silva, S.; Wang, J.; Darzi, Y.; Faust, K.; Kurilshikov, A.; Bonder, M.-J.; Valles-Colomer, M.; Vandeputte, D.; et al. Population-level analysis of gut microbiome variation. Science 2016, 352, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Zhernakova, A.; Kurilshikov, A.; Bonder, M.J.; Tigchelaar, E.F.; Schirmer, M.; Vatanen, T.; Mujagic, Z.; Vila, A.V.; Falony, G.; Vieira-Silva, S.; et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 2016, 352, 565–569. [Google Scholar] [CrossRef]
- Kurilshikov, A.; van den Munckhof, I.C.L.; Chen, L.; Bonder, M.J.; Schraa, K.; Rutten, J.H.W.; Riksen, N.P.; de Graaf, J.; Oosting, M.; Sanna, S.; et al. Gut Microbial Associations to Plasma Metabolites Linked to Cardiovascular Phenotypes and Risk. Circ. Res. 2019, 124, 1808–1820. [Google Scholar] [CrossRef]
- Martinez, K.B.; Pierre, J.F.; Chang, E.B. The Gut Microbiota: The Gateway to Improved Metabolism. Gastroenterol. Clin. North Am. 2016, 45, 601–614. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Bäckhed, F.; Fulton, L.; Gordon, J.I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 2008, 3, 213–223. [Google Scholar] [CrossRef]
- John, G.K.; Mullin, G.E. The Gut Microbiome and Obesity. Curr. Oncol. Rep. 2016, 18, 45. [Google Scholar] [CrossRef] [PubMed]
- Gérard, P. Gut microbiota and obesity. Cell. Mol. Life Sci. 2016, 73, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Gils Contreras, A.; Bonada Sanjaume, A.; Montero Jaime, M.; Rabassa Soler, A.; Sabench Pereferrer, F.; Molina López, A.; Becerra Tomás, N.; Del Castillo Déjardin, D.; Salas-Salvadó, J. Effects of Two Preoperatory Weight Loss Diets on Hepatic Volume, Metabolic Parameters, and Surgical Complications in Morbid Obese Bariatric Surgery Candidates: A Randomized Clinical Trial. Obes. Surg. 2018, 28, 3756–3768. [Google Scholar] [CrossRef] [PubMed]
- Gérard, P. Gut Microbiome and Obesity. How to Prove Causality? Ann. Am. Thorac. Soc. 2017, 14, S354–S356. [Google Scholar] [CrossRef]
- Van Nieuwenhove, Y.; Dambrauskas, Z.; Campillo-Soto, A.; van Dielen, F.; Wiezer, R.; Janssen, I.; Kramer, M.; Thorell, A. Preoperative very low-calorie diet and operative outcome after laparoscopic gastric bypass: A randomized multicenter study. Arch. Surg. 2011, 146, 1300–1305. [Google Scholar] [CrossRef]
- Sivakumar, J.; Chong, L.; Ward, S.; Sutherland, T.R.; Read, M.; Hii, M.W. Body Composition Changes Following a Very-Low-Calorie Pre-Operative Diet in Patients Undergoing Bariatric Surgery. Obes. Surg. 2020, 30, 119–126. [Google Scholar] [CrossRef]
- Fried, M.; Yumuk, V.; Oppert, J.M.; Scopinaro, N.; Torres, A.; Weiner, R.; Yashkov, Y.; Frühbeck, G. Interdisciplinary European guidelines on metabolic and bariatric surgery. Obes. Surg. 2014, 24, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Welbourn, R.; Hollyman, M.; Kinsman, R.; Dixon, J.; Liem, R.; Ottosson, J.; Ramos, A.; Våge, V.; Al-Sabah, S.; Brown, W.; et al. Bariatric Surgery Worldwide: Baseline Demographic Description and One-Year Outcomes from the Fourth IFSO Global Registry Report 2018. Obes. Surg. 2019, 29, 782–795. [Google Scholar] [CrossRef]
- Angrisani, L.; Santonicola, A.; Iovino, P.; Vitiello, A.; Higa, K.; Himpens, J.; Buchwald, H.; Scopinaro, N. IFSO Worldwide Survey 2016: Primary, Endoluminal, and Revisional Procedures. Obes. Surg. 2018, 28, 3783–3794. [Google Scholar] [CrossRef]
- Toolabi, K.; Sarkardeh, M.; Vasigh, M.; Golzarand, M.; Vezvaei, P.; Kooshki, J. Comparison of Laparoscopic Roux-en-Y Gastric Bypass and Laparoscopic Sleeve Gastrectomy on Weight Loss, Weight Regain, and Remission of Comorbidities: A 5 Years of Follow-up Study. Obes. Surg. 2020, 30, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Sharples, A.J.; Mahawar, K. Systematic Review and Meta-Analysis of Randomised Controlled Trials Comparing Long-Term Outcomes of Roux-En-Y Gastric Bypass and Sleeve Gastrectomy. Obes. Surg. 2020, 30, 664–672. [Google Scholar] [CrossRef]
