Association of Coffee, Decaffeinated Coffee and Caffeine Intake from Coffee with Cognitive Performance in Older Adults: National Health and Nutrition Examination Survey (NHANES) 2011–2014.
Abstract
1. Introduction
2. Materials and Methods
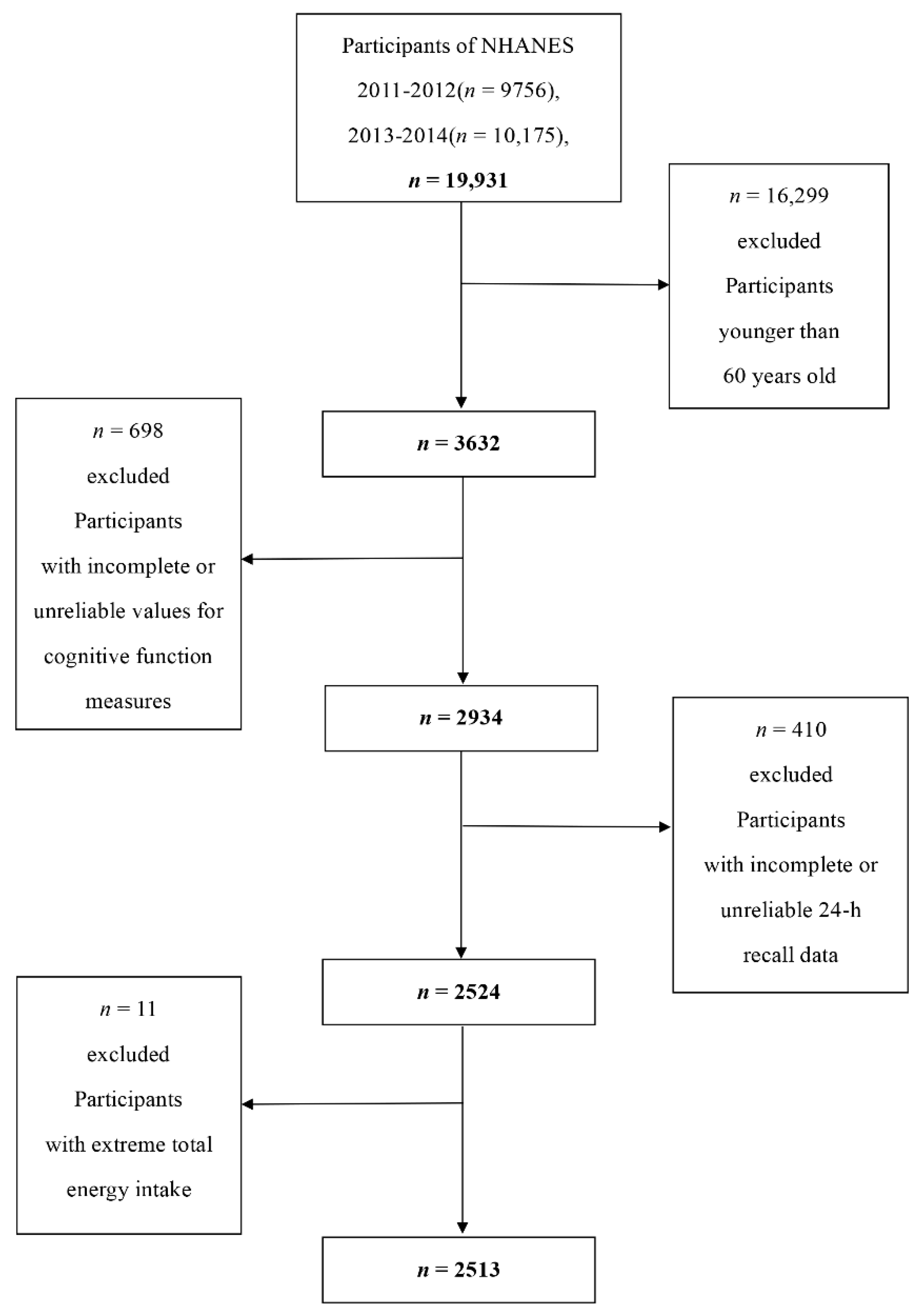
2.1. Data Collection and Study Population
2.2. Cognitive Performance Assessment
2.3. Dietary Intake Assessment
2.4. Covariates
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Butt, M.S.; Sultan, M.T. Coffee and its consumption: Benefits and risks. Crit. Rev. Food Sci. Nutr. 2011, 51, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.M. Caffeine—Its identity, dietary sources, intake and biological effects. Nutr. Rev. 1978, 36, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Heckman, M.A.; Weil, J.; Gonzalez de Mejia, E. Caffeine (1, 3, 7-trimethylxanthine) in foods: A comprehensive review on consumption, functionality, safety, and regulatory matters. J. Food Sci. 2010, 75, R77–R87. [Google Scholar] [CrossRef] [PubMed]
- Nehlig, A. Are we dependent upon coffee and caffeine? A review on human and animal data. Neurosci. Biobehav. Rev. 1999, 23, 563–576. [Google Scholar] [CrossRef]
- Grosso, G.; Godos, J.; Galvano, F.; Giovannucci, E.L. Coffee, Caffeine, and Health Outcomes: An Umbrella Review. Ann. Rev. Nutr. 2017, 37, 131–156. [Google Scholar] [CrossRef]
- Bohn, S.K.; Blomhoff, R.; Paur, I. Coffee and cancer risk, epidemiological evidence, and molecular mechanisms. Mol. Nutr. Food Res. 2014, 58, 915–930. [Google Scholar] [CrossRef]
- Hebert, L.E.; Weuve, J.; Scherr, P.A.; Evans, D.A. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013, 80, 1778–1783. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. The Healthy Brain Initiative: The Public Health Road Map for State and National Partnerships, 2013–2018, Interim Progress Report; CDC: Atlanta, GA, USA, 2015. Available online: https://www.cdc.gov/aging/pdf/2013-healthy-brain-initiative-interim-report.pdf (accessed on 2 February 2020).
