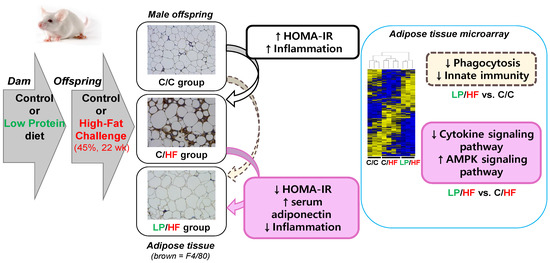
Maternal Protein Restriction Altered Insulin Resistance and Inflammation-Associated Gene Expression in Adipose Tissue of Young Adult Mouse Offspring in Response to a High-Fat Diet
Abstract
1. Introduction.
2. Materials and Methods
2.1. Animals and Diets
2.2. Serum Biochemical Analyses
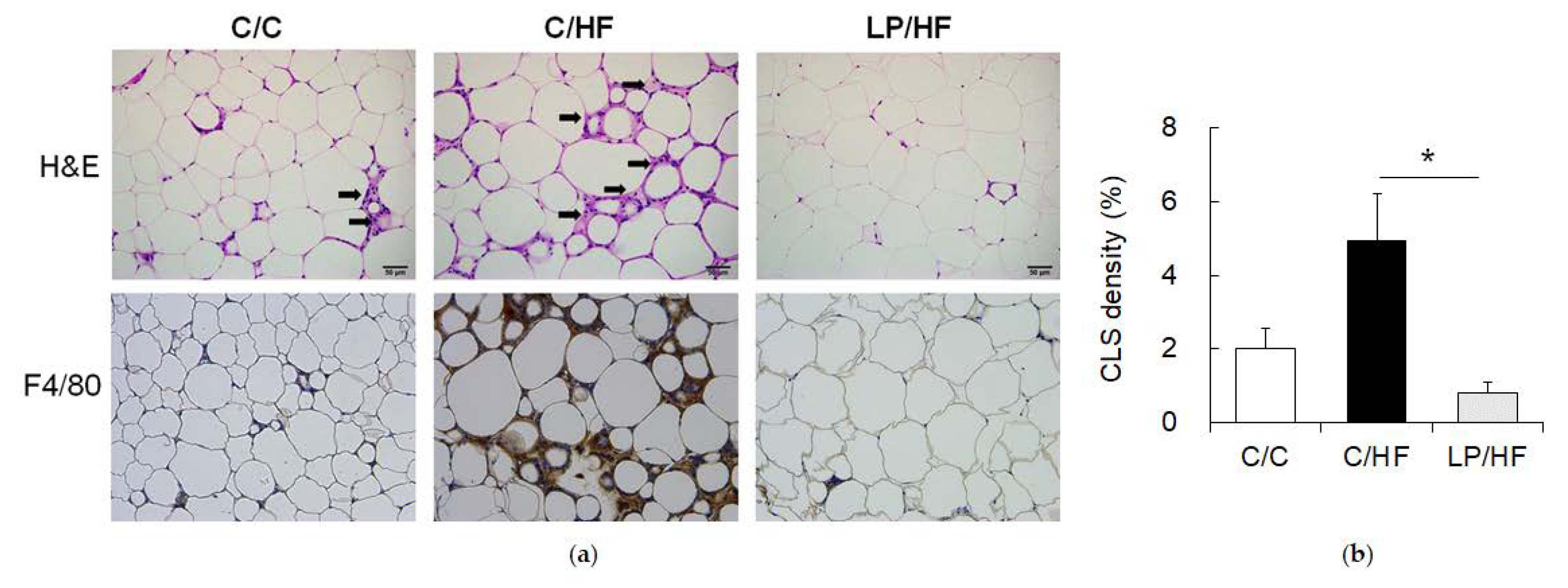
2.3. Epididymal Adipose Tissue Histology Examination
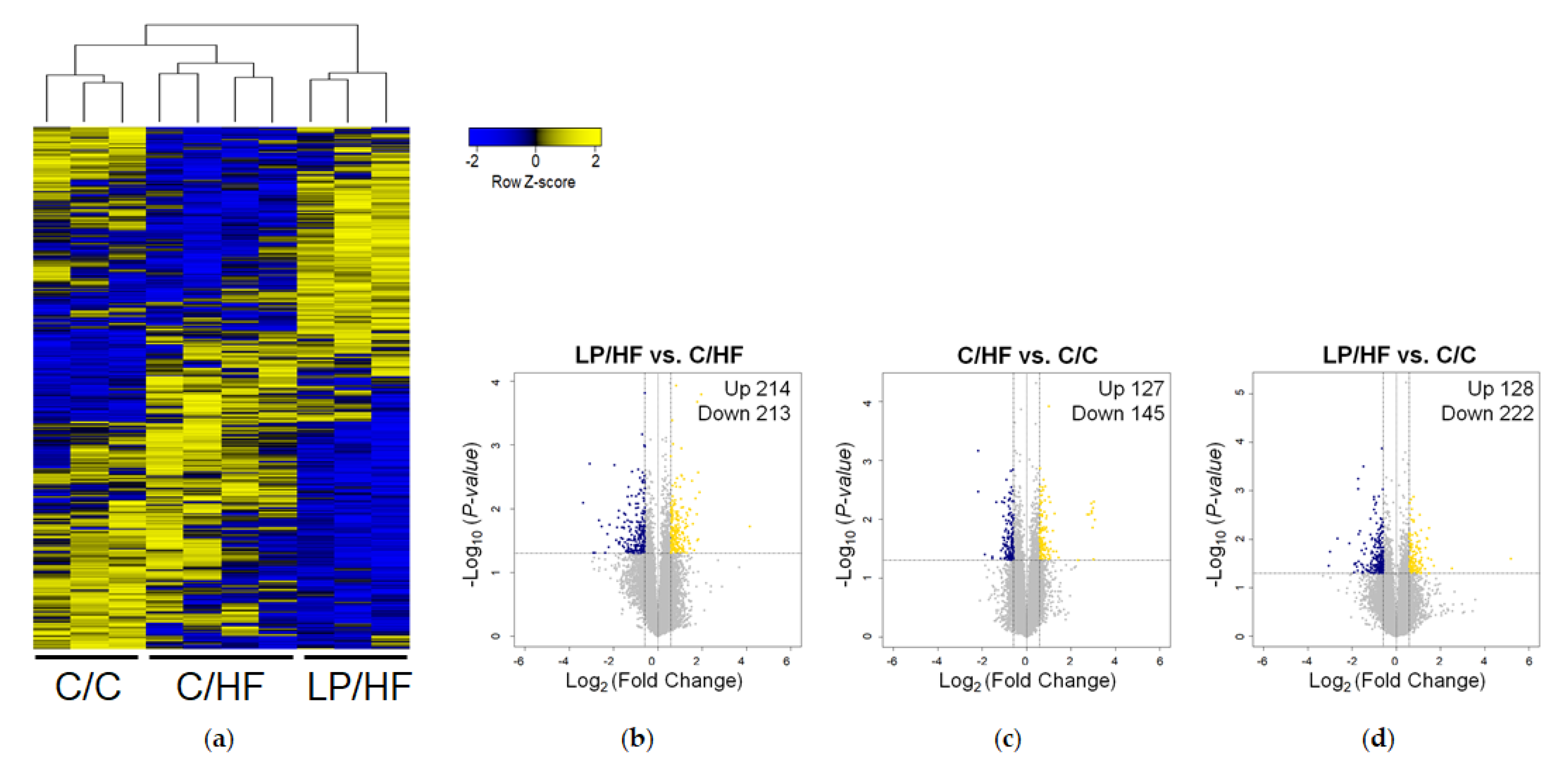
2.4. Microarray Hybridization And Data Analysis
2.5. Statistical Analysis
3. Results
3.1. Maternal Protein Restriction Affects Body Weight and Adiposity of Adult Offspring Fed a High-Fat Diet
3.2. Maternal Protein Restriction Alleviated Systemic Insulin Resistance and Adipose Tissue Inflammation of Adult Offspring Fed a High-Fat Diet
3.3. Maternal Diet Altered Gene Expression of Epididymal Adipose Tissue of Adult Offspring Fed a High-Fat Diet
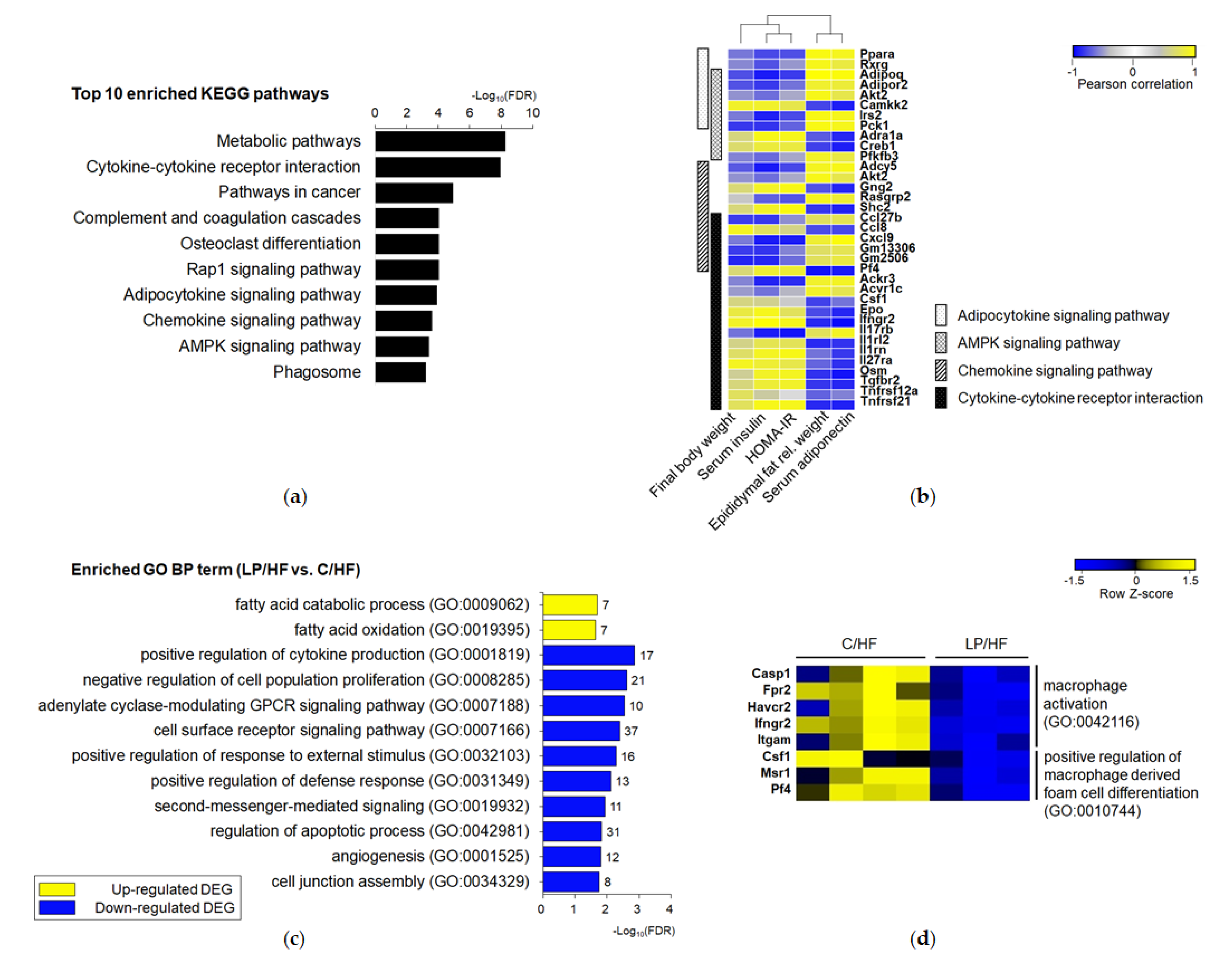
3.4. Maternal Protein Restriction Regulated Gene Expression Involved in Adipokine Signaling and Inflammation of Epididymal Adipose Tissue of Adult Offspring Fed a High-Fat Diet
3.5. Adipose Tissue of Offspring Exposed to Maternal LP and Postweaning HF Diet Showed a Different Gene Profile from Those of Offspring Exposed to a Life-Long Control Diet
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Warner, M.J.; Ozanne, S.E. Mechanisms involved in the developmental programming of adulthood disease. Biochem. J. 2010, 427, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, A.C.; van der Meulen, J.H.; Michels, R.P.; Osmond, C.; Barker, D.J.; Hales, C.N.; Bleker, O.P. Glucose tolerance in adults after prenatal exposure to famine. Lancet 1998, 351, 173–177. [Google Scholar] [CrossRef]
- Hales, C.N.; Desai, M.; Ozanne, S.E. The Thrifty Phenotype hypothesis: How does it look after 5 years? Diabet. Med. 1997, 14, 189–195. [Google Scholar] [CrossRef]
- Jahan-Mihan, A.; Rodriguez, J.; Christie, C.; Sadeghi, M.; Zerbe, T. The Role of Maternal Dietary Proteins in Development of Metabolic Syndrome in Offspring. Nutrients 2015, 7, 9185–9217. [Google Scholar] [CrossRef] [PubMed]
- Ozanne, S.E.; Hales, C.N. The long-term consequences of intra-uterine protein malnutrition for glucose metabolism. Proc. Nutr. Soc. 1999, 58, 615–619. [Google Scholar] [CrossRef]