- Raygor, V.; Garcia, L.; Maron, D.J.; Morton, J.M. The Comparative Effect of Roux-en-Y Gastric Bypass and Sleeve Gastrectomy on 10-Year and Lifetime Atherosclerotic Cardiovascular Disease Risk. Obes. Surg. 2019, 29, 3111–3117. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Varela, J.E. Bariatric surgery for obesity and metabolic disorders: State of the art. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 160–169. [Google Scholar] [CrossRef]
- Baldwin, D.; Chennakesavalu, M.; Gangemi, A. Systematic review and meta-analysis of Roux-en-Y gastric bypass against laparoscopic sleeve gastrectomy for amelioration of NAFLD using four criteria. Surg. Obes. Relat. Dis. 2019, 15, 2123–2130. [Google Scholar] [CrossRef]
- Fakhry, T.K.; Mhaskar, R.; Schwitalla, T.; Muradova, E.; Gonzalvo, J.P.; Murr, M.M. Bariatric surgery improves nonalcoholic fatty liver disease: A contemporary systematic review and meta-analysis. Surg. Obes. Relat. Dis. 2019, 15, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Cherla, D.V.; Rodriguez, N.A.; Vangoitsenhoven, R.; Singh, T.; Mehta, N.; McCullough, A.J.; Brethauer, S.A.; Schauer, P.R.; Aminian, A. Impact of sleeve gastrectomy and Roux-en-Y gastric bypass on biopsy-proven non-alcoholic fatty liver disease. Surg. Endosc. 2020, 34, 2266–2272. [Google Scholar] [CrossRef]
- Sridharan, K.; Kalayarasan, R.; Kamalanathan, S.; Sahoo, J.; Kar, S.S.; Nandhini, L.P.; Palui, R.; Durgia, H. Change in insulin resistance, beta cell function, glucagon-like peptide-1 and calcitonin levels two weeks after bariatric surgery. Diabetes Metab. Syndr. 2019, 13, 2142–2147. [Google Scholar] [CrossRef]
- Carreau, A.-M.; Noll, C.; Blondin, D.P.; Frisch, F.; Nadeau, M.; Pelletier, M.; Phoenix, S.; Cunnane, S.C.; Guérin, B.; Turcotte, E.E.; et al. Bariatric Surgery Rapidly Decreases Cardiac Dietary Fatty Acid Partitioning and Hepatic Insulin Resistance Through Increased Intra-abdominal Adipose Tissue Storage and Reduced Spillover in Type 2 Diabetes. Diabetes 2020, 69, 567–577. [Google Scholar] [CrossRef]
- Feng, W.; Yin, T.; Chu, X.; Shan, X.; Jiang, C.; Wang, Y.; Qian, Y.; Zhu, D.; Sun, X.; Bi, Y. Metabolic effects and safety of Roux-en-Y gastric bypass surgery vs. conventional medication in obese Chinese patients with type 2 diabetes. Diabetes. Metab. Res. Rev. 2019, 35, e3138. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Le, Q.A. Effectiveness of bariatric surgical procedures: A systematic review and network meta-analysis of randomized controlled trials. Medicine (Baltimore) 2017, 96, e8632. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Repiso, C.; Moreno-Indias, I.; de Hollanda, A.; Martín-Núñez, G.M.; Vidal, J.; Tinahones, F.J. Gut microbiota specific signatures are related to the successful rate of bariatric surgery. Am. J. Transl. Res. 2019, 11, 942–952. [Google Scholar] [PubMed]
- De Hollanda, A.; Ruiz, T.; Jiménez, A.; Flores, L.; Lacy, A.; Vidal, J. Patterns of Weight Loss Response Following Gastric Bypass and Sleeve Gastrectomy. Obes. Surg. 2015, 25, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Manning, S.; Pucci, A.; Carter, N.C.; Elkalaawy, M.; Querci, G.; Magno, S.; Tamberi, A.; Finer, N.; Fiennes, A.G.; Hashemi, M.; et al. Early postoperative weight loss predicts maximal weight loss after sleeve gastrectomy and Roux-en-Y gastric bypass. Surg. Endosc. 2015, 29, 1484–1491. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, C.F.; de Oliveira, A.P.R.P.; Brochado, M.J.F.; Pinhel, M.A.S.; de Oliveira, B.A.P.; Marchini, J.S.; Dos Santos, J.E.; Salgado, W.; Cury, N.M.; de Araújo, L.F.; et al. The Ala55Val and -866GA polymorphisms of the UCP2 gene could be biomarkers for weight loss in patients who had Roux-en-Y gastric bypass. Nutrition 2017, 33, 326–330. [Google Scholar] [CrossRef]