- Wu, L.; Sun, D.; He, Y. Coffee intake and the incident risk of cognitive disorders: A dose-response meta-analysis of nine prospective cohort studies. Clin. Nutr. 2017, 36, 730–736. [Google Scholar] [CrossRef]
- Liu, Q.P.; Wu, Y.F.; Cheng, H.Y.; Xia, T.; Ding, H.; Wang, H.; Wang, Z.M.; Xu, Y. Habitual coffee consumption and risk of cognitive decline/dementia: A systematic review and meta-analysis of prospective cohort studies. Nutrition 2016, 32, 628–636. [Google Scholar] [CrossRef]
- Haller, S.; Montandon, M.L.; Rodriguez, C.; Herrmann, F.R.; Giannakopoulos, P. Impact of Coffee, Wine, and Chocolate Consumption on Cognitive Outcome and MRI Parameters in Old Age. Nutrients 2018, 10, 1391. [Google Scholar] [CrossRef]
- Ross, G.W.; Abbott, R.D.; Petrovitch, H.; Morens, D.M.; Grandinetti, A.; Tung, K.H.; Tanner, C.M.; Masaki, K.H.; Blanchette, P.L.; Curb, J.D.; et al. Association of coffee and caffeine intake with the risk of Parkinson disease. JAMA 2000, 283, 2674–2679. [Google Scholar] [CrossRef] [PubMed]
- Panza, F.; Solfrizzi, V.; Barulli, M.R.; Bonfiglio, C.; Guerra, V.; Osella, A.; Seripa, D.; Sabba, C.; Pilotto, A.; Logroscino, G. Coffee, tea, and caffeine consumption and prevention of late-life cognitive decline and dementia: A systematic review. J. Nutr. Health Aging 2015, 19, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, B.B.; Battig, K.; Holmen, J.; Nehlig, A.; Zvartau, E.E. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol. Rev. 1999, 51, 83–133. [Google Scholar] [PubMed]
- Arendash, G.W.; Mori, T.; Cao, C.; Mamcarz, M.; Runfeldt, M.; Dickson, A.; Rezai-Zadeh, K.; Tane, J.; Citron, B.A.; Lin, X.; et al. Caffeine reverses cognitive impairment and decreases brain amyloid-beta levels in aged Alzheimer’s disease mice. J. Alzheimer’s Dis. 2009, 17, 661–680. [Google Scholar] [CrossRef] [PubMed]
- Iranpour, S.; Sabour, S. Inverse association between caffeine intake and depressive symptoms in US adults: Data from National Health and Nutrition Examination Survey (NHANES) 2005–2006. Psychiatry Res. 2019, 271, 732–739. [Google Scholar] [CrossRef]
- Santos, C.; Lunet, N.; Azevedo, A.; de Mendonca, A.; Ritchie, K.; Barros, H. Caffeine intake is associated with a lower risk of cognitive decline: A cohort study from Portugal. J. Alzheimer’s Dis. 2010, 20, S175–S185. [Google Scholar] [CrossRef]
- Haskell, C.F.; Kennedy, D.O.; Milne, A.L.; Wesnes, K.A.; Scholey, A.B. The effects of L-theanine, caffeine and their combination on cognition and mood. Biol. Psychol. 2008, 77, 113–122. [Google Scholar] [CrossRef]
- Ritchie, K.; Carriere, I.; de Mendonca, A.; Portet, F.; Dartigues, J.F.; Rouaud, O.; Barberger-Gateau, P.; Ancelin, M.L. The neuroprotective effects of caffeine: A prospective population study (the Three City Study). Neurology 2007, 69, 536–545. [Google Scholar] [CrossRef]
- Arab, L.; Biggs, M.L.; O’Meara, E.S.; Longstreth, W.T.; Crane, P.K.; Fitzpatrick, A.L. Gender differences in tea, coffee, and cognitive decline in the elderly: The Cardiovascular Health Study. J. Alzheimer’s Dis. 2011, 27, 553–566. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Gamaldo, A.A.; Beydoun, H.A.; Tanaka, T.; Tucker, K.L.; Talegawkar, S.A.; Ferrucci, L.; Zonderman, A.B. Caffeine and alcohol intakes and overall nutrient adequacy are associated with longitudinal cognitive performance among U.S. adults. J. Nutr. 2014, 144, 890–901. [Google Scholar] [CrossRef]
- Ritchie, K.; Artero, S.; Portet, F.; Brickman, A.; Muraskin, J.; Beanino, E.; Ancelin, M.L.; Carriere, I. Caffeine, cognitive functioning, and white matter lesions in the elderly: Establishing causality from epidemiological evidence. J. Alzheimer’s Dis. 2010, 20, S161–S166. [Google Scholar] [CrossRef] [PubMed]
- Haskell-Ramsay, C.F.; Jackson, P.A.; Forster, J.S.; Dodd, F.L.; Bowerbank, S.L.; Kennedy, D.O. The Acute Effects of Caffeinated Black Coffee on Cognition and Mood in Healthy Young and Older Adults. Nutrients 2018, 10, 1386. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.J.; Kim, J.; Shim, J.; Kim, C.Y.; Jang, J.H.; Lee, K.W.; Lee, H.J. Decaffeinated coffee prevents scopolamine-induced memory impairment in rats. Behav. Brain Res. 2013, 245, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Kozlow, M.; Kritz-Silverstein, D.; Barrett-Connor, E.; Morton, D. Coffee consumption and cognitive function among older adults. Am. J. Epidemiol. 2002, 156, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Camfield, D.A.; Silber, B.Y.; Scholey, A.B.; Nolidin, K.; Goh, A.; Stough, C. A randomised placebo-controlled trial to differentiate the acute cognitive and mood effects of chlorogenic acid from decaffeinated coffee. PLoS ONE 2013, 8, e82897. [Google Scholar] [CrossRef]
- Riedel, W.; Hogervorst, E.; Leboux, R.; Verhey, F.; van Praag, H.; Jolles, J. Caffeine attenuates scopolamine-induced memory impairment in humans. Psychopharmacology 1995, 122, 158–168. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kwak, S.M.; Myung, S.K. Caffeine intake from coffee or tea and cognitive disorders: A meta-analysis of observational studies. Neuroepidemiology 2015, 44, 51–63. [Google Scholar] [CrossRef]
- National Health and Nutrition Examination Survey. Questionnaires, Datasets, and Related Documentation. Available online: https://wwwn.cdc.gov/nchs/ (accessed on 15 January 2020).