- Shepherd, P.R.; Crowther, N.J.; Desai, M.; Hales, C.N.; Ozanne, S.E. Altered adipocyte properties in the offspring of protein malnourished rats. Br. J. Nutr. 1997, 78, 121–129. [Google Scholar] [CrossRef][Green Version]
- Ozanne, S.E.; Smith, G.D.; Tikerpae, J.; Hales, C.N. Altered regulation of hepatic glucose output in the male offspring of protein-malnourished rat dams. Am. J. Physiol. 1996, 270, E559–E564. [Google Scholar] [CrossRef]
- Ozanne, S.E.; Wang, C.L.; Coleman, N.; Smith, G.D. Altered muscle insulin sensitivity in the male offspring of protein-malnourished rats. Am. J. Physiol. 1996, 271, E1128–E1134. [Google Scholar] [CrossRef]
- Souza-Mello, V.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. Hepatic structural alteration in adult programmed offspring (severe maternal protein restriction) is aggravated by post-weaning high-fat diet. Br. J. Nutr. 2007, 98, 1159–1169. [Google Scholar] [CrossRef]
- Wilson, M.R.; Hughes, S.J. The effect of maternal protein deficiency during pregnancy and lactation on glucose tolerance and pancreatic islet function in adult rat offspring. J. Endocrinol. 1997, 154, 177–185. [Google Scholar] [CrossRef]
- Xie, L.; Zhang, K.; Rasmussen, D.; Wang, J.; Wu, D.; Roemmich, J.N.; Bundy, A.; Johnson, W.T.; Claycombe, K. Effects of prenatal low protein and postnatal high fat diets on visceral adipose tissue macrophage phenotypes and IL-6 expression in Sprague Dawley rat offspring. PLoS ONE 2017, 12, e0169581. [Google Scholar] [CrossRef]
- Han, R.; Li, A.; Li, L.; Kitlinska, J.B.; Zukowska, Z. Maternal low-protein diet up-regulates the neuropeptide Y system in visceral fat and leads to abdominal obesity and glucose intolerance in a sex- and time-specific manner. FASEB J. 2012, 26, 3528–3536. [Google Scholar] [CrossRef] [PubMed]
- Holness, M.J.; Sugden, M.C. Antecedent protein restriction exacerbates development of impaired insulin action after high-fat feeding. Am. J. Physiol. 1999, 276, E85–E93. [Google Scholar] [CrossRef] [PubMed]