- Barhouch, A.S.; Padoin, A.V.; Casagrande, D.S.; Chatkin, R.; Süssenbach, S.P.; Pufal, M.A.; Rossoni, C.; Mottin, C.C. Predictors of Excess Weight Loss in Obese Patients After Gastric Bypass: A 60-Month Follow-up. Obes. Surg. 2016, 26, 1178–1185. [Google Scholar] [CrossRef]
- Faria, G.; Preto, J.; Almeida, A.B.; Guimarães, J.T.; Calhau, C.; Taveira-Gomes, A. Fasting glycemia: A good predictor of weight loss after RYGB. Surg. Obes. Relat. Dis. 2014, 10, 419–424. [Google Scholar] [CrossRef]
- Contreras, J.E.; Santander, C.; Court, I.; Bravo, J. Correlation between age and weight loss after bariatric surgery. Obes. Surg. 2013, 23, 1286–1289. [Google Scholar] [CrossRef]
- Melton, G.B.; Steele, K.E.; Schweitzer, M.A.; Lidor, A.O.; Magnuson, T.H. Suboptimal weight loss after gastric bypass surgery: Correlation of demographics, comorbidities, and insurance status with outcomes. J. Gastrointest. Surg. 2008, 12, 250–255. [Google Scholar] [CrossRef]
- Furet, J.-P.; Kong, L.-C.; Tap, J.; Poitou, C.; Basdevant, A.; Bouillot, J.-L.; Mariat, D.; Corthier, G.; Doré, J.; Henegar, C.; et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: Links with metabolic and low-grade inflammation markers. Diabetes 2010, 59, 3049–3057. [Google Scholar] [CrossRef] [PubMed]
- Fouladi, F.; Brooks, A.E.; Fodor, A.A.; Carroll, I.M.; Bulik-Sullivan, E.C.; Tsilimigras, M.C.B.; Sioda, M.; Steffen, K.J. The Role of the Gut Microbiota in Sustained Weight Loss Following Roux-en-Y Gastric Bypass Surgery. Obes. Surg. 2019, 29, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, Z.E.; DiBaise, J.K.; Isern, N.G.; Hoyt, D.W.; Marcus, A.K.; Kang, D.-W.; Crowell, M.D.; Rittmann, B.E.; Krajmalnik-Brown, R. Distinctive microbiomes and metabolites linked with weight loss after gastric bypass, but not gastric banding. ISME J. 2017, 11, 2047–2058. [Google Scholar] [CrossRef] [PubMed]
- Graessler, J.; Qin, Y.; Zhong, H.; Zhang, J.; Licinio, J.; Wong, M.-L.; Xu, A.; Chavakis, T.; Bornstein, A.B.; Ehrhart-Bornstein, M.; et al. Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: Correlation with inflammatory and metabolic parameters. Pharm. J. 2013, 13, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Tremaroli, V.; Karlsson, F.; Werling, M.; Ståhlman, M.; Kovatcheva-Datchary, P.; Olbers, T.; Fändriks, L.; Le Roux, C.W.; Nielsen, J.; Bäckhed, F. Roux-en-Y Gastric Bypass and Vertical Banded Gastroplasty Induce Long-Term Changes on the Human Gut Microbiome Contributing to Fat Mass Regulation. Cell Metab. 2015, 22, 228–238. [Google Scholar] [CrossRef]
- Palleja, A.; Kashani, A.; Allin, K.H.; Nielsen, T.; Zhang, C.; Li, Y.; Brach, T.; Liang, S.; Feng, Q.; Jørgensen, N.B.; et al. Roux-en-Y gastric bypass surgery of morbidly obese patients induces swift and persistent changes of the individual gut microbiota. Genome Med. 2016, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Patrone, V.; Vajana, E.; Minuti, A.; Callegari, M.L.; Federico, A.; Loguercio, C.; Dallio, M.; Tolone, S.; Docimo, L.; Morelli, L. Postoperative Changes in Fecal Bacterial Communities and Fermentation Products in Obese Patients Undergoing Bilio-Intestinal Bypass. Front. Microbiol. 2016, 7, 200. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.; Tsai, P.; Jüllig, M.; Liu, A.; Plank, L.; Booth, M. Differential Changes in Gut Microbiota After Gastric Bypass and Sleeve Gastrectomy Bariatric Surgery Vary According to Diabetes Remission. Obes. Surg. 2017, 27, 917–925. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Prifti, E.; Belda, E.; Ichou, F.; Kayser, B.D.; Dao, M.C.; Verger, E.O.; Hedjazi, L.; Bouillot, J.-L.; Chevallier, J.-M.; et al. Major microbiota dysbiosis in severe obesity: Fate after bariatric surgery. Gut 2019, 68, 70–82. [Google Scholar] [CrossRef]
