Determining Malnutrition Assessment Criteria to Predict One-Year Mortality for Locally Advanced Head and Neck Cancer Patients Undergoing Concurrent Chemoradiotherapy
Abstract
1. Introduction
2. Materials and Methods
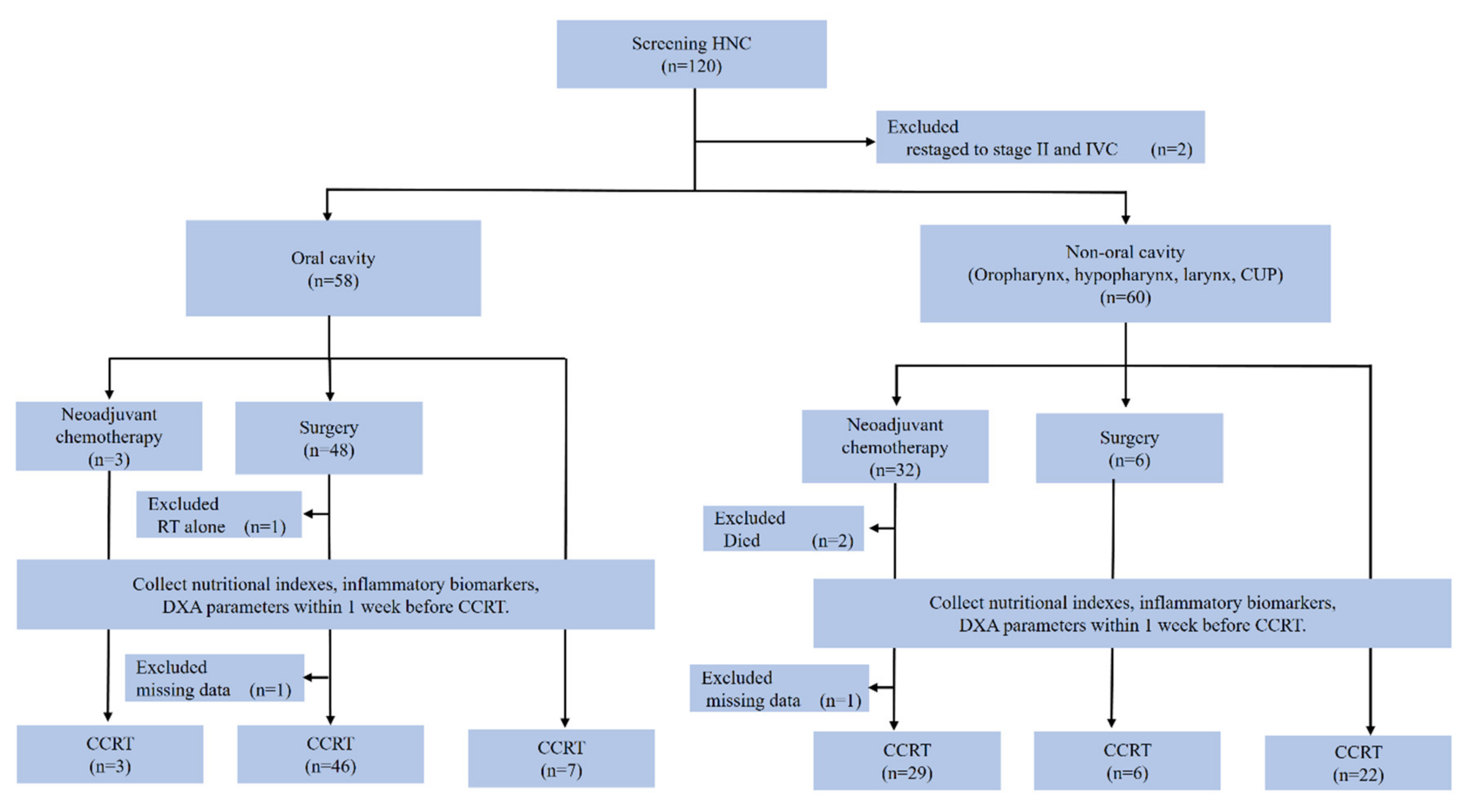
2.1. Study Design and Patients
2.2. CCRT Schedule
2.3. Clinicopathological Data
2.4. Malnutrition Assessment Methods
2.5. Statistical Analysis
3. Results
3.1. Patients
3.2. Parameters Predicting One Year Mortality
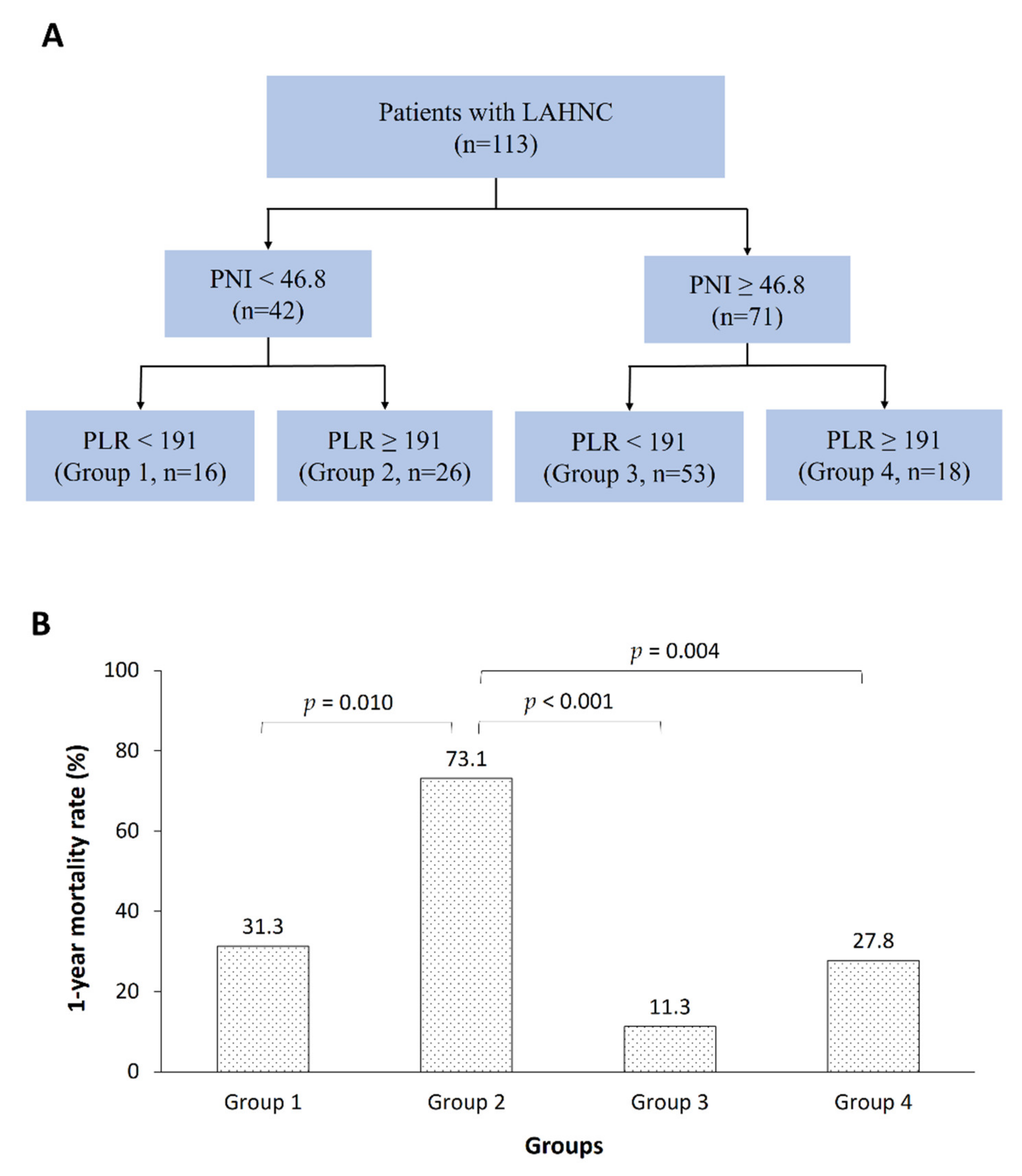
3.3. Relationships between the PNI or PLR and LBMI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ravasco, P.; Monteiro-Grillo, I.; Marques Vidal, P.; Camilo, M.E. Impact of nutrition on outcome: A prospective randomized controlled trial in patients with head and neck cancer undergoing radiotherapy. Head Neck 2005, 27, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Alshadwi, A.; Nadershah, M.; Carlson, E.R.; Young, L.S.; Burke, P.A.; Daley, B.J. Nutritional considerations for head and neck cancer patients: A review of the literature. J. Oral. Maxillofac. Surg. 2013, 71, 1853–1860. [Google Scholar] [CrossRef] [PubMed]
- Gorenc, M.; Kozjek, N.R.; Strojan, P. Malnutrition and cachexia in patients with head and neck cancer treated with (chemo)radiotherapy. Rep. Pract. Oncol. Radiother. 2015, 20, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Senesse, P.; Gioulbasanis, I.; Antoun, S.; Bozzetti, F.; Deans, C. Diagnostic criteria for the classification of cancer-associated weight loss. J. Clin. Oncol. 2015, 33, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Talwar, B.; Donnelly, R.; Skelly, R.; Donaldson, M. Nutritional management in head and neck cancer: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 2016, 130, S32–S40. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Keshavarz-Fathi, M.; Baracos, V.; Arends, J.; Mahmoudi, M.; Rezaei, N. Cancer cachexia: Diagnosis, assessment, and treatment. Crit. Rev. Oncol. Hematol. 2018, 127, 91–104. [Google Scholar] [CrossRef]
- Dechaphunkul, T.; Martin, L.; Alberda, C.; Olson, K.; Baracos, V.; Gramlich, L. Malnutrition assessment in patients with cancers of the head and neck: A call to action and consensus. Crit. Rev. Oncol. Hematol. 2013, 88, 459–476. [Google Scholar] [CrossRef]
