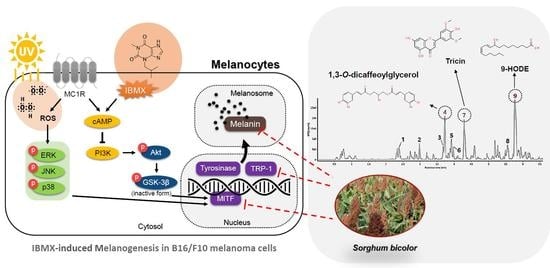
Anti-Melanogenic Effect of Ethanolic Extract of Sorghum bicolor on IBMX–Induced Melanogenesis in B16/F10 Melanoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Preparation
2.3. In Vitro Antioxidant Activity
2.3.1. Total Phenolic Contents (TPC)
2.3.2. Radical Scavenging Activity
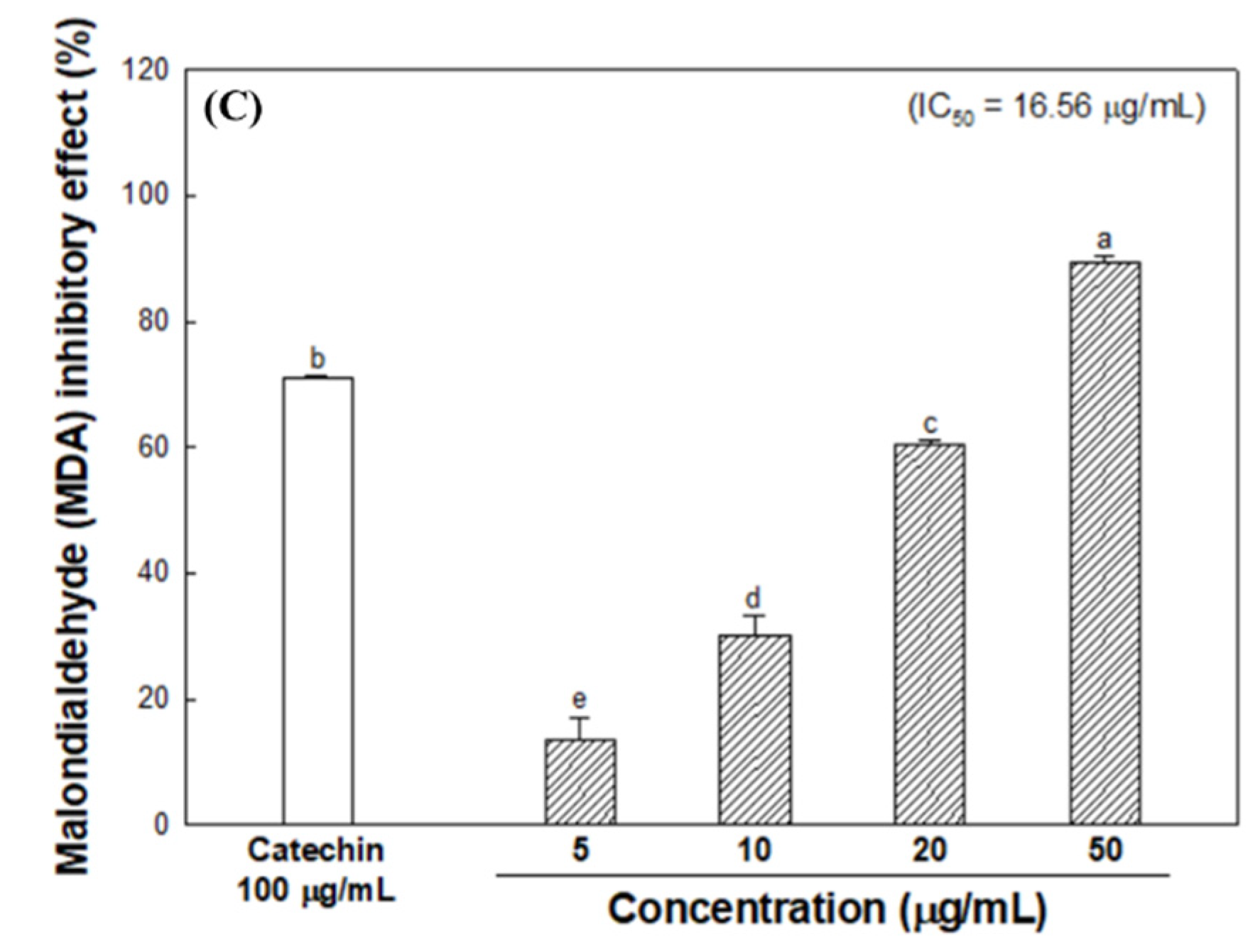
2.3.3. Inhibitory Effect on Lipid Peroxidation
2.4. Tyrosinase Inhibitory Effect
2.5. α-Glucosidase Inhibitory Effect
2.6. Cell Viability Assay
2.7. Measurement of Cellular Melanin Contents
2.8. Western Blot Analysis