- Paganelli, F.L.; Luyer, M.; Hazelbag, C.M.; Uh, H.-W.; Rogers, M.R.C.; Adriaans, D.; Berbers, R.-M.; Hendrickx, A.P.A.; Viveen, M.C.; Groot, J.A.; et al. Roux-Y Gastric Bypass and Sleeve Gastrectomy directly change gut microbiota composition independent of surgery type. Sci. Rep. 2019, 9, 10979. [Google Scholar] [CrossRef]
- Liou, A.P.; Paziuk, M.; Luevano, J.-M.; Machineni, S.; Turnbaugh, P.J.; Kaplan, L.M. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci. Transl. Med. 2013, 5, 178ra41. [Google Scholar] [CrossRef]
- Sánchez-Alcoholado, L.; Gutiérrez-Repiso, C.; Gómez-Pérez, A.M.; García-Fuentes, E.; Tinahones, F.J.; Moreno-Indias, I. Gut microbiota adaptation after weight loss by Roux-en-Y gastric bypass or sleeve gastrectomy bariatric surgeries. Surg. Obes. Relat. Dis. 2019, 15, 1888–1895. [Google Scholar] [CrossRef] [PubMed]
- Palmisano, S.; Campisciano, G.; Silvestri, M.; Guerra, M.; Giuricin, M.; Casagranda, B.; Comar, M.; de Manzini, N. Changes in Gut Microbiota Composition after Bariatric Surgery: A New Balance to Decode. J. Gastrointest. Surg. 2019. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.-C.; Tap, J.; Aron-Wisnewsky, J.; Pelloux, V.; Basdevant, A.; Bouillot, J.-L.; Zucker, J.-D.; Doré, J.; Clément, K. Gut microbiota after gastric bypass in human obesity: Increased richness and associations of bacterial genera with adipose tissue genes. Am. J. Clin. Nutr. 2013, 98, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Li, J.V.; Ashrafian, H.; Bueter, M.; Kinross, J.; Sands, C.; Le Roux, C.W.; Bloom, S.R.; Darzi, A.; Athanasiou, T.; Marchesi, J.R.; et al. Metabolic Surgery Profoundly Influences Gut Microbial-Host Metabolic Crosstalk. Gut 2011, 60, 1214–1223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; DiBaise, J.K.; Zuccolo, A.; Kudrna, D.; Braidotti, M.; Yu, Y.; Parameswaran, P.; Crowell, M.D.; Wing, R.; Rittmann, B.E.; et al. Human gut microbiota in obesity and after gastric bypass. Proc. Natl. Acad. Sci. USA 2009, 106, 2365–2370. [Google Scholar] [CrossRef]
- Kikuchi, R.; Irie, J.; Yamada-Goto, N.; Kikkawa, E.; Seki, Y.; Kasama, K.; Itoh, H. The Impact of Laparoscopic Sleeve Gastrectomy with Duodenojejunal Bypass on Intestinal Microbiota Differs from that of Laparoscopic Sleeve Gastrectomy in Japanese Patients with Obesity. Clin. Drug Investig. 2018, 38, 545–552. [Google Scholar] [CrossRef]
- Wang, F.-G.; Bai, R.-X.; Yan, W.-M.; Yan, M.; Dong, L.-Y.; Song, M.-M. Differential composition of gut microbiota among healthy volunteers, morbidly obese patients and post-bariatric surgery patients. Exp. Ther. Med. 2019, 17, 2268–2278. [Google Scholar] [CrossRef]
- Campisciano, G.; Cason, C.; Palmisano, S.; Giuricin, M.; Rizzardi, A.; Croce, L.S.; de Manzini, N.; Comar, M. Bariatric surgery drives major rearrangements of the intestinal microbiota including the biofilm composition. Front. Biosci. (Elite Ed) 2018, 10, 495–505. [Google Scholar]
- Liu, R.; Hong, J.; Xu, X.; Feng, Q.; Zhang, D.; Gu, Y.; Shi, J.; Zhao, S.; Liu, W.; Wang, X.; et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med. 2017, 23, 859–868. [Google Scholar] [CrossRef]
- Damms-Machado, A.; Mitra, S.; Schollenberger, A.E.; Kramer, K.M.; Meile, T.; Königsrainer, A.; Huson, D.H.; Bischoff, S.C. Effects of surgical and dietary weight loss therapy for obesity on gut microbiota composition and nutrient absorption. Biomed Res. Int. 2015, 2015, 806248. [Google Scholar] [CrossRef] [PubMed]