- Datema, F.R.; Ferrier, M.B.; Baatenburg de Jong, R.J. Impact of severe malnutrition on short-term mortality and overall survival in head and neck cancer. Oral Oncol. 2011, 47, 910–914. [Google Scholar] [CrossRef]
- Chang, P.H.; Yeh, K.Y.; Huang, J.S.; Lai, C.H.; Wu, T.H.; Lan, Y.J. Pretreatment performance status and nutrition are associated with early mortality of locally advanced head and neck cancer patients undergoing concurrent chemoradiation. Eur. Arch. Otorhinolaryngol. 2013, 270, 1909–1915. [Google Scholar] [CrossRef]
- Dixon, L.; Garcez, K.; Lee, L.W.; Sykes, A.; Slevin, N.; Thomson, D. Ninety Day Mortality After Radical Radiotherapy for Head and Neck Cancer. Clin. Oncol. 2017, 29, 835–840. [Google Scholar] [CrossRef]
- Spencer, K.; Ellis, R.; Birch, R.; Dugdale, E.; Turner, R.; Sebag-Montefiore, D. Caution is required in the implementation of 90-day mortality indicators for radiotherapy in a curative setting: A retrospective population-based analysis of over 16,000 episodes. Radiother. Oncol. 2017, 125, 140–146. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kanotra, S.P.; Kanotra, S.; Gupta, A.; Paul, J. Chemoradiation in Advanced Head and Neck Cancers: A Comparison of two Radiosensitizers, Paclitaxel and Cisplatin. Indian J. Otolaryngol. Head Neck Surg. 2011, 63, 229–236. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nedergaard, A.; Karsdal, M.A.; Sun, S.; Henriksen, K. Serological muscle loss biomarkers: An overview of current concepts and future possibilities. J. Cachexia Sarcopenia Muscle 2013, 4, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.S.; Chaw, C.; Kovarik, J.; Aslam, S.; Jackson, A.; Kelly, J. Primary Concurrent Chemoradiation in Head and Neck Cancers with Weekly Cisplatin Chemotherapy: Analysis of Compliance, Toxicity and Survival. Int. Arch. Otorhinolaryngol. 2017, 21, 171–177. [Google Scholar] [CrossRef]
- Arends, J.; Bodoky, G.; Bozzetti, F.; Fearon, K.; Muscaritoli, M.; Selga, G. ESPEN Guidelines on Enteral Nutrition: Non-surgical oncology. Clin. Nutr. 2006, 25, 245–259. [Google Scholar] [CrossRef]
