A Novel Combination of Vitamin C, Curcumin and Glycyrrhizic Acid Potentially Regulates Immune and Inflammatory Response Associated with Coronavirus Infections: A Perspective from System Biology Analysis
Abstract
1. Introduction
2. Method
2.1. Gene Target Acquisition and Screening
2.2. Hub Target Identification and Protein–Protein Interaction (PPI) Analysis
2.3. Distribution Analysis of Targets in Tissues/System and Gene Ontology (GO) Enrichment and Analysis
2.4. Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Analysis
3. Results
3.1. Hub Target Identification and Analysis
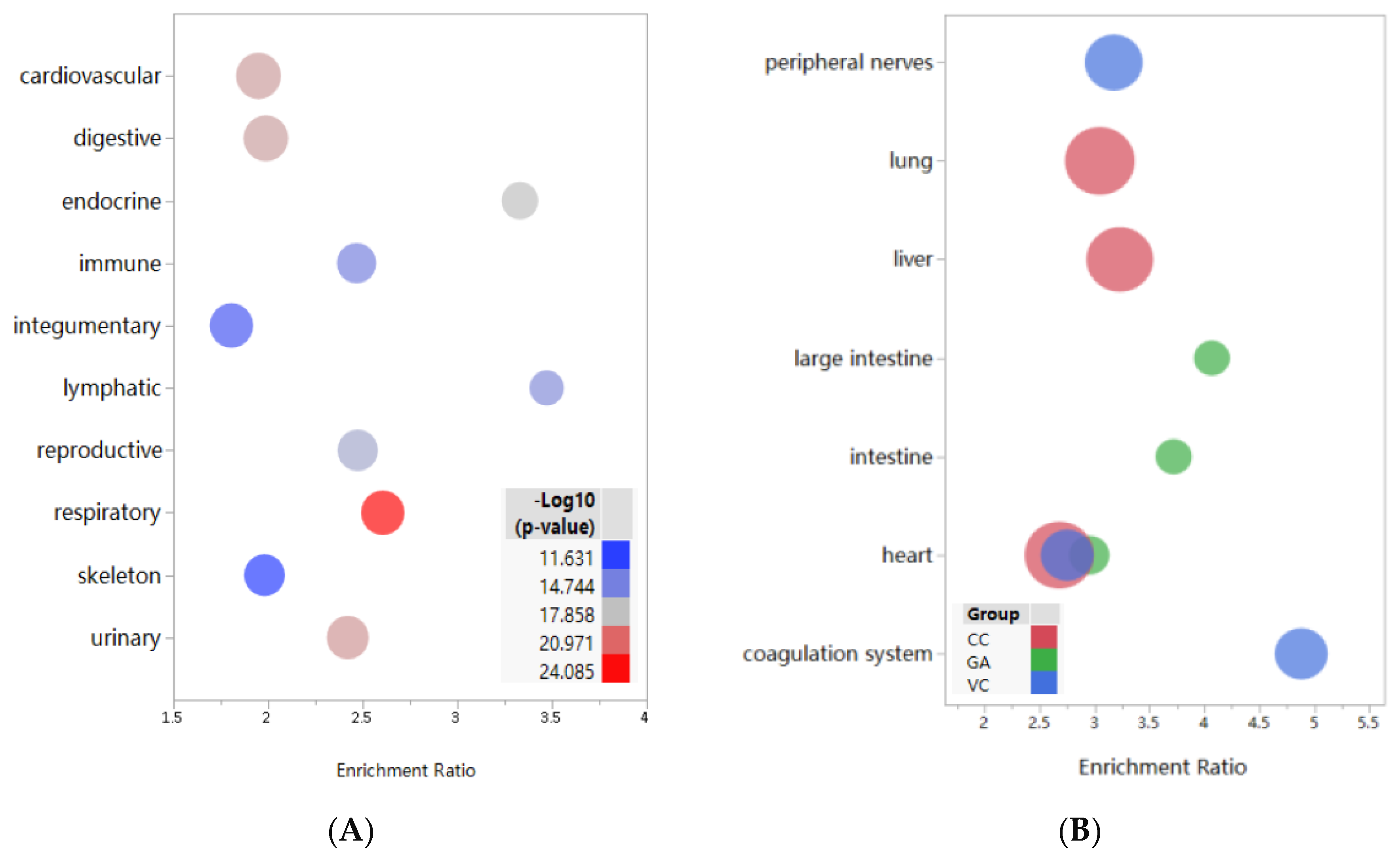
3.2. Enrichment and Analysis of Target Distribution in Tissues and Systems
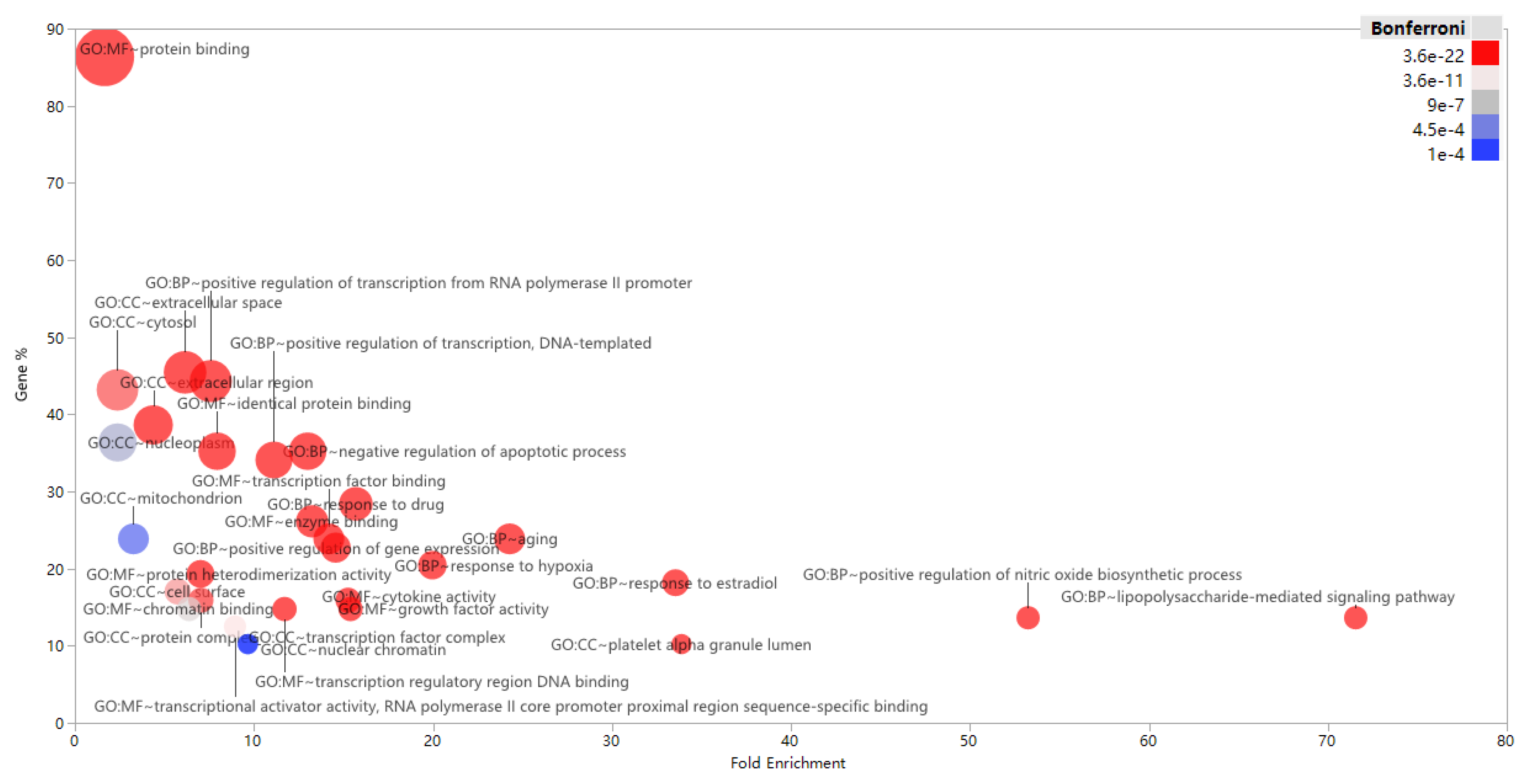
3.3. Enrichment and Analysis of GO Term
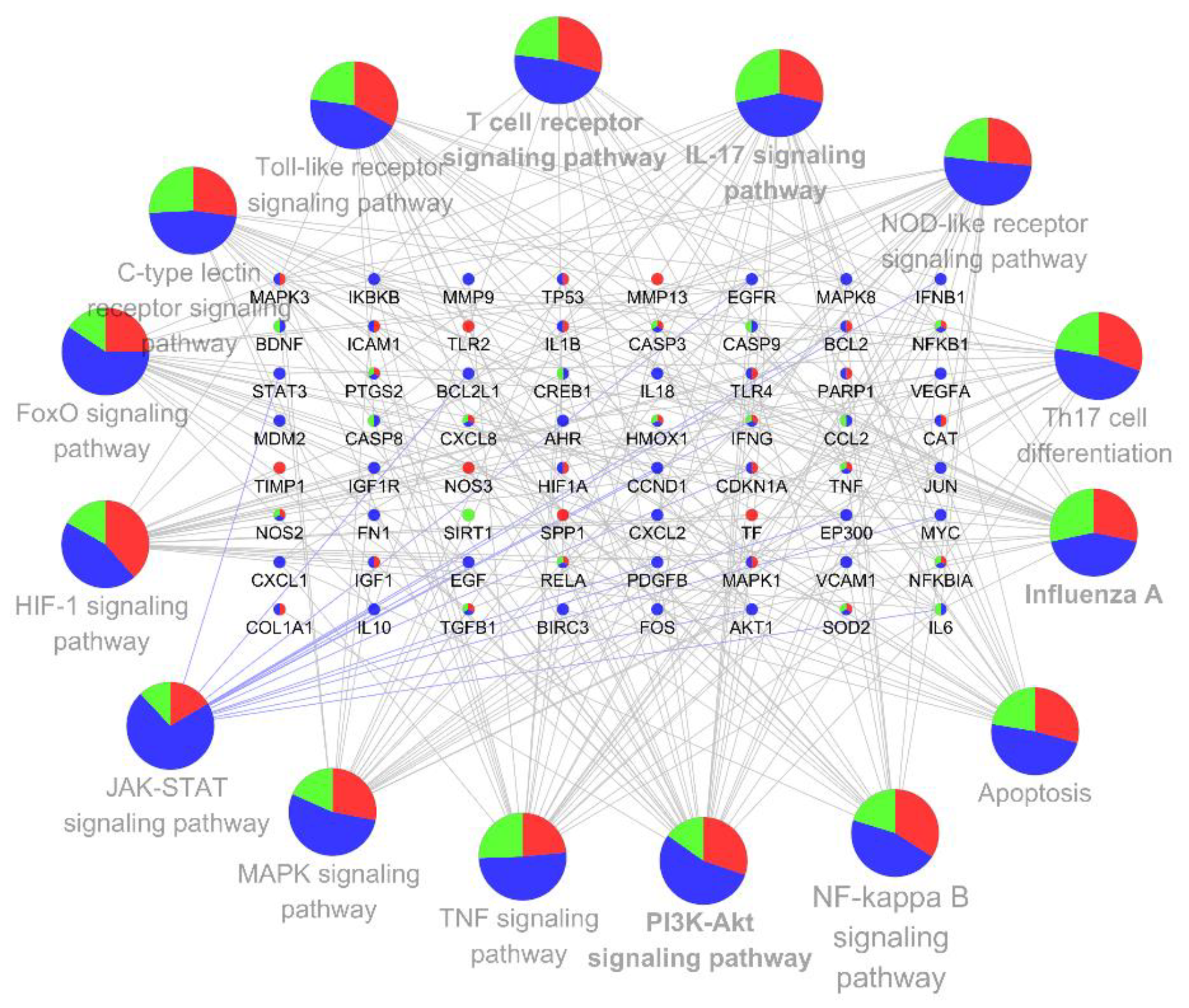
3.4. KEGG Pathway Enrichment and Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fehr, A.R.; Perlman, S. Coronaviruses: An Overview of Their Replication and Pathogenesis. In Coronaviruses; Maier, H.J., Bickerton, E., Britton, P., Eds.; Springer New York: New York, NY, USA, 2015; Volume 1282, pp. 1–23. ISBN 978-1-4939-2437-0. [Google Scholar]
- Xu, B.; Kraemer, M.U.G.; Xu, B.; Gutierrez, B.; Mekaru, S.; Sewalk, K.; Loskill, A.; Wang, L.; Cohn, E.; Hill, S.; et al. Open access epidemiological data from the COVID-19 outbreak. Lancet Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Channappanavar, R.; Perlman, S. Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017, 39, 529–539. [Google Scholar] [CrossRef]
- Li, G.; Fan, Y.; Lai, Y.; Han, T.; Li, Z.; Zhou, P.; Pan, P.; Wang, W.; Hu, D.; Liu, X.; et al. Coronavirus infections and immune responses. J. Med. Virol. 2020, 92, 424–432. [Google Scholar] [CrossRef]
- Zheng, J.; Perlman, S. Immune responses in influenza A virus and human coronavirus infections: An ongoing battle between the virus and host. Curr. Opin. Virol. 2018, 28, 43–52. [Google Scholar] [CrossRef]
- Lai, C.-C.; Shih, T.-P.; Ko, W.-C.; Tang, H.-J.; Hsueh, P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and corona virus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 2020, 105924. [Google Scholar] [CrossRef]
- Zumla, A.; Chan, J.F.W.; Azhar, E.I.; Hui, D.S.C.; Yuen, K.-Y. Coronaviruses—Drug discovery and therapeutic options. Nat. Rev. Drug Discov. 2016, 15, 327–347. [Google Scholar] [CrossRef]
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517S–520S. [Google Scholar] [CrossRef]
- Chang, S.K.; Alasalvar, C.; Shahidi, F. Review of dried fruits: Phytochemicals, antioxidant efficacies, and health benefits. J. Funct. Foods 2016, 21, 113–132. [Google Scholar] [CrossRef]
- Oh, J.; Wall, E.H.; Bravo, D.M.; Hristov, A.N. Host-mediated effects of phytonutrients in ruminants: A review. J. Dairy Sci. 2017, 100, 5974–5983. [Google Scholar] [CrossRef]
- Gupta, C.; Prakash, D. Phytonutrients as therapeutic agents. J. Complement. Integr. Med. 2014, 11, 151–169. [Google Scholar] [CrossRef]
- Wu, D.; Lewis, E.D.; Pae, M.; Meydani, S.N. Nutritional Modulation of Immune Function: Analysis of Evidence, Mechanisms, and Clinical Relevance. Front. Immunol. 2019, 9. [Google Scholar] [CrossRef]
- Wintergerst, E.S.; Maggini, S.; Hornig, D.H. Immune-enhancing role of vitamin C and zinc and effect on clinical conditions. Ann. Nutr. Metab. 2006, 50, 85–94. [Google Scholar] [CrossRef]