- Sanmiguel, C.P.; Jacobs, J.; Gupta, A.; Ju, T.; Stains, J.; Coveleskie, K.; Lagishetty, V.; Balioukova, A.; Chen, Y.; Dutson, E.; et al. Surgically Induced Changes in Gut Microbiome and Hedonic Eating as Related to Weight Loss: Preliminary Findings in Obese Women Undergoing Bariatric Surgery. Psychosom. Med. 2017, 79, 880–887. [Google Scholar] [CrossRef]
- Ward, E.K.; Schuster, D.P.; Stowers, K.H.; Royse, A.K.; Ir, D.; Robertson, C.E.; Frank, D.N.; Austin, G.L. The effect of PPI use on human gut microbiota and weight loss in patients undergoing laparoscopic Roux-en-Y gastric bypass. Obes. Surg. 2014, 24, 1567–1571. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Dallio, M.; Tolone, S.; Gravina, A.G.; Patrone, V.; Romano, M.; Tuccillo, C.; Mozzillo, A.L.; Amoroso, V.; Misso, G.; et al. Gastrointestinal Hormones, Intestinal Microbiota and Metabolic Homeostasis in Obese Patients: Effect of Bariatric Surgery. In Vivo 2016, 30, 321–330. [Google Scholar] [PubMed]
- Cortez, R.V.; Petry, T.; Caravatto, P.; Pessôa, R.; Sanabani, S.S.; Martinez, M.B.; Sarian, T.; Salles, J.E.; Cohen, R.; Taddei, C.R. Shifts in intestinal microbiota after duodenal exclusion favor glycemic control and weight loss: A randomized controlled trial. Surg. Obes. Relat. Dis. 2018, 14, 1748–1754. [Google Scholar] [CrossRef]
- Pajecki, D.; de Oliveira, L.C.; Sabino, E.C.; de Souza-Basqueira, M.; Dantas, A.C.B.; Nunes, G.C.; de Cleva, R.; Santo, M.A. Changes in the intestinal microbiota of superobese patients after bariatric surgery. Clinics (Sao Paulo) 2019, 74, e1198. [Google Scholar] [CrossRef]
- Lee, C.J.; Florea, L.; Sears, C.L.; Maruthur, N.; Potter, J.J.; Schweitzer, M.; Magnuson, T.; Clark, J.M. Changes in Gut Microbiome after Bariatric Surgery Versus Medical Weight Loss in a Pilot Randomized Trial. Obes. Surg. 2019, 29, 3239–3245. [Google Scholar] [CrossRef]
- Shen, N.; Caixàs, A.; Ahlers, M.; Patel, K.; Gao, Z.; Dutia, R.; Blaser, M.J.; Clemente, J.C.; Laferrère, B. Longitudinal changes of microbiome composition and microbial metabolomics after surgical weight loss in individuals with obesity. Surg. Obes. Relat. Dis. 2019, 15, 1367–1373. [Google Scholar] [CrossRef]
- Al Assal, K.; Prifti, E.; Belda, E.; Sala, P.; Clément, K.; Dao, M.-C.; Doré, J.; Levenez, F.; Taddei, C.R.; Fonseca, D.C.; et al. Gut Microbiota Profile of Obese Diabetic Women Submitted to Roux-en-Y Gastric Bypass and Its Association with Food Intake and Postoperative Diabetes Remission. Nutrients 2020, 12, 278. [Google Scholar] [CrossRef]
- Janmohammadi, P.; Sajadi, F.; Alizadeh, S.; Daneshzad, E. Comparison of Energy and Food Intake Between Gastric Bypass and Sleeve Gastrectomy: A Meta-analysis and Systematic Review. Obes. Surg. 2019, 29, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Reichmann, M.T.F.; Todeschini, S.; Setter, N.; Vilela, R.M.; Radominski, R.B. Comparación de la ingesta dietética entre las mujeres en el postoperatorio tardío después del bypass gástrico en Y de Roux con la pirámide nutricional bariátrica. Nutr. Hosp. 2019, 36, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Soares, F.L.; Bissoni de Sousa, L.; Corradi-Perini, C.; Ramos da Cruz, M.R.; Nunes, M.G.J.; Branco-Filho, A.J. Food quality in the late postoperative period of bariatric surgery: An evaluation using the bariatric food pyramid. Obes. Surg. 2014, 24, 1481–1486. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, C.-Q.; Shan, C.-X.; Chen, Y.; Li, H.-H.; Huang, Z.-P.; Zou, D.-J. Gut microbiota after Roux-en-Y gastric bypass and sleeve gastrectomy in a diabetic rat model: Increased diversity and associations of discriminant genera with metabolic changes. Diabetes Metab. Res. Rev. 2017, 33. [Google Scholar] [CrossRef] [PubMed]
- Haange, S.-B.; Jehmlich, N.; Krügel, U.; Hintschich, C.; Wehrmann, D.; Hankir, M.; Seyfried, F.; Froment, J.; Hübschmann, T.; Müller, S.; et al. Gastric bypass surgery in a rat model alters the community structure and functional composition of the intestinal microbiota independently of weight loss. Microbiome 2020, 8, 13. [Google Scholar] [CrossRef]