- Evans, W.J.; Morley, J.E.; Argiles, J.; Bales, C.; Baracos, V.; Guttridge, D. Cachexia: A new definition. Clin. Nutr. 2008, 27, 793–799. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Wendrich, A.W.; Swartz, J.E.; Bril, S.I.; Wegner, I.; de Graeff, A.; Smid, E.J. Low skeletal muscle mass is a predictive factor for chemotherapy dose-limiting toxicity in patients with locally advanced head and neck cancer. Oral Oncol. 2017, 71, 26–33. [Google Scholar] [CrossRef]
- Pring, E.T.; Malietzis, G.; Kennedy, R.H.; Athanasiou, T.; Jenkins, J.T. Cancer cachexia and myopenia - Update on management strategies and the direction of future research for optimizing body composition in cancer - A narrative review. Cancer Treat Rev. 2018, 70, 245–254. [Google Scholar] [CrossRef]
- Wang, C.H.; Wang, H.M.; Pang, Y.P.; Yeh, K.Y. Early nutritional support in non-metastatic stage IV oral cavity cancer patients undergoing adjuvant concurrent chemoradiotherapy: Analysis of treatment tolerance and outcome in an area endemic for betel quid chewing. Supportive Care Cancer 2012, 20, 1169–1174. [Google Scholar] [CrossRef]
- Boje, C.R.; Dalton, S.O.; Primdahl, H.; Kristensen, C.A.; Andersen, E.; Johansen, J. Evaluation of comorbidity in 9388 head and neck cancer patients: A national cohort study from the DAHANCA database. Radiother. Oncol. 2014, 110, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Bruixola, G.; Caballero, J.; Papaccio, F.; Petrillo, A.; Iranzo, A.; Civera, M. Prognostic Nutritional Index as an independent prognostic factor in locoregionally advanced squamous cell head and neck cancer. ESMO Open 2018, 3, e000425. [Google Scholar] [CrossRef] [PubMed]
- Aziz, E.F.; Javed, F.; Pratap, B.; Musat, D.; Nader, A.; Pulimi, S. Malnutrition as assessed by nutritional risk index is associated with worse outcome in patients admitted with acute decompensated heart failure: An ACAP-HF data analysis. Heart Int. 2011, 6, e2. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Capra, S.; Ferguson, M. Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur. J. Clin. Nutr. 2002, 56, 779–785. [Google Scholar] [CrossRef]
- Noori, N.; Kovesdy, C.P.; Dukkipati, R.; Kim, Y.; Duong, U.; Bross, R. Survival predictability of lean and fat mass in men and women undergoing maintenance hemodialysis. Am. J. Clin. Nutr. 2010, 92, 1060–1070. [Google Scholar] [CrossRef]