- Centers for Disease, Control, and Prevention. National Health and Nutrition Examination Survey. Survey Methods and Analytic Guidelines. Available online: https://www.cdc.gov/nchs/nhanes/index.htm (accessed on 15 January 2020).
- CDC. National Health and Nutrition Examination Survey 2011–2012 Data Documentation, Codebook and Frequencies. Cognitive Functioning. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/CFQ_G.htm (accessed on 15 January 2020).
- Fillenbaum, G.G.; van Belle, G.; Morris, J.C.; Mohs, R.C.; Mirra, S.S.; Davis, P.C.; Tariot, P.N.; Silverman, J.M.; Clark, C.M.; Welsh-Bohmer, K.A.; et al. Consortium to Establish a Registry for Alzheimer’s Disease (CERAD): The first twenty years. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2008, 4, 96–109. [Google Scholar] [CrossRef]
- Gao, S.; Jin, Y.; Unverzagt, F.W.; Liang, C.; Hall, K.S.; Ma, F.; Murrell, J.R.; Cheng, Y.; Matesan, J.; Bian, J.; et al. Hypertension and cognitive decline in rural elderly Chinese. J. Am. Geriatr. Soc. 2009, 57, 1051–1057. [Google Scholar] [CrossRef]
- Clark, L.J.; Gatz, M.; Zheng, L.; Chen, Y.L.; McCleary, C.; Mack, W.J. Longitudinal verbal fluency in normal aging, preclinical, and prevalent Alzheimer’s disease. Am. J. Alzheimer’s Dis. Other Dement. 2009, 24, 461–468. [Google Scholar] [CrossRef]
- Canning, S.J.; Leach, L.; Stuss, D.; Ngo, L.; Black, S.E. Diagnostic utility of abbreviated fluency measures in Alzheimer disease and vascular dementia. Neurology 2004, 62, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, J. Digit Symbol Substitution Test: The Case for Sensitivity over Specificity in Neuropsychological Testing. J. Clin. Psychopharmacol. 2018, 38, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Xun, P.; Jacobs, D.R., Jr.; Zhu, N.; Daviglus, M.L.; Reis, J.P.; Steffen, L.M.; Van Horn, L.; Sidney, S.; He, K. Intake of niacin, folate, vitamin B-6, and vitamin B-12 through young adulthood and cognitive function in midlife: The Coronary Artery Risk Development in Young Adults (CARDIA) study. Am. J. Clin. Nutr. 2017, 106, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- National Health and Nutrition Examination Survey 2013–2014 Data Documentation, Codebook, and Frequencies, Cognitive Functioning (CFQ_H). Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2013–2014/CFQ_H.htm#CFDDS (accessed on 30 January 2020).
- Chen, S.P.; Bhattacharya, J.; Pershing, S. Association of Vision Loss with Cognition in Older Adults. JAMA Ophthalmol. 2017, 135, 963–970. [Google Scholar] [CrossRef]
- Li, S.; Sun, W.; Zhang, D. Association of Zinc, Iron, Copper, and Selenium Intakes with Low Cognitive Performance in Older Adults: A Cross-Sectional Study from National Health and Nutrition Examination Survey (NHANES). J. Alzheimer’s Dis. 2019, 72, 1145–1157. [Google Scholar] [CrossRef]
- The Examination Protocol and Data Collection Methods. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2011–2012/manuals/mec_in_person_dietary_procedures_manual_jan_2012.pdf (accessed on 31 January 2020).
- USDA’s Food and Nutrient Database for Dietary Studies (FNDDS) 2011–2012. Available online: http://www.ars.usda.gov/ba/bhnrc/fsrg (accessed on 13 February 2020).
- National Health and Nutrition Examination Survey. Task 3: Key Concepts about Food Frequency Data Files. Available online: https://www.cdc.gov/nchs/tutorials/Dietary/SurveyOrientation/DietaryDataStructureContents/Info3.htm (accessed on 4 March 2020).
- National Health and Nutrition Examination Survey. Food Frequency Questionnaire—Raw Questionnaire Responses. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2005–2006/FFQRAW_D.htm#FFQ0123 (accessed on 4 March 2020).
- Sun, B.; Shi, X.; Wang, T.; Zhang, D. Exploration of the Association between Dietary Fiber Intake and Hypertension among U.S. Adults Using 2017 American College of Cardiology/American Heart Association Blood Pressure Guidelines: NHANES 2007–2014. Nutrients 2018, 10, 1091. [Google Scholar] [CrossRef]
- Jaber, R.M.; Mirbolouk, M.; DeFilippis, A.P.; Maziak, W.; Keith, R.; Payne, T.; Stokes, A.; Benjamin, E.; Bhatnagar, A.; Blankstein, R.; et al. Electronic Cigarette Use Prevalence, Associated Factors, and Pattern by Cigarette Smoking Status in the United States From NHANES (National Health and Nutrition Examination Survey) 2013–2014. J. Am. Heart Assoc. 2018, 7, e008178. [Google Scholar] [CrossRef]
- Centers for Disease, Control, and Prevention. National Health and Nutrition Examination Survey. Demographic Data and Related Documentation. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/DEMO_G.htm#INDFMPIR (accessed on 1 February 2020).