- Flint, H.J.; Duncan, S.H.; Scott, K.P.; Louis, P. Links between diet, gut microbiota composition and gut metabolism. Proc. Nutr. Soc. 2015, 74, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Fava, F.; Gitau, R.; Griffin, B.A.; Gibson, G.R.; Tuohy, K.M.; Lovegrove, J.A. The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome ‘at-risk’ population. Int. J. Obes. (Lond) 2013, 37, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef]
- Mills, S.; Stanton, C.; Lane, J.A.; Smith, G.J.; Ross, R.P. Precision Nutrition and the Microbiome, Part I: Current State of the Science. Nutrients 2019, 11, 923. [Google Scholar] [CrossRef]
- Ravussin, Y.; Koren, O.; Spor, A.; LeDuc, C.; Gutman, R.; Stombaugh, J.; Knight, R.; Ley, R.E.; Leibel, R.L. Responses of gut microbiota to diet composition and weight loss in lean and obese mice. Obesity (Silver Spring) 2012, 20, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Louis, S.; Tappu, R.-M.; Damms-Machado, A.; Huson, D.H.; Bischoff, S.C. Characterization of the Gut Microbial Community of Obese Patients Following a Weight-Loss Intervention Using Whole Metagenome Shotgun Sequencing. PLoS ONE 2016, 11, e0149564. [Google Scholar] [CrossRef]
- Heinsen, F.-A.; Fangmann, D.; Müller, N.; Schulte, D.M.; Rühlemann, M.C.; Türk, K.; Settgast, U.; Lieb, W.; Baines, J.F.; Schreiber, S.; et al. Beneficial Effects of a Dietary Weight Loss Intervention on Human Gut Microbiome Diversity and Metabolism Are Not Sustained during Weight Maintenance. Obes. Facts 2016, 9, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Ott, B.; Skurk, T.; Hastreiter, L.; Lagkouvardos, I.; Fischer, S.; Büttner, J.; Kellerer, T.; Clavel, T.; Rychlik, M.; Haller, D.; et al. Effect of caloric restriction on gut permeability, inflammation markers, and fecal microbiota in obese women. Sci. Rep. 2017, 7, 11955. [Google Scholar] [CrossRef]
- Lewis, M.C.; Phillips, M.L.; Slavotinek, J.P.; Kow, L.; Thompson, C.H.; Toouli, J. Change in liver size and fat content after treatment with Optifast very low calorie diet. Obes. Surg. 2006, 16, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Chakravartty, S.; Vivian, G.; Mullholland, N.; Shaikh, H.; McGrath, J.; Sidhu, P.S.; Jaffer, O.; Patel, A.G. Preoperative liver shrinking diet for bariatric surgery may impact wound healing: A randomized controlled trial. Surg. Obes. Relat. Dis. 2019, 15, 117–125. [Google Scholar] [CrossRef] [PubMed]
| Reference | Subjects | Type of Surgery (n) | Sample Size (n) | Time Points | Pre-BS Dietary Intake | Post-BS Dietary Intake | Impact on Diversity and Gene Richness | Changes in Relative Abundance | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Phylum | Class/Order/Family | Genus | Species | ||||||||
| Zhang 2009 [67] | Normal weight, obese, post-BS | RYGB | 6 RYGB 3 NW 3 MO 3 | 8–15 mo post-BS | - | - | - | ↑ Verrucomicrobia ↑ Fusobacteria ↓ Firmicutes | ↑ Gammaproteobacteria ↑ Prevotellaceae ↑ Fusobacteriaceae ↑ Enterobacteriaceae ↓ Clostridia | ↓ Lachnospira ↑ Akkermansia | |
| Furet 2010 [52] | Post-BS (7 T2DM), lean controls | RYGB | 43 RYGB 30 NW 13 | Pre-BS, 3, 6 mo post-BS | 1-h questioning period | 1-h questioning period | - | ↑ Bacteroidetes | ↑ Bacteroides/Prevotella ratio ↓ Bifidobacterium ↓ Lactobacillus ↓ Leuconostoc ↓ Pediococcus | ↑ Escherichia coli ↑ Faecalibacterium prausnitzii | |
| Kong 2013 [65] | Morbidly obese women | RYGB | 30 | Pre- BS, 3, 6 mo post-bs | 1-h questioning period | 1-h questioning period | ↑ GM richness | ↑ Proteobacteria ↓ Firmicutes | ↑ Alistipes ↑ Escherichia ↑ Bacteroides ↓ Bifidobacterium ↓ Lactobacillus ↓ Dorea ↓ Blautia | ||