- Chang, J.S.; Kim, T.H.; Kim, H.; Choi, E.H.; Kim, N.; Kong, I.D. Qualitative muscle mass index as a predictor of skeletal muscle function deficit in Asian older adults. Geriatr. Gerontol. Int. 2017, 17, 99–107. [Google Scholar] [CrossRef]
- Onodera, T.; Goseki, N.; Kosaki, G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 1984, 85, 1001–1005. [Google Scholar]
- Palumbo, J.S.; Talmage, K.E.; Massari, J.V.; La Jeunesse, C.M.; Flick, M.J.; Kombrinck, K.W. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood 2005, 105, 178–185. [Google Scholar] [CrossRef]
- Du, X.J.; Tang, L.L.; Mao, Y.P.; Guo, R.; Sun, Y.; Lin, A.H. Value of the prognostic nutritional index and weight loss in predicting metastasis and long-term mortality in nasopharyngeal carcinoma. J. Transl. Med. 2015, 13, 364. [Google Scholar] [CrossRef]
- Gupta, D.; Lis, C.G. Pretreatment serum albumin as a predictor of cancer survival: A systematic review of the epidemiological literature. Nutr. J. 2010, 9, 69. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Cropet, C.; Van Glabbeke, M.; Sebban, C.; Le Cesne, A.; Judson, I. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009, 69, 5383–5391. [Google Scholar] [CrossRef] [PubMed]
- Gay, L.J.; Felding-Habermann, B. Contribution of platelets to tumour metastasis. Nat. Rev. Cancer 2011, 11, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Wojtukiewicz, M.Z.; Sierko, E.; Hempel, D.; Tucker, S.C.; Honn, K.V. Platelets and cancer angiogenesis nexus. Cancer Metastasis Rev. 2017, 36, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.H.; Hsieh, J.C.; Yeh, K.Y.; Chen, E.Y.; Yang, S.W.; Huang, J.S. Prognostic nutritional index relevance in chemoradiotherapy for advanced oral cavity, oropharyngeal and hypopharyngeal cancer. Asia Pac. J. Clin. Nutr. 2018, 27, 996–1001. [Google Scholar] [PubMed]
- Rassouli, A.; Saliba, J.; Castano, R.; Hier, M.; Zeitouni, A.G. Systemic inflammatory markers as independent prognosticators of head and neck squamous cell carcinoma. Head Neck 2015, 37, 103–110. [Google Scholar] [CrossRef]
- Tangthongkum, M.; Tiyanuchit, S.; Kirtsreesakul, V.; Supanimitjaroenporn, P.; Sinkitjaroenchai, W. Platelet to lymphocyte ratio and red cell distribution width as prognostic factors for survival and recurrence in patients with oral cancer. Eur. Arch. Otorhinolaryngol. 2017, 274, 3985–3992. [Google Scholar] [CrossRef]