- National Health and Nutrition Examination Survey. Tutorials. Module 3: Weighting. Available online: https://wwwn.cdc.gov/nchs/nhanes/tutorials/module3.aspx (accessed on 3 February 2020).
- Christensen, K.; Gleason, C.E.; Mares, J.A. Dietary carotenoids and cognitive function among US adults, NHANES 2011–2014. Nutr. Neurosci. 2018, 1–9. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Wang, W.; Zhang, D. Association between Dietary Protein Intake and Cognitive Function in Adults Aged 60 Years and Older. J. Nutr. Health Aging 2020, 24, 223–229. [Google Scholar] [CrossRef]
- van Gelder, B.M.; Buijsse, B.; Tijhuis, M.; Kalmijn, S.; Giampaoli, S.; Nissinen, A.; Kromhout, D. Coffee consumption is inversely associated with cognitive decline in elderly European men: The FINE Study. Eur. J. Clin. Nutr. 2007, 61, 226–232. [Google Scholar] [CrossRef]
- Rinne, J.O.; Portin, R.; Ruottinen, H.; Nurmi, E.; Bergman, J.; Haaparanta, M.; Solin, O. Cognitive impairment and the brain dopaminergic system in Parkinson disease: [18F] fluorodopa positron emission tomographic study. Arch. Neurol. 2000, 57, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, M.J. Does caffeine intake enhance absolute levels of cognitive performance? Psychopharmacology 1993, 110, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Taylor, A.E.; Karhunen, V.; Zhan, Y.; Rovio, S.P.; Lahti, J.; Sjogren, P.; Byberg, L.; Lyall, D.M.; Auvinen, J.; et al. Habitual coffee consumption and cognitive function: A Mendelian randomization meta-analysis in up to 41,5530 participants. Sci. Rep. 2018, 8, 7526. [Google Scholar] [CrossRef] [PubMed]
- Araujo, L.F.; Mirza, S.S.; Bos, D.; Niessen, W.J.; Barreto, S.M.; van der Lugt, A.; Vernooij, M.W.; Hofman, A.; Tiemeier, H.; Ikram, M.A. Association of Coffee Consumption with MRI Markers and Cognitive Function: A Population-Based Study. J. Alzheimer’s Dis 2016, 53, 451–461. [Google Scholar] [CrossRef]
- Araujo, L.F.; Giatti, L.; Reis, R.C.; Goulart, A.C.; Schmidt, M.I.; Duncan, B.B.; Ikram, M.A.; Barreto, S.M. Inconsistency of Association between Coffee Consumption and Cognitive Function in Adults and Elderly in a Cross-Sectional Study (ELSA-Brasil). Nutrients 2015, 7, 9590–9601. [Google Scholar] [CrossRef]
- Laitala, V.S.; Kaprio, J.; Koskenvuo, M.; Raiha, I.; Rinne, J.O.; Silventoinen, K. Coffee drinking in middle age is not associated with cognitive performance in old age. Am. J. Clin. Nutr. 2009, 90, 640–646. [Google Scholar] [CrossRef]
- Santos, C.; Costa, J.; Santos, J.; Vaz-Carneiro, A.; Lunet, N. Caffeine intake and dementia: Systematic review and meta-analysis. J. Alzheimer’s Dis. 2010, 20, S187–S204. [Google Scholar] [CrossRef]