- Claycombe, K.J.; Uthus, E.O.; Roemmich, J.N.; Johnson, L.K.; Johnson, W.T. Prenatal low-protein and postnatal high-fat diets induce rapid adipose tissue growth by inducing Igf2 expression in Sprague Dawley rat offspring. J. Nutr. 2013, 143, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Claycombe, K.J.; Vomhof-DeKrey, E.E.; Garcia, R.; Johnson, W.T.; Uthus, E.; Roemmich, J.N. Decreased beige adipocyte number and mitochondrial respiration coincide with increased histone methyl transferase (G9a) and reduced FGF21 gene expression in Sprague-Dawley rats fed prenatal low protein and postnatal high-fat diets. J. Nutr. Biochem. 2016, 31, 113–121. [Google Scholar] [CrossRef]
- Dumortier, O.; Roger, E.; Pisani, D.F.; Casamento, V.; Gautier, N.; Lebrun, P.; Johnston, H.; Lopez, P.; Amri, E.Z.; Jousse, C.; et al. Age-Dependent Control of Energy Homeostasis by Brown Adipose Tissue in Progeny Subjected to Maternal Diet-Induced Fetal Programming. Diabetes 2017, 66, 627–639. [Google Scholar] [CrossRef]
- Sellayah, D.; Dib, L.; Anthony, F.W.; Watkins, A.J.; Fleming, T.P.; Hanson, M.A.; Cagampang, F.R. Effect of maternal protein restriction during pregnancy and postweaning high-fat feeding on diet-induced thermogenesis in adult mouse offspring. Eur. J. Nutr. 2014, 53, 1523–1531. [Google Scholar] [CrossRef]
- Allman, B.R.; Andres, A.; Borsheim, E. The Association of Maternal Protein Intake during Pregnancy in Humans with Maternal and Offspring Insulin Sensitivity Measures. Curr. Dev. Nutr. 2019, 3, nzz055. [Google Scholar] [CrossRef]
- Shiell, A.W.; Campbell, D.M.; Hall, M.H.; Barker, D.J. Diet in late pregnancy and glucose-insulin metabolism of the offspring 40 years later. BJOG 2000, 107, 890–895. [Google Scholar] [CrossRef]
- Maslova, E.; Rytter, D.; Bech, B.H.; Henriksen, T.B.; Rasmussen, M.A.; Olsen, S.F.; Halldorsson, T.I. Maternal protein intake during pregnancy and offspring overweight 20 y later. Am. J. Clin. Nutr. 2014, 100, 1139–1148. [Google Scholar] [CrossRef]
- Maslova, E.; Hansen, S.; Grunnet, L.G.; Strom, M.; Bjerregaard, A.A.; Hjort, L.; Kampmann, F.B.; Madsen, C.M.; Baun Thuesen, A.C.; Bech, B.H.; et al. Maternal protein intake in pregnancy and offspring metabolic health at age 9-16 y: Results from a Danish cohort of gestational diabetes mellitus pregnancies and controls. Am. J. Clin. Nutr. 2017, 106, 623–636. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Reilly, S.M.; Saltiel, A.R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 2017, 13, 633–643. [Google Scholar] [CrossRef]