| Graessler 2013 [55] | Morbidly obese subjects | RYGB | 6 | Pre- BS, 3 mo post-bs | - | - | - | ↑ Proteobacteria ↑ Fusobacteria ↑ Verrucomicrobia ↓ Bacteroidetes ↓ Firmicutes ↓ Actinobacteria ↓ Cyanobacteria ↑ Bacteroidetes/Firmicutes ratio | ↑ Enterobacter ↑ Neurospora ↑ Citrobacter ↑ Veillonella ↑ Salmonella ↓ Faecalibacterium ↓ Coprococcus ↓ Helicobacter ↓ Anaerostipes ↓ Nakamurella | ↑ Enterobacter cancerogenus ↑ Veillonella parvula ↑ Veillonella dispar ↑ Shigella boydii ↑ Salmonella enerica ↓ Lactobacillus reuteri ↓ Treponema pallidum ↓ Mycobacterium kansasii ↓ Faecalibacterium prausnitzii ↓ Clostridium comes | |
| Ward 2014 [74] | Severely obese subjects | RYGB | 8 | Pre- BS, 6 mo post-bs | - | - | - | PPI Users: ↑ Bacteroidetes ↑ Proteobacteria PPI non-users: ↓ Verrucomicrobia ↓ Firmicutes ↓ Proteobacteria | |||
| Tremaroli 2015 [56] | Post-BS women, non-operated severely obese women | RYGB VGB | 21 RYGB 7 VGB 7 MO 7 | 9.4 y post-BS | - | - | - | ↑ Proteobacteria ↓ Firmicutes | ↑ Gammaproteobacteria | ↑ Escherichia coli ↓ Clostridium difficile ↓ Clostridium hiranonis ↓ Gemella sanguinis | |
| Federico 2016 [75] | Severely obese and normal weight | BIB | 56 BIB 28 NW 28 | Pre- BS, 6 mo post-bs | 7 d food records | 7 d food records | - | ↑ Lactobacillus crispatus ↑ Streptococcus spp. ↑ Megasphaera sp. | |||
| Palleja 2016 [57] | Morbidly obese subjects | RYGB | 13 | Pre- BS, 3, 12 mo post-bs | Weight loss diet (8% weight loss) | - | ↑ species richness ↑ gene richness | ↑ Proteobacteria ↑ Fusobacteria | ↑ Escherichia coli ↑ Klebsiella pneumonia ↑ Akkermansia muciniphila ↓ Faecalibacterium prausnitzii ↓ Anaerotruncus colihominis ↓ Megasphaeara micronuciformis ↑ Alistipes spp. ↑ Streptococcus spp. ↑ Veillonella spp. | ||
| Patrone 2016 [58] | Severely obese | BIB | 11 | Pre- BS, 6 mo post-bs | Assessment of dietary habits | Assessment of dietary habits | ↓ Species richness | ↓ Lachnospiraceae ↓ Clostridiaceae ↓ Ruminococca-ceae ↓ Eubacteriaceae ↓ Coriobacteriaceae ↑ Lactobacillus ↑ Megasphaera ↑ Acidaminococcus | |||
| Ilhan 2017 [54] | Pre-BS obese, normal weight, post-RYGB and post-LAGB | RYGB LABG | 63 RYGB 24 LAGB 14 NW 10 Preb-Ob 15 | 35 ± 8 mo post-BS | 4 d food diaries and FFQ | ↑ α-diversity | ↑ Gammaproteobacteria ↑ Bacilli ↑ Flavobacteria ↑ Fusobacteria | ↑ Escherichia ↑ Veillonella ↑ Streptococcus ↑ Trabulsiella ↑ Haemophilus ↑ Coprococcus ↑ Enterococcus ↓ Oscillospira ↓ Coprobacillus ↓ Bacteroides | |||
| Murphy 2017 [59] | Obese T2DM subjects | RYGB SG | 14 RYGB 7 SG 7 | 1 w pre-BS, 1 y post-BS | 2 w Optifast 3 d food diary | 3 d food diary | ↑ α-diversity | ↑ Firmicutes ↑ Actinobacteria ↓ Bacteroidetes | |||
| Aron-Wisnewsky 2018 [60] | Severely obese subjects | RYGB agb | 61 RYGB 41 Agb 20 | Pre- BS, 1, 3, 12 mo post-bs | Equilibrate diet | - | ↑ Microbial gene richness | ↑ GU:99 Roseburia ↑ GU:225 Butyricimonas virosa ↑ GU:359 Butyricimonas | |||
| Campisciano 2018 [70] | Obese patients, normal weight controls | LGB SG | 40 Sg 10 LGB 10 NW 20 | Pre- BS, 3 mo post-bs | - | - | ↑ α-diversity | ↑ Proteobacteria ↑ Firmicutes ↓ Actinobacteria ↓ Bacteroidetes ↑ Firmicutes/Bacteroidetes ratio | ↑ Prevotella/bacteroides ratio ↑ Prevotella ↓ Bacteroides | ↑ Bifidobacterium vulgatus ↑ Hafnia alvei ↓ Bifidobacterium uniformis | |
| Cortez 2018 [76] | Overweight, class I or II obesity T2DM patients, medical care | DJB | 21 DJB 11 SC 10 | Pre-BS, 6, 12 mo post-BS | - | SC: diet formulated using total energy expenditure | ↓ α-diversity | ↑ Bacteroidetes ↑ Verrucomicrobia | ↑ Bacteroides ↑ Akkermansia ↑ Dialister | ↑ Akkermansia muciniphila | |