- James, J.E.; Kristjansson, A.L.; Sigfusdottir, I.D. A gender-specific analysis of adolescent dietary caffeine, alcohol consumption, anger, and violent behavior. Subst. Use Misuse 2015, 50, 257–267. [Google Scholar] [CrossRef]
- Carrillo, J.A.; Benitez, J. CYP1A2 activity, gender and smoking, as variables influencing the toxicity of caffeine. Br. J. Clin. Pharmacol. 1996, 41, 605–608. [Google Scholar] [CrossRef]
- Relling, M.V.; Lin, J.S.; Ayers, G.D.; Evans, W.E. Racial and gender differences in N-acetyltransferase, xanthine oxidase, and CYP1A2 activities. Clin. Pharmacol. Ther. 1992, 52, 643–658. [Google Scholar] [CrossRef]
- Temple, J.L.; Ziegler, A.M. Gender Differences in Subjective and Physiological Responses to Caffeine and the Role of Steroid Hormones. J. Caffeine Res. 2011, 1, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Knuppel, S.; Norman, K.; Boeing, H. Is a Single 24-hour Dietary Recall per Person Sufficient to Estimate the Population Distribution of Usual Dietary Intake? J. Nutr. 2019, 149, 1491–1492. [Google Scholar] [CrossRef] [PubMed]
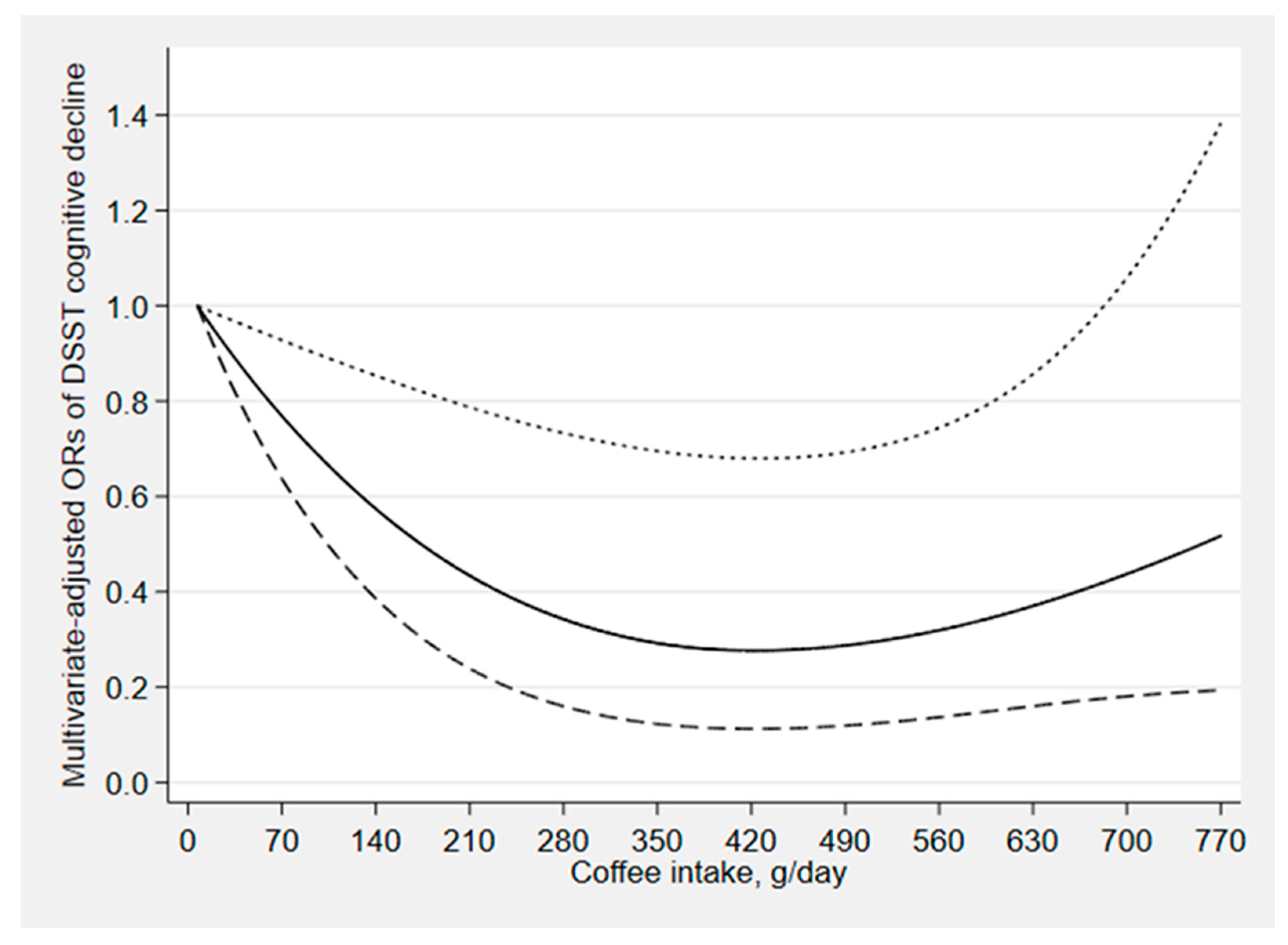
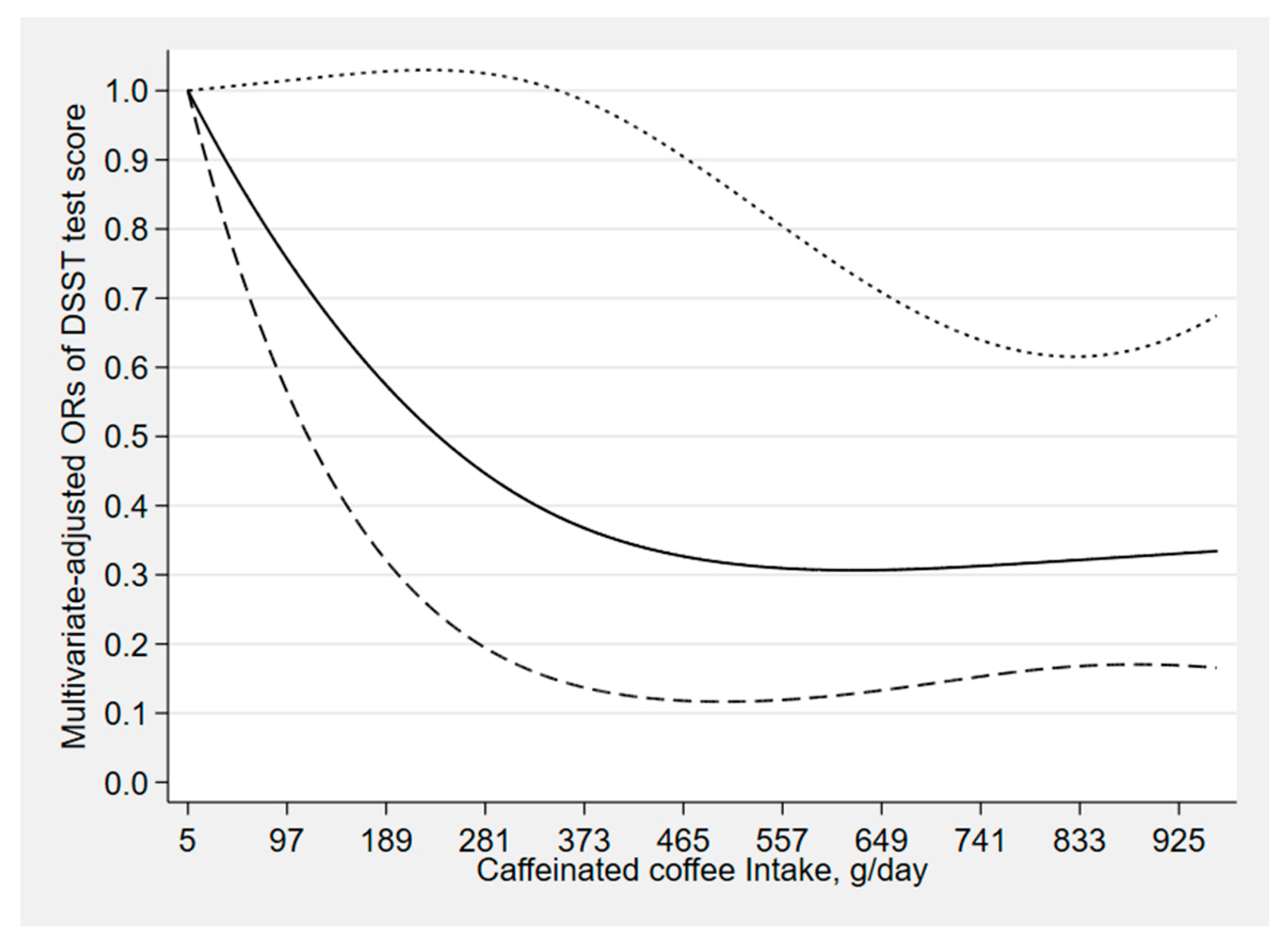
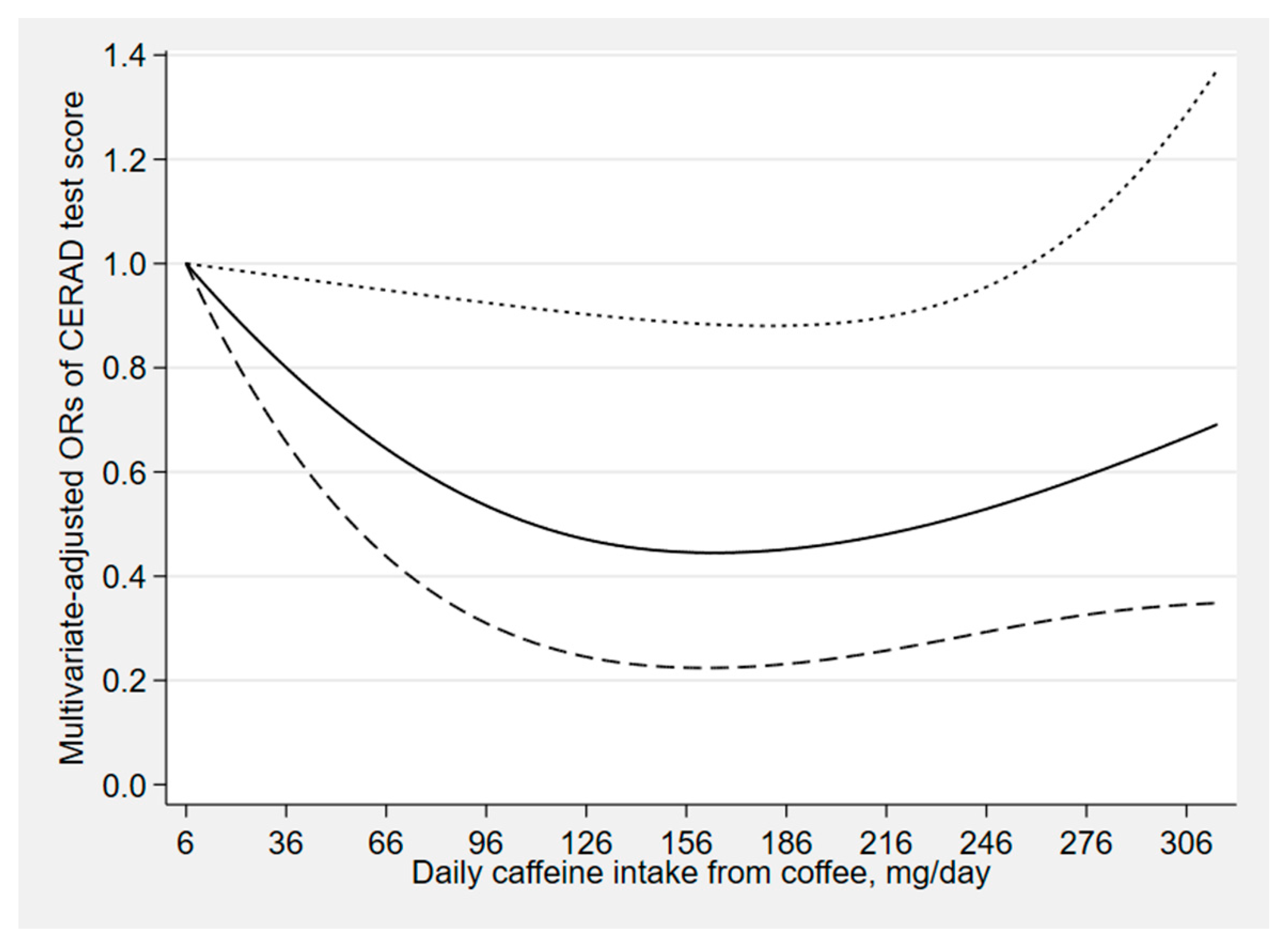
| CERAD Test | Animal Fluency Test | Digit Symbol Test | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of Subjects (N) | Normal Cognitive Performance | Low Cognitive Performance | p Value | Normal Cognitive Performance | Low Cognitive Performance | p Value | Normal Cognitive Performance | Low Cognitive Performance | p Value | |
| Number of subjects (%) 1 | 1800(71.6) | 713(28.4) | 1789(71.2) | 724(28.8) | 1857(73.9) | 656(26.1) | ||||
| Age (%) 1 | 2513 | 0.336 | 0.016 | 0.920 | ||||||
| 60–70 years | 959(53.3) | 403(56.5) | 961(53.7) | 401(55.4) | 1009(54.3) | 353(53.8) | ||||
| 70–80 years | 546(30.3) | 200(28.1) | 557(31.1) | 189(26.1) | 552(29.7) | 194(29.6) | ||||
| ≥80 years | 295(16.4) | 110(15.4) | 271(15.1) | 134(18.5) | 296(15.9) | 109(16.6) | ||||
| Gender (%) 1 | 2513 | <0.01 | 0.741 | <0.01 | ||||||
| Male | 782(43.4) | 432(60.6) | 868(48.5) | 346(47.8) | 843(45.4) | 371(56.6) | ||||
| Female | 1018(56.6) | 281(39.4) | 921(51.5) | 378(52.2) | 1014(54.6) | 285(43.4) | ||||
| Race (%) 1 | 2513 | <0.01 | <0.01 | <0.01 | ||||||
| Mexican American | 129(7.2) | 82(11.5) | 149(8.3) | 62(8.6) | 120(6.5) | 91(13.9) | ||||
| Other Hispanic | 143(7.9) | 101(14.2) | 151(8.4) | 93(12.8) | 120(6.5) | 124(18.9) | ||||
| Non-Hispanic White | 985(54.7) | 279(39.1) | 1031(57.6) | 233(32.2) | 1093(58.9) | 171(26.1) | ||||