- Oster, M.; Murani, E.; Metges, C.C.; Ponsuksili, S.; Wimmers, K. Transcriptional response of skeletal muscle to a low-protein gestation diet in porcine offspring accumulates in growth- and cell cycle-regulating pathways. Physiol. Genom. 2012, 44, 811–818. [Google Scholar] [CrossRef][Green Version]
- Doring, F.; Luersen, K.; Schmelzer, C.; Hennig, S.; Lang, I.S.; Gors, S.; Rehfeldt, C.; Otten, W.; Metges, C.C. Influence of maternal low protein diet during pregnancy on hepatic gene expression signature in juvenile female porcine offspring. Mol. Nutr. Food Res. 2013, 57, 277–290. [Google Scholar] [CrossRef]
- Mortensen, O.H.; Olsen, H.L.; Frandsen, L.; Nielsen, P.E.; Nielsen, F.C.; Grunnet, N.; Quistorff, B. A maternal low protein diet has pronounced effects on mitochondrial gene expression in offspring liver and skeletal muscle; protective effect of taurine. J. Biomed. Sci. 2010, 17 (Suppl. 1), S38. [Google Scholar] [CrossRef][Green Version]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Sondergaard, E.; Espinosa De Ycaza, A.E.; Morgan-Bathke, M.; Jensen, M.D. How to Measure Adipose Tissue Insulin Sensitivity. J. Clin. Endocrinol. Metab. 2017, 102, 1193–1199. [Google Scholar] [CrossRef]
- Grubbs, F.E. Procedures for Detecting Outlying Observations in Samples. Technometrics 1969, 11, 1–21. [Google Scholar] [CrossRef]
- Ajuogu, P.K.; Wolden, M.; McFarlane, J.R.; Hart, R.A.; Carlson, D.J.; Van der Touw, T.; Smart, N.A. Effect of low- and high-protein maternal diets during gestation on reproductive outcomes in the rat: A systematic review and meta-analysis. J. Anim. Sci. 2020, 98. [Google Scholar] [CrossRef]
- Tao, C.; Sifuentes, A.; Holland, W.L. Regulation of glucose and lipid homeostasis by adiponectin: Effects on hepatocytes, pancreatic beta cells and adipocytes. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Russo, L.; Lumeng, C.N. Properties and functions of adipose tissue macrophages in obesity. Immunology 2018, 155, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Jablonski, K.A.; Amici, S.A.; Webb, L.M.; Ruiz-Rosado Jde, D.; Popovich, P.G.; Partida-Sanchez, S.; Guerau-de-Arellano, M. Novel Markers to Delineate Murine M1 and M2 Macrophages. PLoS ONE 2015, 10, e0145342. [Google Scholar] [CrossRef] [PubMed]