2.9. UPLC-IMS-QTOF/MS2 Analysis
2.10. Statistical Analysis
3. Results
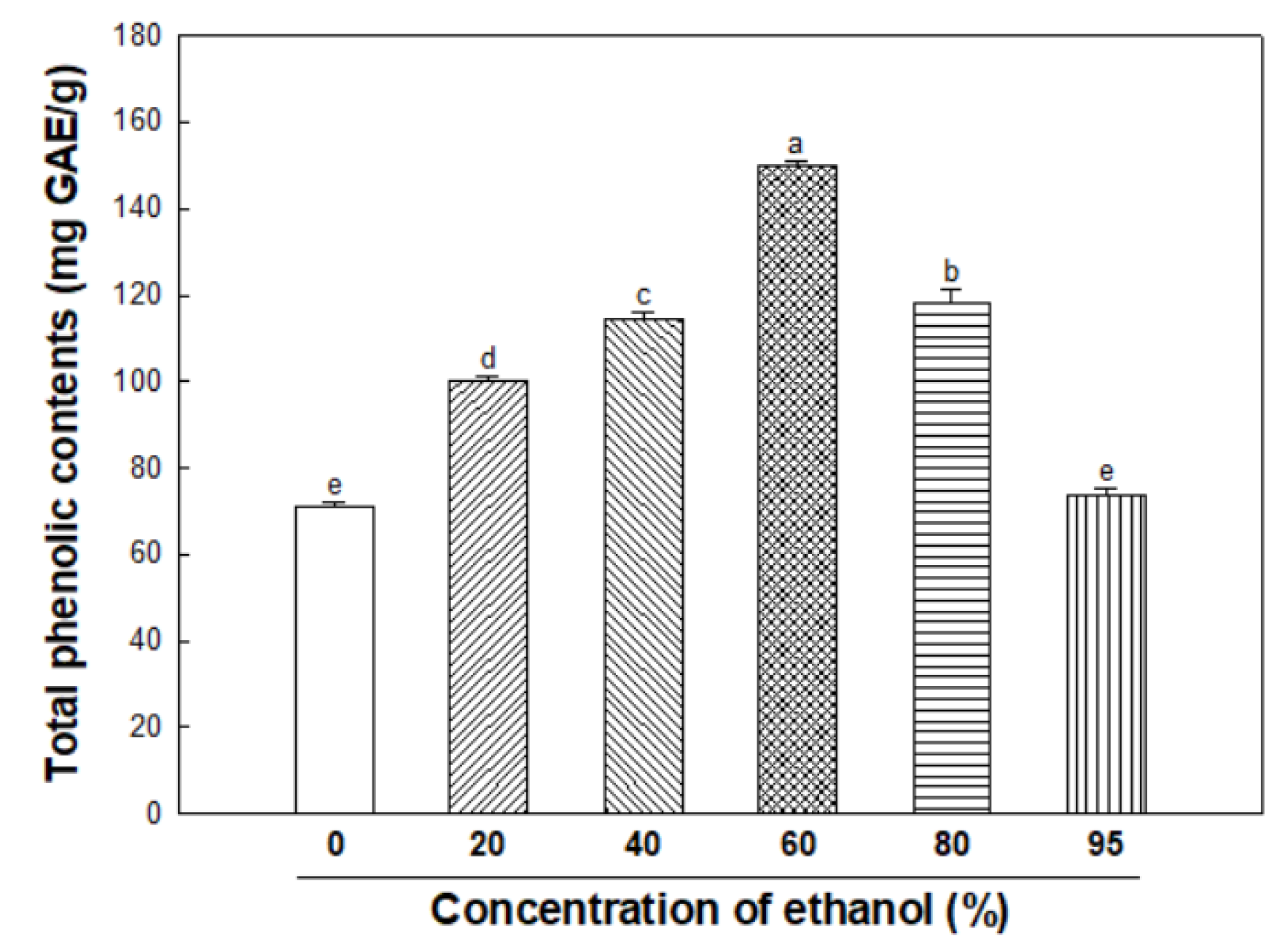
3.1. Total Phenolic Contents
3.2. Radical Scavenging Activity
3.3. Inhibitory Effect of Melanogenesis-Mediated Enzymes
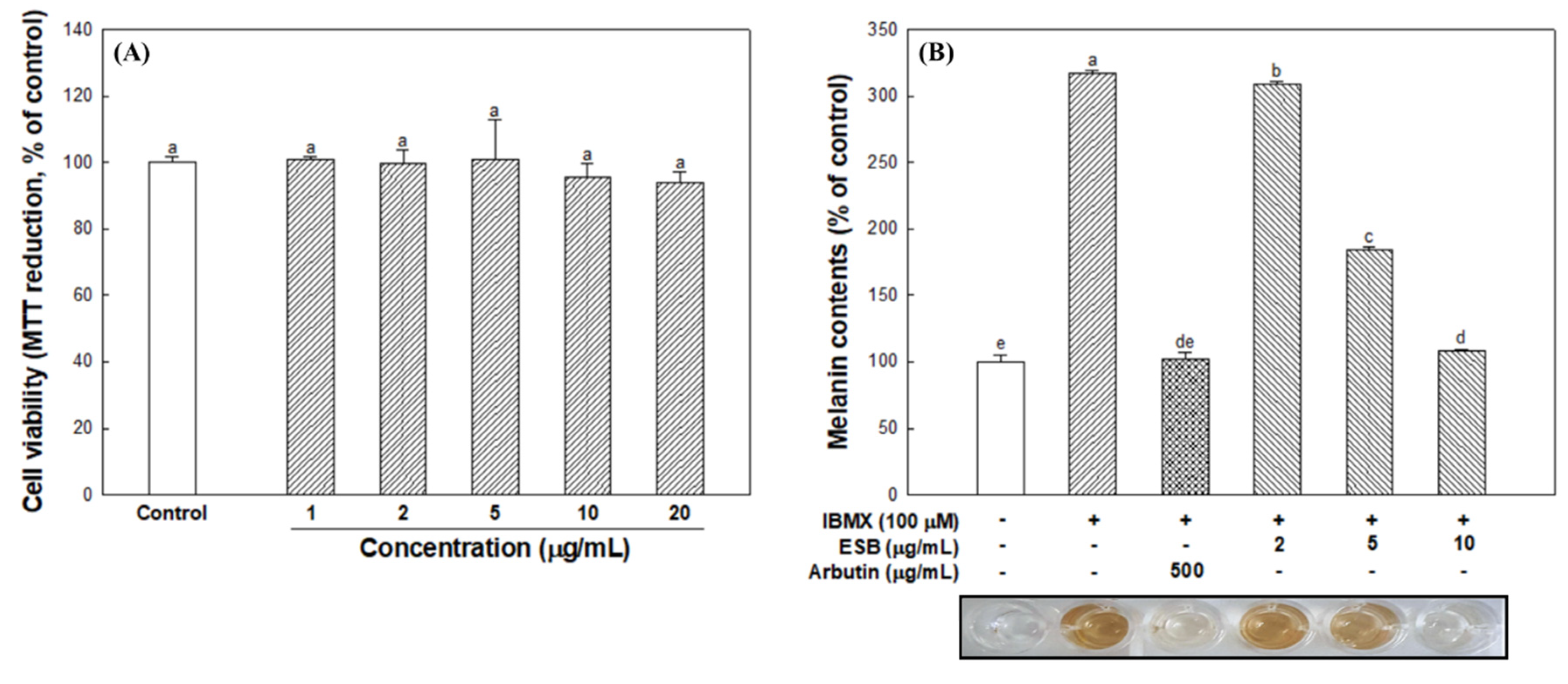
3.4. Cell Viability and Cellular Melanin Synthesis