| Non-Hispanic Black | 384(21.3) | 204(28.6) | 333(18.6) | 255(35.2) | 342(18.4) | 246(37.5) | ||||
| Other race | 159(8.8) | 47(6.6) | 125(7.0) | 81(11.2) | 182(9.8) | 24(3.7) | ||||
| Educational level (%) 1 | 2511 | <0.01 | <0.01 | <0.01 | ||||||
| Below high school | 320(17.8) | 277(38.9) | 329(18.4) | 268(37.1) | 244(13.1) | 353(54.0) | ||||
| High school | 423(23.5) | 174(24.4) | 405(22.6) | 192(26.6) | 441(23.7) | 156(23.9) | ||||
| Above high school | 1056(58.7) | 261(36.7) | 1055(59.0) | 262(36.3) | 1172(63.1) | 145(22.2) | ||||
| Material status (%) 1 | 2510 | 0.889 | 0.032 | <0.01 | ||||||
| Married/living with partner | 1056(58.7) | 416(58.4) | 1072(60.0) | 400(55.3) | 1144(61.7) | 328(50.1) | ||||
| Widowed/divorced/separated/never married | 742(41.3) | 296(41.6) | 715(40.0) | 323(44.7) | 711(38.3) | 327(49.9) | ||||
| Poverty–income ratio (%) 1 | 2324 | <0.01 | <0.01 | <0.01 | ||||||
| ≤0.99 | 224(13.4) | 144(22.0) | 217(13.0) | 151(22.9) | 185(10.7) | 183(30.6) | ||||
| ≥1 | 1445(86.6) | 511(78.0) | 1449(87.0) | 507(77.1) | 1540(89.3) | 416(69.4) | ||||
| Body mass index (%) 1 | 2483 | 0.055 | 0.638 | 0.498 | ||||||
| < 25 kg/m2 | 460(25.9) | 200(28.3) | 464(26.1) | 196 (27.7) | 487(26.4) | 173(27.2) | ||||
| 25-30 kg/m2 | 603(33.9) | 259(36.7) | 625(35.2) | 237(33.5) | 653(35.4) | 209(32.8) | ||||
| ≥30 kg/m2 | 714(40.2) | 247(35.0) | 686(38.6) | 275(38.8) | 706(38.2) | 255(40.0) | ||||
| Smoking status (%) 1 | 2510 | 0.003 | 0.018 | <0.01 | ||||||
| Never | 904(50.3) | 339(47.5) | 874(48.9) | 369(51.0) | 927(49.9) | 316(48.2) | ||||
| Former | 708(39.4) | 265(37.2) | 719(40.2) | 254(35.0) | 743(40.0) | 230(35.1) | ||||
| Current | 187(10.3) | 109(15.3) | 195(10.9) | 101(14.0) | 186(10.1) | 110(16.7) | ||||
| Hypertension (%) 1 | 2510 | 1123(62.4) | 445(62.6) | 0.939 | 1077(60.3) | 491(67.9) | <0.01 | 1119(60.4) | 449(68.4) | <0.01 |
| Diabetes (%) 1 | 2511 | 392(21.8) | 197(27.6) | 0.002 | 377(21.1) | 212(29.3) | <0.01 | 373(20.1) | 216(33.0) | <0.01 |
| Had at least 12 alcohol drinks/year (%) 1 | 2495 | 1237(69.0) | 491(69.9) | 0.643 | 1270(71.4) | 458(64.0) | <0.01 | 1322(71.5) | 406(62.8) | <0.01 |
| Ever told you had a stroke (%) 1 | 2508 | 107(6.0) | 59(8.3) | 0.036 | 100(5.6) | 66(9.1) | 0.001 | 91(4.9) | 75(11.4) | <0.01 |
| Coffee intake (g/day) 2 | 2513 | 255.77(499.25) | 228.9(442.5) | 0.017 | 273.8(510) | 212.8(397.5) | <0.01 | 277.5(510) | 208.43(393.2) | <0.01 |
| Caffeinated coffee intake (g/day) 2 | 2513 | 240(472.5) | 211(429.1) | 0.025 | 247.5(480) | 195.5(363.5) | <0.01 | 251.6(480) | 192.4(370.975) | <0.01 |
| Decaffeinated coffee intake (g/day) 2 | 2513 | 74.1(221) | 63(180.6) | 0.919 | 71.1(207) | 70.4(218) | 0.327 | 71.7(208) | 68.7(219) | 0.412 |