- Scheuerer, B.; Ernst, M.; Durrbaum-Landmann, I.; Fleischer, J.; Grage-Griebenow, E.; Brandt, E.; Flad, H.D.; Petersen, F. The CXC-chemokine platelet factor 4 promotes monocyte survival and induces monocyte differentiation into macrophages. Blood 2000, 95, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Ikeda, K.; Suganami, T.; Komiya, C.; Ochi, K.; Shirakawa, I.; Hamaguchi, M.; Nishimura, S.; Manabe, I.; Matsuda, T.; et al. Macrophage-inducible C-type lectin underlies obesity-induced adipose tissue fibrosis. Nat. Commun. 2014, 5, 4982. [Google Scholar] [CrossRef]
- Meissburger, B.; Stachorski, L.; Roder, E.; Rudofsky, G.; Wolfrum, C. Tissue inhibitor of matrix metalloproteinase 1 (TIMP1) controls adipogenesis in obesity in mice and in humans. Diabetologia 2011, 54, 1468–1479. [Google Scholar] [CrossRef]
- Lee, M.R.; Lim, C.J.; Lee, Y.H.; Park, J.G.; Sonn, S.K.; Lee, M.N.; Jung, I.H.; Jeong, S.J.; Jeon, S.; Lee, M.; et al. The adipokine Retnla modulates cholesterol homeostasis in hyperlipidemic mice. Nat. Commun. 2014, 5, 4410. [Google Scholar] [CrossRef]
- Gosby, A.K.; Maloney, C.A.; Caterson, I.D. Elevated insulin sensitivity in low-protein offspring rats is prevented by a high-fat diet and is associated with visceral fat. Obesity (Silver Spring) 2010, 18, 1593–1600. [Google Scholar] [CrossRef]
- Sellayah, D.; Cagampang, F.R. The Divergent Effect of Maternal Protein Restriction during Pregnancy and Postweaning High-Fat Diet Feeding on Blood Pressure and Adiposity in Adult Mouse Offspring. Nutrients 2018, 10, 1832. [Google Scholar] [CrossRef]
- Badoud, F.; Perreault, M.; Zulyniak, M.A.; Mutch, D.M. Molecular insights into the role of white adipose tissue in metabolically unhealthy normal weight and metabolically healthy obese individuals. FASEB J. 2015, 29, 748–758. [Google Scholar] [CrossRef]
- Desai, M.; Guang, H.; Ferelli, M.; Kallichanda, N.; Lane, R.H. Programmed upregulation of adipogenic transcription factors in intrauterine growth-restricted offspring. Reprod. Sci. 2008, 15, 785–796. [Google Scholar] [CrossRef]
- Ferland-McCollough, D.; Fernandez-Twinn, D.S.; Cannell, I.G.; David, H.; Warner, M.; Vaag, A.A.; Bork-Jensen, J.; Brons, C.; Gant, T.W.; Willis, A.E.; et al. Programming of adipose tissue miR-483-3p and GDF-3 expression by maternal diet in type 2 diabetes. Cell Death Differ. 2012, 19, 1003–1012. [Google Scholar] [CrossRef]