- Ang, A.; Pullar, J.M.; Currie, M.J.; Vissers, M.C.M. Vitamin C and immune cell function in inflammation and cancer. Biochem. Soc. Trans. 2018, 46, 1147–1159. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y. Potential interventions for novel coronavirus in China: A systematic review. J. Med. Virol. 2020, 92, 479–490. [Google Scholar] [CrossRef]
- Hu, C. Chapter 21—Historical Change of Raw Materials and Claims of Health Food Regulations in China. In Nutraceutical and Functional Food Regulations in the United States and Around the World, 2nd ed.; Bagchi, D., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 363–388. ISBN 978-0-12-405870-5. [Google Scholar]
- Pompei, R.; Laconi, S.; Ingianni, A. Antiviral properties of glycyrrhizic acid and its semisynthetic derivatives. Mini. Rev. Med. Chem. 2009, 9, 996–1001. [Google Scholar] [CrossRef]
- Ming, L.J.; Yin, A.C.Y. Therapeutic effects of glycyrrhizic acid. Nat. Prod. Commun. 2013, 8, 415–418. [Google Scholar] [CrossRef]
- Li, J.; Cao, H.; Liu, P.; Cheng, G.; Sun, M. Glycyrrhizic acid in the treatment of liver diseases: Literature review. Biomed. Res. Int. 2014, 2014, 872139. [Google Scholar] [CrossRef]
- Luo, H.; Tang, Q.; Shang, Y.; Liang, S.; Yang, M.; Robinson, N.; Liu, J. Can Chinese Medicine Be Used for Prevention of Corona Virus Disease 2019 (COVID-19)? A Review of Historical Classics, Research Evidence and Current Prevention Programs. Chin. J. Integr. Med. 2020, 26, 243–250. [Google Scholar] [CrossRef]
- Chen, H.; Du, Q. Potential natural compounds for preventing 2019-nCoV infection. Preprints 2020, 2020010358. [Google Scholar] [CrossRef]
- Deguchi, A. Curcumin targets in inflammation and cancer. Endocr. Metab. Immune Disord. Drug Targets 2015, 15, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Ramirez-Tortosa, M. Curcumin and Health. Molecules 2016, 21, 264. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Clifton, P. Curcumin, Cardiometabolic Health and Dementia. Int. J. Environ. Res. Public Health 2018, 15, 2093. [Google Scholar] [CrossRef] [PubMed]
- Lelli, D.; Sahebkar, A.; Johnston, T.P.; Pedone, C. Curcumin use in pulmonary diseases: State of the art and future perspectives. Pharmacol. Res. 2017, 115, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Bright, J.J. Curcumin and autoimmune disease. Adv. Exp. Med. Biol. 2007, 595, 425–451. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, P.P.; Helson, L. Curcumin Suppression of Cytokine Release and Cytokine Storm. A Potential Therapy for Patients with Ebola and Other Severe Viral Infections. In Vivo 2015, 29, 1–4. [Google Scholar]
- Bauvois, B.; Dauzonne, D. Aminopeptidase-N/CD13 (EC 3.4.11.2) inhibitors: Chemistry, biological evaluations, and therapeutic prospects. Med. Res. Rev. 2006, 26, 88–130. [Google Scholar] [CrossRef]
- Yadav, B.S.; Tripathi, V. Recent Advances in the System Biology-based Target Identification and Drug Discovery. CTMC 2018, 18, 1737–1744. [Google Scholar] [CrossRef]
- Zhang, W.; Huai, Y.; Miao, Z.; Qian, A.; Wang, Y. Systems Pharmacology for Investigation of the Mechanisms of Action of Traditional Chinese Medicine in Drug Discovery. Front. Pharmacol. 2019, 10, 743. [Google Scholar] [CrossRef]
- Chen, L.; Hu, C.; Hood, M.; Kan, J.; Gan, X.; Zhang, X.; Zhang, Y.; Du, J. An Integrated Approach Exploring the Synergistic Mechanism of Herbal Pairs in a Botanical Dietary Supplement: A Case Study of a Liver Protection Health Food. Int. J. Genom. 2020, 2020, 1–14. [Google Scholar] [CrossRef]