| Caffeine intake from coffee (mg/day) 2 | 1803 | 131.75 (213.03) | 107 (135) | 0.003 | 140 (149.5) | 99 (129.75) | <0.01 | 140 (147.5) | 95 (126.875) | <0.01 |
| Total energy intake (kcal/day) 2 | 2513 | 1754(816.25) | 1647.5(909.5) | <0.01 | 1796(838) | 1583.5(837) | <0.01 | 1796(815.5) | 1560.25(894) | <0.01 |
| CERAD Test | Animal Fluency Test | DSST | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coffee (g/day) | Case/Participants | Model 1 1 | Model 2 1 | Model 3 1 | Case/Participants | Model 1 1 | Model 2 1 | Model 3 1 | Case/Participants | Model 1 1 | Model 2 1 | Model 3 1 |
| 0 | 210/710 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 219/710 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 198/710 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) |
| 1 to <266.4 | 196/602 | 1.23(0.78–1.97) | 1.32(0.83–2.08) | 1.24(0.72–2.14) | 212/602 | 1.16(0.79–1.69) | 1.11(0.77–1.61) | 0.98(0.66–1.44) | 205/602 | 1.39(0.98–1.97) | 1.36(0.96–1.93) | 1.19(0.74–1.90) |
| 266.4 to <495 | 165/607 | 0.68(0.44–1.06) | 0.68(0.42–1.08) | 0.71(0.44–1.13) | 166/607 | 0.70(0.48–1.03) | 0.66(0.46–0.95) | 0.85(0.61–1.18) | 148/607 | 0.74(0.50–0.91) * | 0.71(0.47–0.87) * | 0.56(0.35–0.89) * |
| ≥495 | 142/594 | 0.80(0.50–1.27) | 0.75(0.45–1.23) | 0.89(0.52–1.54) | 127/594 | 0.71(0.48–1.07) | 0.72(0.48–1.06) | 1.13(0.73–1.76) | 105/594 | 0.57(0.40–0.81) * | 0.56(0.39–0.79) * | 1.03(0.67–1.62) |
| CERAD Test | Animal Fluency Test | DSST | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case/Participants | Model 1 1 | Model 2 1 | Model 3 1 | Case/Participants | Model 1 1 | Model 2 1 | Model 3 1 | Case/Participants | Model 1 1 | Model 2 1 | Model 3 1 | |
| Caffeinated coffee (g/day) | ||||||||||||
| 0 | 291/958 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 297/958 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 276/958 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) |
| 1 to <384.8 | 226/784 | 0.91(0.65–1.28) | 0.93(0.66–1.32) | 0.94(0.65–1.35) | 256/784 | 0.96(0.71–1.32) | 0.94(0.70–1.27) | 0.99(0.71–1.40) | 224/784 | 1.03(0.74–1.44) | 1.01(0.71–1.45) | 1.04(0.71–1.52) |
| ≥384.8 | 196/771 | 0.78(0.50–1.21) | 0.72(0.45–1.16) | 0.82(0.51–1.30) | 171/771 | 0.76(0.50–1.14) | 0.77(0.51–1.16) | 0.92(0.61–1.39) | 156/771 | 0.58(0.42–0.81) ** | 0.56(0.41–0.80) ** | 0.68(0.48–0.97) * |
| Decaffeinated coffee (g/day) | ||||||||||||
| 0 | 591/2094 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 593/2094 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 537/2094 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) |