- Mao, Y.; Fu, Y.; Gao, Y.; Yang, A.; Zhang, Q. Platelet-to-lymphocyte ratio predicts long-term survival in laryngeal cancer. Eur. Arch. Otorhinolaryngol. 2018, 275, 553–559. [Google Scholar] [CrossRef]
- Zhang, W.; Ye, B.; Liang, W.; Ren, Y. Preoperative prognostic nutritional index is a powerful predictor of prognosis in patients with stage III ovarian cancer. Sci. Rep. 2017, 7, 9548. [Google Scholar] [CrossRef]
- Cho, U.; Park, H.S.; Im, S.Y.; Yoo, C.Y.; Jung, J.H.; Suh, Y.J. Prognostic value of systemic inflammatory markers and development of a nomogram in breast cancer. PLoS ONE 2018, 13, e0200936. [Google Scholar]
- Tan, B.H.; Brammer, K.; Randhawa, N.; Welch, N.T.; Parsons, S.L.; James, E.J. Sarcopenia is associated with toxicity in patients undergoing neo-adjuvant chemotherapy for oesophago-gastric cancer. Eur. J. Surg. Oncol. 2015, 41, 333–338. [Google Scholar] [CrossRef]
- Reijnierse, E.M.; Trappenburg, M.C.; Leter, M.J.; Sipila, S.; Stenroth, L.; Narici, M.V. Serum albumin and muscle measures in a cohort of healthy young and old participants. Age 2015, 37, 88. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Kritchevsky, S.B.; Newman, A.B.; Goodpaster, B.H.; Tylavsky, F.A.; Nevitt, M.C. Lower serum albumin concentration and change in muscle mass: The Health, Aging and Body Composition Study. Am. J. Clin. Nutr. 2005, 82, 531–537. [Google Scholar] [PubMed]
- Liaw, F.Y.; Huang, C.F.; Chen, W.L.; Wu, L.W.; Peng, T.C.; Chang, Y.W. Higher Platelet-to-Lymphocyte Ratio Increased the Risk of Sarcopenia in the Community-Dwelling Older Adults. Sci. Rep. 2017, 7, 16609. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhang, W.; Huang, Y.; Chen, W.; Wu, R.; Chen, X. Sarcopenia is associated with the neutrophil/lymphocyte and platelet/lymphocyte ratios in operable gastric cancer patients: A prospective study. Cancer Manag. Res. 2018, 10, 4935–4944. [Google Scholar] [CrossRef] [PubMed]
- Couch, M.E.; Dittus, K.; Toth, M.J.; Willis, M.S.; Guttridge, D.C.; George, J.R. Cancer cachexia update in head and neck cancer: Pathophysiology and treatment. Head Neck 2015, 37, 1057–1072. [Google Scholar] [CrossRef]