- Yue, S.-J.; Liu, J.; Feng, W.-W.; Zhang, F.-L.; Chen, J.-X.; Xin, L.-T.; Peng, C.; Guan, H.-S.; Wang, C.-Y.; Yan, D. System Pharmacology-Based Dissection of the Synergistic Mechanism of Huangqi and Huanglian for Diabetes Mellitus. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.P.; Wiegers, T.C.; Johnson, R.J.; Lay, J.M.; Lennon-Hopkins, K.; Saraceni-Richards, C.; Sciaky, D.; Murphy, C.G.; Mattingly, C.J. Text Mining Effectively Scores and Ranks the Literature for Improving Chemical-Gene-Disease Curation at the Comparative Toxicogenomics Database. PLoS ONE 2013, 8, e58201. [Google Scholar] [CrossRef]
- Davis, A.P.; Grondin, C.J.; Johnson, R.J.; Sciaky, D.; McMorran, R.; Wiegers, J.; Wiegers, T.C.; Mattingly, C.J. The Comparative Toxicogenomics Database: Update 2019. Nucleic Acids Res. 2019, 47, D948–D954. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-Y.; Zhang, Y.-Q.; Liu, Z.-M.; Chen, T.; Lv, C.-Y.; Tang, S.-H.; Zhang, X.-B.; Zhang, W.; Li, Z.-Y.; Zhou, R.-R.; et al. ETCM: An encyclopaedia of traditional Chinese medicine. Nucleic Acids Res. 2019, 47, D976–D982. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H. The Universal Protein Resource (UniProt): An expanding universe of protein information. Nucleic Acids Res. 2006, 34, D187–D191. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Su, G.; Morris, J.H.; Demchak, B.; Bader, G.D. Biological Network Exploration with Cytoscape 3. Curr. Protoc. Bioinform. 2014, 47, 8.13.1–8.13.24. [Google Scholar] [CrossRef]
- Gokhman, D.; Kelman, G.; Amartely, A.; Gershon, G.; Tsur, S.; Carmel, L. Gene ORGANizer: Linking genes to the organs they affect. Nucleic Acids Res. 2017, 45, W138–W145. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.-H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Wunderink, R.G. MERS, SARS and other coronaviruses as causes of pneumonia: MERS, SARS and coronaviruses. Respirology 2018, 23, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Kui, L.; Fang, Y.-Y.; Deng, Y.; Liu, W.; Wang, M.-F.; Ma, J.-P.; Xiao, W.; Wang, Y.-N.; Zhong, M.-H.; Li, C.-H.; et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin. Med. J. (Engl.) 2020. [Google Scholar] [CrossRef]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal Coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020. [Google Scholar] [CrossRef]
- Nakayama, K.; Kataoka, N. Regulation of Gene Expression under Hypoxic Conditions. Int. J. Mol. Sci. 2019, 20, 3278. [Google Scholar] [CrossRef]
- Cong, Y.; Ren, X. Coronavirus entry and release in polarized epithelial cells: A review: Polarized infection of coronaviruses. Rev. Med. Virol. 2014, 24, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Yeung, K.-S.; Yamanaka, G.A.; Meanwell, N.A. Severe acute respiratory syndrome coronavirus entry into host cells: Opportunities for therapeutic intervention. Med. Res. Rev. 2006, 26, 414–433. [Google Scholar] [CrossRef]
- Li, W.-Y.; Ren, J.-H.; Tao, N.-N.; Ran, L.-K.; Chen, X.; Zhou, H.-Z.; Liu, B.; Li, X.-S.; Huang, A.-L.; Chen, J. The SIRT1 inhibitor, nicotinamide, inhibits hepatitis B virus replication in vitro and in vivo. Arch. Virol. 2016, 161, 621–630. [Google Scholar] [CrossRef]
- Deng, J.-J.; Kong, K.-Y.E.; Gao, W.-W.; Tang, H.-M.V.; Chaudhary, V.; Cheng, Y.; Zhou, J.; Chan, C.-P.; Wong, D.K.-H.; Yuen, M.-F.; et al. Interplay between SIRT1 and hepatitis B virus X protein in the activation of viral transcription. Biochim. Biophys. Acta (BBA)—Gene Regul. Mech. 2017, 1860, 491–501. [Google Scholar] [CrossRef]