| >0 | 122/419 | 0.92(0.43–1.96) | 1.02(0.45–2.33) | 1.45(0.75–2.80) | 131/419 | 0.99(0.50–1.95) | 0.92(0.45–1.87) | 1.09(0.54–2.13) | 119/419 | 1.32(0.63–2.77) | 1.31(0.60–2.86) | 1.84(0.68–3.17) |
| CERAD Test | Animal Fluency Test | DSST | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case/Participants | Model 1 1 | Model 2 1 | Model 3 1 | Case/Participants | Model 1 1 | Model 2 1 | Model 3 1 | Case/Participants | Model 1 1 | Model 2 1 | Model 3 1 | |
| Caffeine (mg/day) | ||||||||||||
| <67 | 150/455 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 162/455 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 160/455 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) |
| 67 to <124.5 | 133/450 | 0.77 (0.50–1.20) | 0.74(0.49–1.13) | 0.80(0.48–1.33) | 146/450 | 1.22(0.75–1.96) | 1.24(0.78–1.99) | 1.36(0.80–2.34) | 123/450 | 0.75(0.46–1.20) | 0.74(0.46–1.17) | 0.78(0.45–1.33) |
| 124.5 to <208 | 112/448 | 0.60(0.40–0.91) * | 0.53(0.34–0.83) ** | 0.62(0.38–0.98) * | 108/448 | 0.66(0.44–0.97) * | 0.65(0.45–0.95) * | 0.88(0.59–1.33) | 98/448 | 0.59(0.31–1.13) | 0.55(0.31–0.98) * | 0.67(0.39–1.15) |
| ≥208 | 108/450 | 0.76(0.52–1.11) | 0.67(0.45–0.99) * | 0.92(0.62–1.36) | 89/450 | 0.76(0.43–1.34) | 0.83(0.49–1.40) | 1.28(0.74–2.24) | 77/450 | 0.42(0.25–0.71) ** | 0.39(0.23–0.67) ** | 0.83(0.48–1.47) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Li, S.; Sun, J.; Li, Y.; Zhang, D. Association of Coffee, Decaffeinated Coffee and Caffeine Intake from Coffee with Cognitive Performance in Older Adults: National Health and Nutrition Examination Survey (NHANES) 2011–2014. Nutrients 2020, 12, 840. https://doi.org/10.3390/nu12030840
Dong X, Li S, Sun J, Li Y, Zhang D. Association of Coffee, Decaffeinated Coffee and Caffeine Intake from Coffee with Cognitive Performance in Older Adults: National Health and Nutrition Examination Survey (NHANES) 2011–2014. Nutrients. 2020; 12(3):840. https://doi.org/10.3390/nu12030840
Chicago/Turabian StyleDong, Xue, Shiru Li, Jing Sun, Yan Li, and Dongfeng Zhang. 2020. "Association of Coffee, Decaffeinated Coffee and Caffeine Intake from Coffee with Cognitive Performance in Older Adults: National Health and Nutrition Examination Survey (NHANES) 2011–2014." Nutrients 12, no. 3: 840. https://doi.org/10.3390/nu12030840
APA StyleDong, X., Li, S., Sun, J., Li, Y., & Zhang, D. (2020). Association of Coffee, Decaffeinated Coffee and Caffeine Intake from Coffee with Cognitive Performance in Older Adults: National Health and Nutrition Examination Survey (NHANES) 2011–2014. Nutrients, 12(3), 840. https://doi.org/10.3390/nu12030840