- Kim, J.Y.; van de Wall, E.; Laplante, M.; Azzara, A.; Trujillo, M.E.; Hofmann, S.M.; Schraw, T.; Durand, J.L.; Li, H.; Li, G.; et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Invest. 2007, 117, 2621–2637. [Google Scholar] [CrossRef]
- Luo, T.; Nocon, A.; Fry, J.; Sherban, A.; Rui, X.; Jiang, B.; Xu, X.J.; Han, J.; Yan, Y.; Yang, Q.; et al. AMPK Activation by Metformin Suppresses Abnormal Extracellular Matrix Remodeling in Adipose Tissue and Ameliorates Insulin Resistance in Obesity. Diabetes 2016, 65, 2295–2310. [Google Scholar] [CrossRef]
- Appleton, S.L.; Seaborn, C.J.; Visvanathan, R.; Hill, C.L.; Gill, T.K.; Taylor, A.W.; Adams, R.J.; North West Adelaide Health Study Team. Diabetes and cardiovascular disease outcomes in the metabolically healthy obese phenotype: A cohort study. Diabetes Care 2013, 36, 2388–2394. [Google Scholar] [CrossRef]
- Rey-Lopez, J.P.; de Rezende, L.F.; Pastor-Valero, M.; Tess, B.H. The prevalence of metabolically healthy obesity: A systematic review and critical evaluation of the definitions used. Obes. Rev. 2014, 15, 781–790. [Google Scholar] [CrossRef]
- Muhlhausler, B.; Smith, S.R. Early-life origins of metabolic dysfunction: Role of the adipocyte. Trends Endocrinol. Metab. 2009, 20, 51–57. [Google Scholar] [CrossRef]
- Mancuso, P.; Bouchard, B. The Impact of Aging on Adipose Function and Adipokine Synthesis. Front. Endocrinol. (Lausanne) 2019, 10, 137. [Google Scholar] [CrossRef]
- Bol, V.V.; Delattre, A.I.; Reusens, B.; Raes, M.; Remacle, C. Forced catch-up growth after fetal protein restriction alters the adipose tissue gene expression program leading to obesity in adult mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R291–R299. [Google Scholar] [CrossRef]
- Bagattin, A.; Hugendubler, L.; Mueller, E. Transcriptional coactivator PGC-1alpha promotes peroxisomal remodeling and biogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 20376–20381. [Google Scholar] [CrossRef]
- Hilse, K.E.; Kalinovich, A.V.; Rupprecht, A.; Smorodchenko, A.; Zeitz, U.; Staniek, K.; Erben, R.G.; Pohl, E.E. The expression of UCP3 directly correlates to UCP1 abundance in brown adipose tissue. Biochim. Biophys. Acta 2016, 1857, 72–78. [Google Scholar] [CrossRef]
- Langley, S.C.; Browne, R.F.; Jackson, A.A. Altered glucose tolerance in rats exposed to maternal low protein diets in utero. Comp. Biochem. Physiol. Physiol. 1994, 109, 223–229. [Google Scholar] [CrossRef]