- Tang, H.-M.V.; Gao, W.-W.; Chan, C.-P.; Cheng, Y.; Deng, J.-J.; Yuen, K.-S.; Iha, H.; Jin, D.-Y. SIRT1 Suppresses Human T-Cell Leukemia Virus Type 1 Transcription. J. Virol. 2015, 89, 8623–8631. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-C.; Ho, C.-T.; Chuo, W.-H.; Li, S.; Wang, T.T.; Lin, C.-C. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect. Dis. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, L.; Cui, J.; Song, Y.; Luo, Z.; Chen, J.; Xiong, Y.; Zhang, Q.; Liu, F.; Ho, W.; et al. SIRT1 inhibits EV71 genome replication and RNA translation by interfering with the viral polymerase and 5′UTR RNA. J. Cell Sci. 2016, 129, 4534–4547. [Google Scholar] [CrossRef] [PubMed]
- Saito, K. Studies on glycyrrhizin, an active principle of radix liquiritine. (3.) on the mechanism of detoxicating action. Gunma J. Med. Sci. 1964, 13, 275–282. [Google Scholar]
- Pu, J.-Y.; He, L.; Wu, S.-Y.; Zhang, P.; Huang, X. Anti-virus research of triterpenoids in licorice. Bing Du Xue Bao 2013, 29, 673–679. [Google Scholar]
- Wang, J.; Chen, X.; Wang, W.; Zhang, Y.; Yang, Z.; Jin, Y.; Ge, H.M.; Li, E.; Yang, G. Glycyrrhizic acid as the antiviral component of Glycyrrhiza uralensis Fisch. against coxsackievirus A16 and enterovirus 71 of hand foot and mouth disease. J. Ethnopharmacol. 2013, 147, 114–121. [Google Scholar] [CrossRef]
- Feng Yeh, C.; Wang, K.C.; Chiang, L.C.; Shieh, D.E.; Yen, M.H.; San Chang, J. Water extract of licorice had anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines. J. Ethnopharmacol. 2013, 148, 466–473. [Google Scholar] [CrossRef]
- Hou, S.; Zhang, T.; Li, Y.; Guo, F.; Jin, X. Glycyrrhizic Acid Prevents Diabetic Nephropathy by Activating AMPK/SIRT1/PGC-1 α Signaling in db/db Mice. J. Diabetes Res. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Hou, S.; Zheng, F.; Li, Y.; Gao, L.; Zhang, J. The Protective Effect of Glycyrrhizic Acid on Renal Tubular Epithelial Cell Injury Induced by High Glucose. Int. J. Mol. Sci. 2014, 15, 15026–15043. [Google Scholar] [CrossRef]
- Nelemans, T.; Kikkert, M. Viral Innate Immune Evasion and the Pathogenesis of Emerging RNA Virus Infections. Viruses 2019, 11, 961. [Google Scholar] [CrossRef]
- Channappanavar, R.; Fehr, A.R.; Vijay, R.; Mack, M.; Zhao, J.; Meyerholz, D.K.; Perlman, S. Dysregulated Type I Interferon and Inflammatory Monocyte-Macrophage Responses Cause Lethal Pneumonia in SARS-CoV-Infected Mice. Cell Host Microbe 2016, 19, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Shokri, S.; Mahmoudvand, S.; Taherkhani, R.; Farshadpour, F. Modulation of the immune response by Middle East respiratory syndrome coronavirus. J. Cell. Physiol. 2019, 234, 2143–2151. [Google Scholar] [CrossRef] [PubMed]
- Peiris, M. Pathogenesis of avian flu H5N1 and SARS. Novartis Found. Symp. 2006, 279, 56–219. [Google Scholar]
- Dai, J.; Gu, L.; Su, Y.; Wang, Q.; Zhao, Y.; Chen, X.; Deng, H.; Li, W.; Wang, G.; Li, K. Inhibition of curcumin on influenza A virus infection and influenzal pneumonia via oxidative stress, TLR2/4, p38/JNK MAPK and NF-κB pathways. Int. Immunopharmacol. 2018, 54, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, H.; Bae, S.; Choi, J.; Lim, S.Y.; Lee, N.; Kong, J.M.; Hwang, Y.-I.; Kang, J.S.; Lee, W.J. Vitamin C Is an Essential Factor on the Anti-viral Immune Responses through the Production of Interferon-α/β at the Initial Stage of Influenza A Virus (H3N2) Infection. Immune Netw. 2013, 13, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jang, M.; Kim, Y.; Choi, J.; Jeon, J.; Kim, J.; Hwang, Y.-I.; Kang, J.S.; Lee, W.J. Red ginseng and vitamin C increase immune cell activity and decrease lung inflammation induced by influenza A virus/H1N1 infection. J. Pharm. Pharmacol. 2016, 68, 406–420. [Google Scholar] [CrossRef]