- Pin, F.; Couch, M.E.; Bonetto, A. Preservation of muscle mass as a strategy to reduce the toxic effects of cancer chemotherapy on body composition. Curr. Opin. Support. Palliat. Care. 2018, 12, 420–426. [Google Scholar] [CrossRef]
- Pin, F.; Barreto, R.; Couch, M.E.; Bonetto, A.; O’Connell, T.M. Cachexia induced by cancer and chemotherapy yield distinct perturbations to energy metabolism. J. Cachexia Sarcopenia Muscle 2019, 10, 140–154. [Google Scholar] [CrossRef]
- Lonbro, S.; Dalgas, U.; Primdahl, H.; Johansen, J.; Nielsen, J.L.; Overgaard, J. Lean body mass and muscle function in head and neck cancer patients and healthy individuals--results from the DAHANCA 25 study. Acta Oncol. 2013, 52, 1543–1551. [Google Scholar] [CrossRef]
- Orell-Kotikangas, H.; Osterlund, P.; Makitie, O.; Saarilahti, K.; Ravasco, P.; Schwab, U. Cachexia at diagnosis is associated with poor survival in head and neck cancer patients. Acta Otolaryngol. 2017, 137, 778–785. [Google Scholar] [CrossRef]
- Noronha, V.; Joshi, A.; Patil, V.M.; Agarwal, J.; Ghosh-Laskar, S.; Budrukkar, A. Once-a-Week Versus Once-Every-3-Weeks Cisplatin Chemoradiation for Locally Advanced Head and Neck Cancer: A Phase III Randomized Noninferiority Trial. J. Clin. Oncol. 2018, 36, 1064–1072. [Google Scholar] [CrossRef]
| Variables | Total |
|---|---|
| Numbers (%) or Mean ± SD | |
| Included patient No. | 113 (100) |
| Clinicopathological data | |
| Age (years) | 53.6 ± 8.3 |
| Gender (male : female) | 110 (97.3) : 3 (2.7) |
| Smoking (no : yes) | 8 (7.1) : 105 (92.9) |
| Alcohol (no : yes) | 28 (24.8) : 85 (75.2) |
| Betel nut (no : yes) | 42 (37.2) : 71 (62.8) |
| ECOG performance status (0 : 1 : 2) | 9 (8.0) : 98 (86.7) : 6 (5.3) |
| HN-CCI (0 : 1 : 2) | 72 (63.7) : 33 (29.2) : 8 (7.1) |
| Location (oral cavity : oropharynx : hypopharynx : larynx : unknown primary) | 56 (49.6) : 25 (22.1) : 20 (17.7) : 10 (8.8) : 2 (1.8) |
| Histologic grade (well : moderate : poor : unknown) | 7 (6.2) : 74 (65.5) : 21 (18.6) : 11 (9.7) |
| Tumor (T0 : T1 : T2 : T3 : T4) | 2 (1.8) : 4 (3.5) : 20 (17.7) : 17 (15.0) : 70 (62.0) |
| Lymph node (N0 : N1 : N2 : N3) | 20 (17.7) : 21 (18.6) : 64 (56.6) : 8 (7.1) |
| Stage, AJCC 7th edition (III : IVA : IVB) | 12 (10.6) : 79 (69.9) : 22 (19.5) |
| Neoadjuvant chemotherapy (no : yes) | 81 (71.7) : 32 (28.3) |
| Curative surgery (no : yes) | 61 (54.0) : 52 (46.0) |