- Hales, C.N.; Desai, M.; Ozanne, S.E.; Crowther, N.J. Fishing in the stream of diabetes: From measuring insulin to the control of fetal organogenesis. Biochem. Soc. Trans. 1996, 24, 341–350. [Google Scholar] [CrossRef]
- Charkoftaki, G.; Wang, Y.; McAndrews, M.; Bruford, E.A.; Thompson, D.C.; Vasiliou, V.; Nebert, D.W. Update on the human and mouse lipocalin (LCN) gene family, including evidence the mouse Mup cluster is result of an “evolutionary bloom”. Hum. Genom. 2019, 13, 11. [Google Scholar] [CrossRef]
- Chen, K.; Jih, A.; Osborn, O.; Kavaler, S.T.; Fu, W.; Sasik, R.; Saito, R.; Kim, J.J. Distinct gene signatures predict insulin resistance in young mice with high fat diet-induced obesity. Physiol. Genom. 2018, 50, 144–157. [Google Scholar] [CrossRef]
- Armengaud, J.B.; Dennebouy, Z.; Labes, D.; Fumey, C.; Wilson, A.; Candotti, F.; Yzydorczyk, C.; Simeoni, U. Intra-uterine growth restriction induced by maternal low-protein diet causes long-term alterations of thymic structure and function in adult male rat offspring. Br. J. Nutr. 2020, 123, 892–900. [Google Scholar] [CrossRef]
- Silva, S.V.; Garcia-Souza, E.P.; Moura, A.S.; Barja-Fidalgo, C. Maternal protein restriction during early lactation induces changes on neutrophil activation and TNF-alpha production of adult offspring. Inflammation 2010, 33, 65–75. [Google Scholar] [CrossRef]
- Alheiros-Lira, M.C.; Jurema-Santos, G.C.; da-Silva, H.T.; da-Silva, A.C.; Moreno Senna, S.; Ferreira, E.S.W.T.; Ferraz, J.C.; Leandro, C.G. Effects of high-fat diet on somatic growth, metabolic parameters and function of peritoneal macrophages of young rats submitted to a maternal low-protein diet. Br. J. Nutr. 2017, 117, 796–803. [Google Scholar] [CrossRef]
- Muniyappa, R.; Lee, S.; Chen, H.; Quon, M.J. Current approaches for assessing insulin sensitivity and resistance in vivo: Advantages, limitations, and appropriate usage. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E15–E26. [Google Scholar] [CrossRef]

| C/C | C/HF | LP/HF | |
|---|---|---|---|
| Body weight (g) | |||
| Postnatal day 3 | 2.62 ± 0.09 | 2.06 ± 0.12 § | |
| Postnatal day 20 | 13.20 ± 0.30 | 6.42 ± 0.35 § | |
| At sacrifice (25 weeks) | 55.75 ± 0.96 | 62.12 ± 2.74 | 47.11 ± 0.78 * |