- Ram, A.; Mabalirajan, U.; Das, M.; Bhattacharya, I.; Dinda, A.K.; Gangal, S.V.; Ghosh, B. Glycyrrhizin alleviates experimental allergic asthma in mice. Int. Immunopharmacol. 2006, 6, 1468–1477. [Google Scholar] [CrossRef]
- Wang, Y.; Chai, J.; Sun, M.; He, W.; Hu, X.; Zou, W.; Li, H.; Lu, Y.; Xie, C. Glycyrrhizinic acid modulates the immunity of MRL/lpr mice and related mechanism. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2017, 33, 305–309. [Google Scholar]
- Ma, C.; Ma, Z.; Liao, X.; Liu, J.; Fu, Q.; Ma, S. Immunoregulatory effects of glycyrrhizic acid exerts anti-asthmatic effects via modulation of Th1/Th2 cytokines and enhancement of CD4(+)CD25(+)Foxp3+ regulatory T cells in ovalbumin-sensitized mice. J. Ethnopharmacol. 2013, 148, 755–762. [Google Scholar] [CrossRef]
- Cecere, T.E.; Todd, S.M.; Leroith, T. Regulatory T cells in arterivirus and coronavirus infections: Do they protect against disease or enhance it? Viruses 2012, 4, 833–846. [Google Scholar] [CrossRef]
- Chu, H.; Zhou, J.; Wong, B.H.-Y.; Li, C.; Chan, J.F.-W.; Cheng, Z.-S.; Yang, D.; Wang, D.; Lee, A.C.-Y.; Li, C.; et al. Middle East Respiratory Syndrome Coronavirus Efficiently Infects Human Primary T Lymphocytes and Activates the Extrinsic and Intrinsic Apoptosis Pathways. J. Infect. Dis. 2016, 213, 904–914. [Google Scholar] [CrossRef]
- Chen, J.; Lau, Y.F.; Lamirande, E.W.; Paddock, C.D.; Bartlett, J.H.; Zaki, S.R.; Subbarao, K. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection. J. Virol. 2010, 84, 1289–1301. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Huang, Z.; Ying, G.; Zhang, X.; Ye, W.; Hu, Z.; Hu, C.; Wei, H.; Zeng, Y.; Chi, Y.; et al. Study of the lymphocyte change between COVID-19 and non-COVID-19 pneumonia cases suggesting other factors besides uncontrolled inflammation contributed to multi-organ injury. Preprints 2020. [Google Scholar] [CrossRef]
- National Research Project For SARS Beijing Group Beijing 100020 China. Dynamic changes of T-lymphocytes and immunoglobulins in patients with severe acute respiratory syndrome. Zhonghua Yi Xue Za Zhi 2003, 83, 1014–1017. [Google Scholar]
- Cui, W.; Fan, Y.; Wu, W.; Zhang, F.; Wang, J.; Ni, A. Expression of lymphocytes and lymphocyte subsets in patients with severe acute respiratory syndrome. Clin. Infect. Dis. 2003, 37, 857–859. [Google Scholar] [CrossRef] [PubMed]
- Van Gorkom, G.N.Y.; Klein Wolterink, R.G.J.; Van Elssen, C.H.M.J.; Wieten, L.; Germeraad, W.T.V.; Bos, G.M.J. Influence of Vitamin C on Lymphocytes: An Overview. Antioxidants (Basel) 2018, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Shafabakhsh, R.; Pourhanifeh, M.H.; Mirzaei, H.R.; Sahebkar, A.; Asemi, Z.; Mirzaei, H. Targeting regulatory T cells by curcumin: A potential for cancer immunotherapy. Pharmacol. Res. 2019, 147, 104353. [Google Scholar] [CrossRef]
- Zou, J.Y.; Su, C.H.; Luo, H.H.; Lei, Y.Y.; Zeng, B.; Zhu, H.S.; Chen, Z.G. Curcumin converts Foxp3+ regulatory T cells to T helper 1 cells in patients with lung cancer. J. Cell. Biochem. 2018, 119, 1420–1428. [Google Scholar] [CrossRef]