| Cisplatin dose (mg/m2) | 216 ± 62 |
| Radiation | |
| dose (Gy) | 65.3 ± 10.9 |
| days to finish | 49.4 ± 11.2 |
| Laboratory data | |
| Hemoglobin (g/dL) | 11.9 ± 1.7 |
| WBC count (× 109/L) | 7.4 ± 2.6 |
| Platelet count (× 109/L) | 305.3 ± 130.0 |
| Total lymphocyte count (× 109/L) | 1.7 ± 0.6 |
| Albumin (g/dL) | 3.9 ± 0.4 |
| Nutrition Index | |
| BMI (kg/m2) | 22.9 ± 4.3 |
| BWL (< 10% : ≥ 10%) | 84 (74.3) : 29 (25.7) |
| PNI | 47.9 ± 5.9 |
| NRI (no : mild : moderate : severe) | 68 (60.2) : 11 (9.7) : 27 (23.9) : 7 (6.2) |
| PG-SGA (well (0–3) : moderate (4–8) : severe (≥ 9)) | 88 (77.9) : 24 (21.2) : 1 (0.9) |
| Inflammatory biomarkers | |
| NLR | 3.4 ± 2.9 |
| PLR | 212.3 ± 149.5 |
| LMR | 3.6 ± 2.4 |
| DXA-derived LBMI (kg/m2) | 15.9 ± 2.0 |
| 1-year all-cause mortality rate | 30.9% |
| Variables | Total | |||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| HR (95% CI) | p | HR (95% CI) | p | |
| Age (< 65 y vs. ≥ 65 y) | 1.246 (0.381–4.069) | 0.716 | ||
| Gender (male vs. female) | 1.094 (0.405–2.958) | 0.859 | ||
| Smoking (no vs. yes) | 1.255 (0.301–5.231) | 0.755 | ||
| Alcohol (no vs. yes) | 1.754 (0.728–4.225) | 0.210 | ||
| Betel nut (no vs. yes) | 1.878 (0.879–4.009) | 0.104 | ||
| ECOG PS (< 2 vs. ≥2) | 3.208 (1.123–9.163) | 0.029 | ||
| HN-CCI (< 1 vs. ≥ 1) | 0.564 (0.264–1.204) | 0.139 | ||
| Location (oral cavity vs. non-oral cavity cancer) | 0.377 (0.184–0.771) | 0.008 | ||
| Histologic grade (well vs. moderate/poor) | 0.481 (0.169–1.366) | 0.169 | ||
| Tumor (T0–T2 vs. T3–T4) | 2.576 (0.909–7.300) | 0.075 | ||
| Lymph node (N0–N1 vs. N2–N3) | 1.480 (0.711–3.081) | 0.295 | ||
| Stage (III vs. IV) | 1.315 (0.403–4.295) | 0.650 | ||
| Neoadjuvant chemotherapy (no vs. yes) | 0.950 (0.456–1.978) | 0.891 | ||
| Curative surgery (no vs. yes) | 2.295 (1.155–4.562) | 0.018 | ||
| Cisplatin dose (< 160 vs. ≥ 160 mg/m2) | 0.893 (0.347–2.303) | 0.815 | ||
| Radiation Dose (< 66 vs. ≥ 66 Gy) Days (< 50 vs. ≥ 50 d) | 0.806 (0.413–1.575) 1.320 (0.680–2.561) | 0.528 0.412 | ||
| Hemoglobin (< 10 vs. ≥ 10 g/dL) | 0.423 (0.192–0.931) | 0.033 | ||
| WBC count (< 11.0 vs. ≥ 11.0 × 109/L) | 1.472 (0.611–3.548) | 0.389 | ||
| Platelet count (< 400 vs. ≥ 400 × 109/L) | 1.975 (0.981–3.976) | 0.057 | ||