| Adipose tissue weight (g) | |||
| Epididymal fat | 1.78 ± 0.10 | 1.80 ± 0.27 | 2.48 ± 0.29 |
| Perirenal fat | 0.43 ± 0.03 | 0.64 ± 0.12 | 0.23 ± 0.02 * |
| Retroperitoneal fat | 0.39 ± 0.02 | 0.73 ± 0.12 # | 0.50 ± 0.07 |
| Sum of fat depots | 2.60 ± 0.13 | 3.18 ± 0.48 | 3.21 ± 0.35 |
| Relative adipose tissue weight (g/100 g body weight) | |||
| Epididymal fat | 3.20 ± 0.18 | 2.81 ± 0.32 | 5.25 ± 0.59 * |
| Perirenal fat | 0.77 ± 0.05 | 1.00 ± 0.16 | 0.48 ± 0.04 * |
| Retroperitoneal fat | 0.70 ± 0.04 | 1.14 ± 0.15 # | 1.05 ± 0.14 |
| Sum of fat depots | 4.67 ± 0.22 | 4.96 ± 0.57 | 6.69 ± 0.69 * |
| C/C | C/HF | LP/HF | |
|---|---|---|---|
| Glucose (mg/dL) | 165.6 ± 20.7 | 198.4 ± 24.8 | 180.4 ± 20.1 |
| Insulin (μU/mL) | 13.1 ± 2.0 | 30.4 ± 4.4 # | 7.7 ± 0.6 * |
| HOMA-IR 1 | 5.03 ± 0.80 | 13.79 ± 2.40 # | 4.65 ± 1.34 * |
| Free fatty acid (mmol/L) | ND | 0.88 ± 0.06 | 1.04 ± 0.06 |
| Adipo-IR 2 | ND | 12.11 ± 1.86 | 3.83 ± 0.74 * |
| Adiponectin (μg/mL) | 3.10 ± 0.31 | 2.97 ± 0.19 | 4.89 ± 0.28 * |
| Leptin (ng/mL) | 24.8 ± 5.6 | 61.1 ± 15.7 | 34.6 ± 7.6 |
| MCP-1 (pg/mL) | 110.4 ± 16.0 | 117.9 ± 12.9 | 85.9 ± 10.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Choi, A.; Kwon, Y.H. Maternal Protein Restriction Altered Insulin Resistance and Inflammation-Associated Gene Expression in Adipose Tissue of Young Adult Mouse Offspring in Response to a High-Fat Diet. Nutrients 2020, 12, 1103. https://doi.org/10.3390/nu12041103
Kim J, Choi A, Kwon YH. Maternal Protein Restriction Altered Insulin Resistance and Inflammation-Associated Gene Expression in Adipose Tissue of Young Adult Mouse Offspring in Response to a High-Fat Diet. Nutrients. 2020; 12(4):1103. https://doi.org/10.3390/nu12041103
Chicago/Turabian StyleKim, Juhae, Alee Choi, and Young Hye Kwon. 2020. "Maternal Protein Restriction Altered Insulin Resistance and Inflammation-Associated Gene Expression in Adipose Tissue of Young Adult Mouse Offspring in Response to a High-Fat Diet" Nutrients 12, no. 4: 1103. https://doi.org/10.3390/nu12041103
APA StyleKim, J., Choi, A., & Kwon, Y. H. (2020). Maternal Protein Restriction Altered Insulin Resistance and Inflammation-Associated Gene Expression in Adipose Tissue of Young Adult Mouse Offspring in Response to a High-Fat Diet. Nutrients, 12(4), 1103. https://doi.org/10.3390/nu12041103