- Rahimi, K.; Ahmadi, A.; Hassanzadeh, K.; Soleimani, Z.; Sathyapalan, T.; Mohammadi, A.; Sahebkar, A. Targeting the balance of T helper cell responses by curcumin in inflammatory and autoimmune states. Autoimmun. Rev. 2019, 18, 738–748. [Google Scholar] [CrossRef]
- Han, S.; Sun, L.; He, F.; Che, H. Anti-allergic activity of glycyrrhizic acid on IgE-mediated allergic reaction by regulation of allergy-related immune cells. Sci. Rep. 2017, 7, 7222. [Google Scholar] [CrossRef]
- Wu, Q.; Tang, Y.; Hu, X.; Wang, Q.; Lei, W.; Zhou, L.; Huang, J. Regulation of Th1/Th2 balance through OX40/OX40L signalling by glycyrrhizic acid in a murine model of asthma. Respirology 2016, 21, 102–111. [Google Scholar] [CrossRef]
- Lau, S.K.P.; Lau, C.C.Y.; Chan, K.-H.; Li, C.P.Y.; Chen, H.; Jin, D.-Y.; Chan, J.F.W.; Woo, P.C.Y.; Yuen, K.-Y. Delayed induction of proinflammatory cytokines and suppression of innate antiviral response by the novel Middle East respiratory syndrome coronavirus: Implications for pathogenesis and treatment. J. Gen. Virol. 2013, 94, 2679–2690. [Google Scholar] [CrossRef] [PubMed]
- Kindrachuk, J.; Ork, B.; Hart, B.J.; Mazur, S.; Holbrook, M.R.; Frieman, M.B.; Traynor, D.; Johnson, R.F.; Dyall, J.; Kuhn, J.H.; et al. Antiviral Potential of ERK/MAPK and PI3K/AKT/mTOR Signaling Modulation for Middle East Respiratory Syndrome Coronavirus Infection as Identified by Temporal Kinome Analysis. Antimicrob. Agents Chemother. 2015, 59, 1088–1099. [Google Scholar] [CrossRef] [PubMed]
- DeDiego, M.L.; Nieto-Torres, J.L.; Regla-Nava, J.A.; Jimenez-Guardeño, J.M.; Fernandez-Delgado, R.; Fett, C.; Castaño-Rodriguez, C.; Perlman, S.; Enjuanes, L. Inhibition of NF-κB-mediated inflammation in severe acute respiratory syndrome coronavirus-infected mice increases survival. J. Virol. 2014, 88, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Dosch, S.F.; Mahajan, S.D.; Collins, A.R. SARS coronavirus spike protein-induced innate immune response occurs via activation of the NF-κB pathway in human monocyte macrophages in vitro. Virus Res. 2009, 142, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Peteranderl, C.; Herold, S. The Impact of the Interferon/TNF-Related Apoptosis-Inducing Ligand Signaling Axis on Disease Progression in Respiratory Viral Infection and Beyond. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef]
- Mizutani, T. Signal Transduction in SARS-CoV-Infected Cells. Ann. N. Y. Acad. Sci. 2007, 1102, 86–95. [Google Scholar] [CrossRef]
- Liang, T.; Chen, X.; Su, M.; Chen, H.; Lu, G.; Liang, K. Vitamin C exerts beneficial hepatoprotection against Concanavalin A-induced immunological hepatic injury in mice through inhibition of NF-κB signal pathway. Food Funct. 2014, 5, 2175–2182. [Google Scholar] [CrossRef]
- Zhu, H.; Bian, C.; Yuan, J.; Chu, W.; Xiang, X.; Chen, F.; Wang, C.; Feng, H.; Lin, J.-K. Curcumin attenuates acute inflammatory injury by inhibiting the TLR4/MyD88/NF-κB signaling pathway in experimental traumatic brain injury. J. Neuroinflammation 2014, 11, 59. [Google Scholar] [CrossRef]
- Kong, F.; Ye, B.; Cao, J.; Cai, X.; Lin, L.; Huang, S.; Huang, W.; Huang, Z. Curcumin Represses NLRP3 Inflammasome Activation via TLR4/MyD88/NF-κB and P2X7R Signaling in PMA-Induced Macrophages. Front. Pharmacol. 2016, 7, 369. [Google Scholar] [CrossRef] [PubMed]