| TLC (< 1.5 vs. ≥ 1.5 × 109/L) | 0.297 (0.149–0.590) | 0.001 | ||
| Albumin (< 3.5 vs. ≥ 3.5 g/dL) | 0.294 (0.141–0.617) | 0.001 | ||
| BMI (< 18.5 vs. ≥ 18.5 kg/m2) | 0.296 (0.144–0.608) | 0.001 | ||
| BWL (< 10% vs. ≥ 10%) | 1.640 (0.815–3.298) | 0.165 | ||
| PNI (< 46.8 vs. ≥ 46.8) * | 0.199 (0.097–0.409) | <0.001 | 0.276 (0.131–0.582) | 0.001# |
| NRI (< 97.5 vs. ≥ 97.5) | 0.387 (0.199–0.753) | 0.005 | ||
| PG-SGA (well vs. moderate/severe) | 1.791 (0.876–3.661) | 0.110 | ||
| NLR (< 3.5 vs. ≥ 3.5) * | 4.078 (2.089–7.962) | <0.001 | ||
| PLR (< 191 vs. ≥ 191) * | 4.585 (2.238–9.393) | <0.001 | 3.205 (1.520–6.757) | 0.002# |
| LMR (< 2.1 vs. ≥ 2.1) * | 0.384 (0.193–0.764) | 0.006 | ||
| LBMI (< 14.4 vs. ≥ 14.4 kg/m2) † | 0.496 (0.249–0.986) | 0.045 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ling, H.H.; Yeh, K.-Y.; Ng, S.-H.; Wang, C.-H.; Lai, C.-H.; Wu, T.-H.; Chang, P.-H.; Chou, W.-C.; Chen, F.-P.; Lin, Y.-C. Determining Malnutrition Assessment Criteria to Predict One-Year Mortality for Locally Advanced Head and Neck Cancer Patients Undergoing Concurrent Chemoradiotherapy. Nutrients 2020, 12, 836. https://doi.org/10.3390/nu12030836
Ling HH, Yeh K-Y, Ng S-H, Wang C-H, Lai C-H, Wu T-H, Chang P-H, Chou W-C, Chen F-P, Lin Y-C. Determining Malnutrition Assessment Criteria to Predict One-Year Mortality for Locally Advanced Head and Neck Cancer Patients Undergoing Concurrent Chemoradiotherapy. Nutrients. 2020; 12(3):836. https://doi.org/10.3390/nu12030836
Chicago/Turabian StyleLing, Hang Huong, Kun-Yun Yeh, Shu-Hang Ng, Cheng-Hsu Wang, Chien-Hong Lai, Tsung-Han Wu, Pei-Hung Chang, Wen-Chi Chou, Fang-Ping Chen, and Yu-Ching Lin. 2020. "Determining Malnutrition Assessment Criteria to Predict One-Year Mortality for Locally Advanced Head and Neck Cancer Patients Undergoing Concurrent Chemoradiotherapy" Nutrients 12, no. 3: 836. https://doi.org/10.3390/nu12030836
APA StyleLing, H. H., Yeh, K.-Y., Ng, S.-H., Wang, C.-H., Lai, C.-H., Wu, T.-H., Chang, P.-H., Chou, W.-C., Chen, F.-P., & Lin, Y.-C. (2020). Determining Malnutrition Assessment Criteria to Predict One-Year Mortality for Locally Advanced Head and Neck Cancer Patients Undergoing Concurrent Chemoradiotherapy. Nutrients, 12(3), 836. https://doi.org/10.3390/nu12030836