| Paganelli 2019 [61] | Morbidly obese | RYGB SG | 45 Sg 22 RYGB 23 | Before VLCD, 2 w after VLCD, 1 w, 3, 6 mo post-bs | 2 w modifast (500 kcal/d) | - | Post-VLCD: ↓ α-diversity 3 and 6 mo: ↑ α-diversity to baseline level | Post-VLCD: ↑ Rikenellaceae ↓ Streptococcaceae ↓ Ruminococcaceae post-BS: ↑ Streptococcaceae ↑ Enterobacteriaceae ↓ Bifidobacteriaceae | |||
| Sanchez-Alcoholado 2019 [63] | Severely obese patients | RYGB SG | 28 RYGB 14 SG 14 | Pre- BS, 3 mo post-bs | - | - | ~α-diversity | ↑ Proteobacteria ↑ Fusobacteria | ↑ Fusobacteriaceae ↑ Clostridiaceae ↑ Enterobacteriaceae ↓ Bifidobacteriaceae ↓ Peptostrepto-coccaceae | ↓ Bifidobacterium ↓ Collinsella ↑ Slackia ↑ Clostridium ↑ Veillonella ↑ Granucatiella ↑ Oscillospira ↑ Fusobacterium ↑ Granucatiella | |
| Pajecki 2019 [77] | Super-obese subjects | RYGB | 9 | Pre- BS, 12, 24 mo post-bs | - | - | ↓ Proteobacteria | ||||
| Lee 2019 [78] | Mildly or moderately obesity with T2DM at 10% of weight loss | RYGB AGB | 12 AGB 4 RYGB 4 MWL 4 | Pre-BS, at 10% of weight loss, 9 mo if 10% was not achieved | - | - | ↑ α-diversity ↑ richness | ↑ Proteobacteria ↑ Actinobacteria | ↑ Faecalibacterium ↑ Akkermansia | ||
| Fouladi 2019 [53] | Post-RYGB with successful or poor weight loss, non-surgical controls | RYGB | 18 SWL 6 PWL 6 NSC 6 | 2–5 years post-BS | -. | 24-h recall for 3 days | ↑ α-diversity ↑ richness PWL vs. NW ↑ diversity | ↑ Micrococcales ↑ Lactobacillales | ↑ Rothia ↑ Streptococcus PWL vs. NW: ↑ Oscullibacter ↑ Lactobacillus ↑ Enterobacter ↑ Akkermansia | ||
| Gutierrez-Repiso 2019 [44] | Post-RYGB with primary failure, weight regain or successful weight loss | RYGB | 24 SWL 6 Primary failure 6 Weight regain 12 | 8.3 ± 1.7 years post-BS | - | - | ~α-diversity | Success vs. weight regain: ↑ Butyrivibrio ↑ Lachnospira ↑ 5–7N15 ↑ Sacina ↑ Alkaliphilus ↑ Pseudo-altermonas ↑ Cetobacterium ↑ AF12 | |||
| Palmisano 2019 [64] | Obese patients, normal weight controls | RYGB SG | 50 RYGB 9 Sg 16 NW 25 | Pre- BS, 3, 6 mo post-bs | Food preferences | Food preferences | ~α-diversity | ↑ Proteobacteria ↑ Fusobacteria ↑ Verrucomicrobia ↓ Bacteroidetes ↓ Firmicutes | ↑ Gammaproteobacteria | 6 mo: ↑ Akkermansia muciniphila ↓ Veillonella atypical ↓ Veillonella dispar ↓ Streptococcus gordonii ↓ Streptococcus australis ↑ Yokenella regensburgei ↑ Fusobacterium varium | |
| Shen 2019 [79] | Severely obese with and without T2DM | RYGB SG | 26 RYGB 19 SG 7 | Pre-BS, 3, 6, 12 mo post-BS | - | - | 6 mo: ↑ for α-diversity ↑ β-diversity 12 mo: Tend to pre-BS levels | 3 and 6 mo: ↑ Verrucomicrobia ↑ Proteobacteria 12 mo: trend diminished | ↑ Akkermansia | ||
| Al Assal 2020 [80] | Obese T2DM women | RYGB | 24 | Pre-BS, 3, 12 mo post-BS | 7 d records (1700 kcal/d) | 7 d records | ↑ GM richness | 3 mo: ↑ Proteobacteria ↑ Firmicutes ↑ Actinobacteria 12 mo: ↓ Firmicutes/bacteroidetes ratio | 3 mo: ↑ Veillonella ↑ Streptococcus ↑ Gemella ↑ Oribacterium ↑ Atopobium ↑ one unclassified Lactobacillus genus ↑ Leptotrichia ↑ Neisseria ↑ one unclassified Pasteurellaceae genus ↓ Faecalibacterium | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crommen, S.; Mattes, A.; Simon, M.-C. Microbial Adaptation Due to Gastric Bypass Surgery: The Nutritional Impact. Nutrients 2020, 12, 1199. https://doi.org/10.3390/nu12041199
Crommen S, Mattes A, Simon M-C. Microbial Adaptation Due to Gastric Bypass Surgery: The Nutritional Impact. Nutrients. 2020; 12(4):1199. https://doi.org/10.3390/nu12041199
Chicago/Turabian StyleCrommen, Silke, Alma Mattes, and Marie-Christine Simon. 2020. "Microbial Adaptation Due to Gastric Bypass Surgery: The Nutritional Impact" Nutrients 12, no. 4: 1199. https://doi.org/10.3390/nu12041199
APA StyleCrommen, S., Mattes, A., & Simon, M.-C. (2020). Microbial Adaptation Due to Gastric Bypass Surgery: The Nutritional Impact. Nutrients, 12(4), 1199. https://doi.org/10.3390/nu12041199