- Vucic, M.; Cojbasic, I.; Vucic, J.; Pavlovic, V. The effect of curcumin and PI3K/Akt inhibitor on monosodium glutamate-induced rat thymocytes toxicity. Gen. Physiol. Biophys. 2018, 37, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Liu, M.; Hong, G.; Li, Y.; Xue, P.; Zheng, M.; Wu, M.; Shen, L.; Yang, M.; Diao, Z.; et al. Curcumin improves LPS-induced preeclampsia-like phenotype in rat by inhibiting the TLR4 signaling pathway. Placenta 2016, 41, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Sun, T. Glycyrrhizin administration ameliorates Streptococcus aureus-induced acute lung injury. Int. Immunopharmacol. 2019, 70, 504–511. [Google Scholar] [CrossRef] [PubMed]
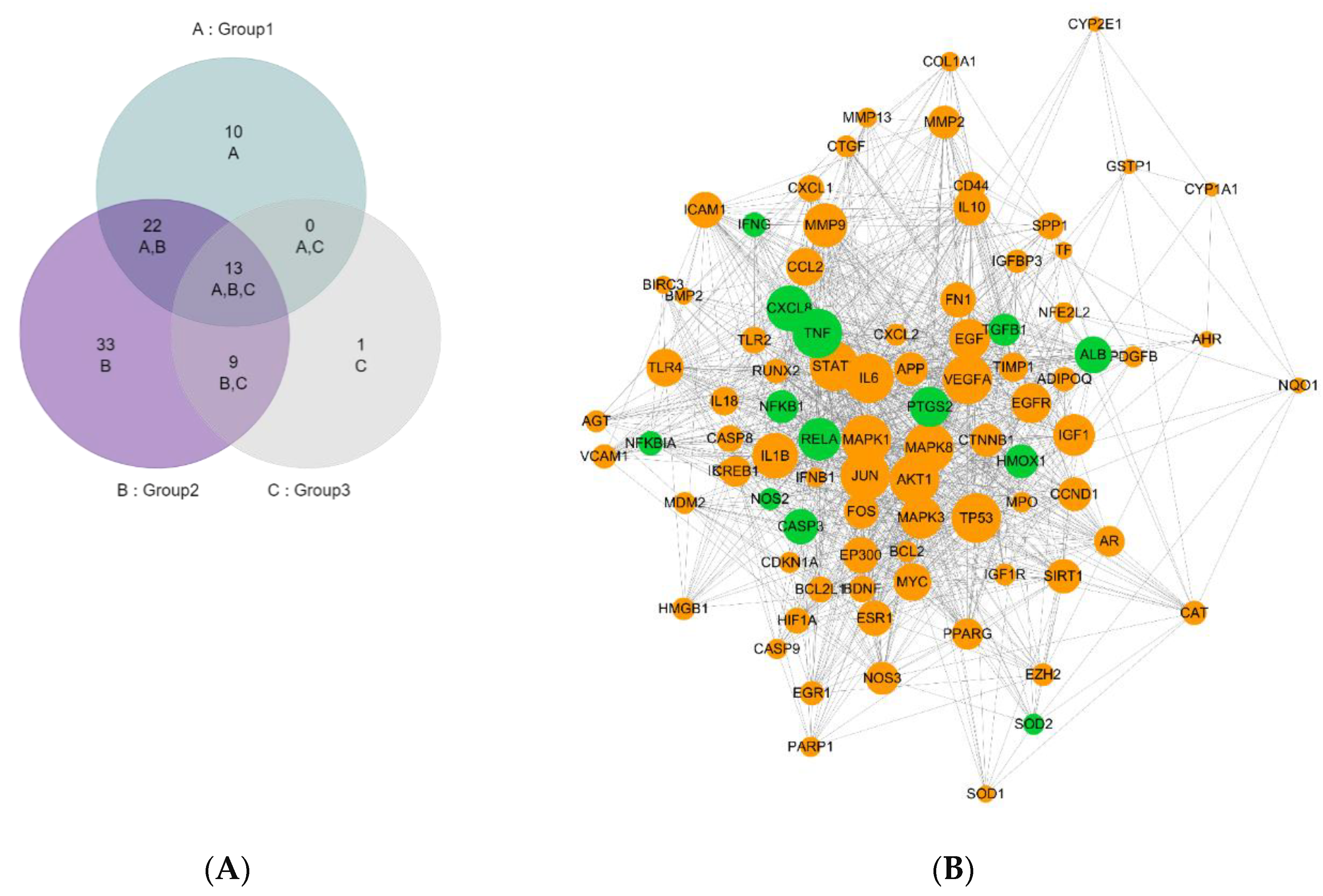

| GENE_SYMBOL | Name | Distribution |
|---|---|---|
| EP300 | E1A binding protein p300 | CC only |
| VCAM1 | vascular cell adhesion molecule 1 | CC only |
| CCN2 | cellular communication network factor 2 | CC only |
| MYC | MYC proto-oncogene, bHLH transcription factor | CC only |
| VEGFA | vascular endothelial growth factor A | CC only |
| ADIPOQ | adiponectin, C1Q and collagen domain containing | CC only |
| IKBKB | inhibitor of nuclear factor kappa B kinase subunit beta | CC only |
| FN1 | fibronectin 1 | CC only |
| ESR1 | estrogen receptor 1 | CC only |
| MAPK8 | mitogen-activated protein kinase 8 | CC only |
| GSTP1 | glutathione S-transferase pi 1 | CC only |
| FOS | Fos proto-oncogene, AP-1 transcription factor subunit | CC only |
| AKT1 | AKT serine/threonine kinase 1 | CC only |
| IFNB1 | interferon beta 1 | CC only |
| MDM2 | MDM2 proto-oncogene | CC only |
| CXCL1 | C-X-C motif chemokine ligand 1 | CC only |
| CXCL2 | C-X-C motif chemokine ligand 2 | CC only |
| PDGFB | platelet derived growth factor subunit B | CC only |
| AHR | aryl hydrocarbon receptor | CC only |
| CYP2E1 | cytochrome P450 family 2 subfamily E member 1 | CC only |
| EGFR | epidermal growth factor receptor | CC only |
| EGR1 | early growth response 1 | CC only |
| IGF1R | insulin like growth factor 1 receptor | CC only |
| BIRC3 | baculoviral IAP repeat containing 3 | CC only |
| IGFBP3 | insulin like growth factor binding protein 3 | CC only |
| STAT3 | signal transducer and activator of transcription 3 | CC only |
| EGF | epidermal growth factor | CC only |
| IL18 | interleukin 18 | CC only |
| CCND1 | cyclin D1 | CC only |
| MMP9 | matrix metallopeptidase 9 | CC only |
| BCL2L1 | BCL2 like 1 | CC only |
| JUN | Jun proto-oncogene, AP-1 transcription factor subunit | CC only |
| IL10 | interleukin 10 | CC only |
| HMGB1 | high mobility group box 1 | CC_GA_intersect |
| IL6 | interleukin 6 | CC_GA_intersect |
| CREB1 | cAMP responsive element binding protein 1 | CC_GA_intersect |
| IFNG | interferon gamma | CC_GA_intersect |
| BDNF | brain derived neurotrophic factor | CC_GA_intersect |
| MMP2 | matrix metallopeptidase 2 | CC_GA_intersect |
| CCL2 | C-C motif chemokine ligand 2 | CC_GA_intersect |
| CASP9 | caspase 9 | CC_GA_intersect |
| AR | androgen receptor | CC_GA_intersect |
| CASP8 | caspase 8 | CC_GA_intersect |
| SIRT1 | silent mating type information regulation 2 homolog 1 | GA only |
| BMP2 | bone morphogenetic protein 2 | VC only |
| TIMP1 | TIMP metallopeptidase inhibitor 1 | VC only |
| TLR2 | toll like receptor 2 | VC only |
| SPP1 | secreted phosphoprotein 1 | VC only |
| MMP13 | matrix metallopeptidase 13 | VC only |
| NOS3 | nitric oxide synthase 3 | VC only |
| TF | transferrin | VC only |
| RUNX2 | RUNX family transcription factor 2 | VC only |
| EZH2 | enhancer of zeste 2 polycomb repressive complex 2 subunit | VC only |
| CD44 | CD44 molecule | VC only |
| HMOX1 | heme oxygenase 1 | VC_CC_GA_intersect |
| RELA | RELA proto-oncogene, NF-κB subunit | VC_CC_GA_intersect |
| TGFB1 | transforming growth factor beta 1 | VC_CC_GA_intersect |
| PTGS2 | prostaglandin-endoperoxide synthase 2 | VC_CC_GA_intersect |
| NFKBIA | NF-κB inhibitor alpha | VC_CC_GA_intersect |
| NFKB1 | nuclear factor kappa B subunit 1 | VC_CC_GA_intersect |
| CXCL8 | C-X-C motif chemokine ligand 8 | VC_CC_GA_intersect |
| SOD2 | superoxide dismutase 2, mitochondrial | VC_CC_GA_intersect |
| ALB | albumin | VC_CC_GA_intersect |
| TNF | tumor necrosis factor | VC_CC_GA_intersect |
| NOS2 | nitric oxide synthase 2 | VC_CC_GA_intersect |
| CASP3 | caspase 3 | VC_CC_GA_intersect |
| PARP1 | poly (ADP-ribose) polymerase 1 | VC_CC_intersect |
| CTNNB1 | catenin beta 1 | VC_CC_intersect |
| NQO1 | NAD(P)H quinone dehydrogenase 1 | VC_CC_intersect |
| NFE2L2 | nuclear factor, erythroid 2 like 2 | VC_CC_intersect |
| PPARG | peroxisome proliferator activated receptor gamma | VC_CC_intersect |
| IL1B | interleukin 1 beta | VC_CC_intersect |
| MAPK3 | mitogen-activated protein kinase 3 | VC_CC_intersect |
| MAPK1 | mitogen-activated protein kinase 1 | VC_CC_intersect |
| MPO | myeloperoxidase | VC_CC_intersect |
| TLR4 | toll like receptor 4 | VC_CC_intersect |
| COL1A1 | collagen type I alpha 1 chain | VC_CC_intersect |
| AGT | angiotensinogen | VC_CC_intersect |
| APP | amyloid beta precursor protein | VC_CC_intersect |
| HIF1A | hypoxia inducible factor 1 alpha subunit | VC_CC_intersect |
| CDKN1A | cyclin dependent kinase inhibitor 1A | VC_CC_intersect |
| IGF1 | insulin like growth factor 1 | VC_CC_intersect |
| SOD1 | superoxide dismutase 1 | VC_CC_intersect |
| CYP1A1 | cytochrome P450 family 1 subfamily A member 1 | VC_CC_intersect |
| BCL2 | BCL2, apoptosis regulator | VC_CC_intersect |
| TP53 | tumor protein p53 | VC_CC_intersect |
| CAT | catalase | VC_CC_intersect |
| ICAM1 | intercellular adhesion molecule 1 | VC_CC_intersect |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Hu, C.; Hood, M.; Zhang, X.; Zhang, L.; Kan, J.; Du, J. A Novel Combination of Vitamin C, Curcumin and Glycyrrhizic Acid Potentially Regulates Immune and Inflammatory Response Associated with Coronavirus Infections: A Perspective from System Biology Analysis. Nutrients 2020, 12, 1193. https://doi.org/10.3390/nu12041193
Chen L, Hu C, Hood M, Zhang X, Zhang L, Kan J, Du J. A Novel Combination of Vitamin C, Curcumin and Glycyrrhizic Acid Potentially Regulates Immune and Inflammatory Response Associated with Coronavirus Infections: A Perspective from System Biology Analysis. Nutrients. 2020; 12(4):1193. https://doi.org/10.3390/nu12041193
Chicago/Turabian StyleChen, Liang, Chun Hu, Molly Hood, Xue Zhang, Lu Zhang, Juntao Kan, and Jun Du. 2020. "A Novel Combination of Vitamin C, Curcumin and Glycyrrhizic Acid Potentially Regulates Immune and Inflammatory Response Associated with Coronavirus Infections: A Perspective from System Biology Analysis" Nutrients 12, no. 4: 1193. https://doi.org/10.3390/nu12041193
APA StyleChen, L., Hu, C., Hood, M., Zhang, X., Zhang, L., Kan, J., & Du, J. (2020). A Novel Combination of Vitamin C, Curcumin and Glycyrrhizic Acid Potentially Regulates Immune and Inflammatory Response Associated with Coronavirus Infections: A Perspective from System Biology Analysis. Nutrients, 12(4), 1193. https://doi.org/10.3390/nu12041193