3.5. Melanogenesis Pathway in B16/F10 Melanoma Cells
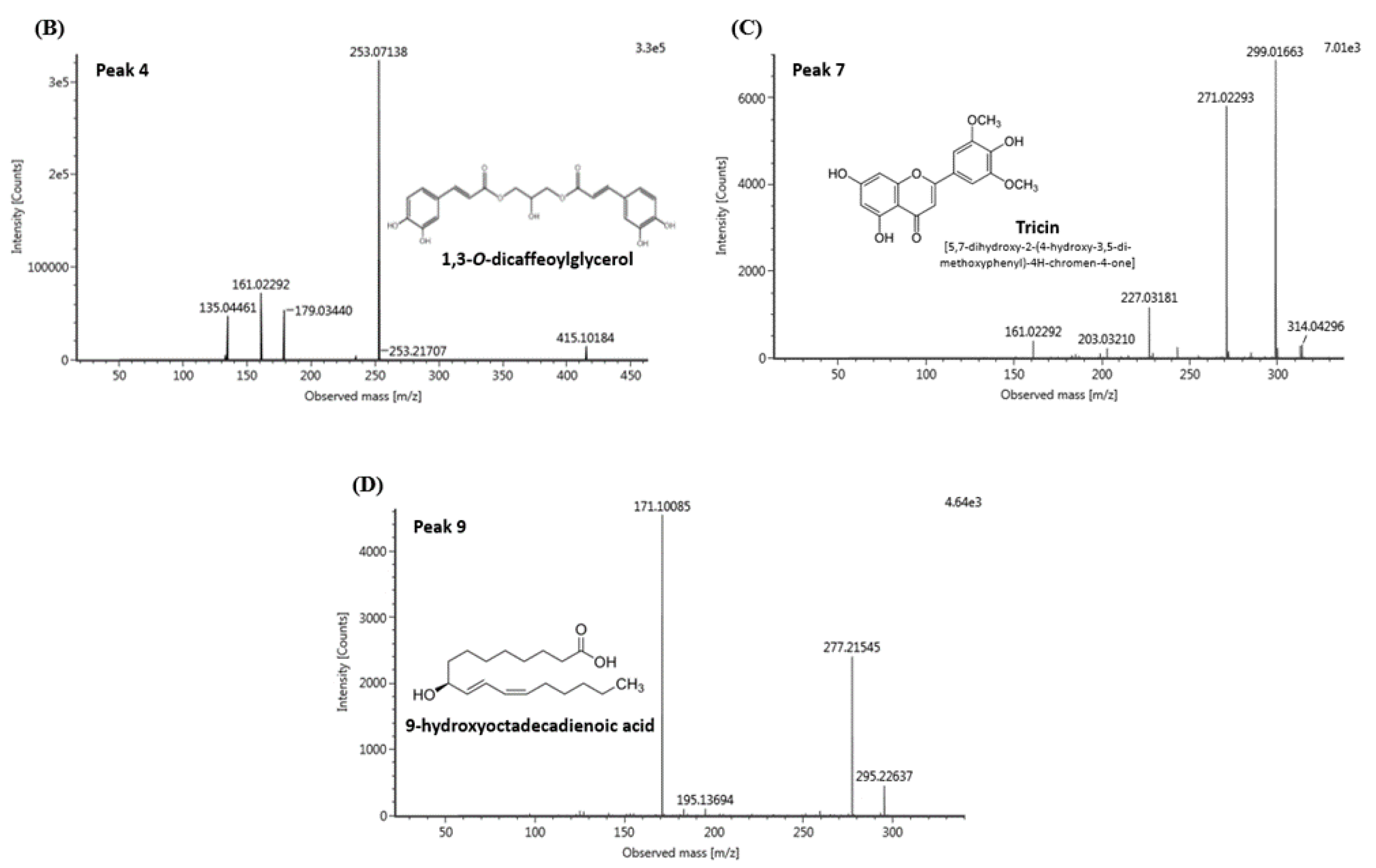
3.6. UPLC-IMS-QTOF/MS2 Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lin, Y.S.; Wu, W.C.; Lin, S.Y.; Hou, W.C. Glycine hydroxamate inhibits tyrosinase activity and melanin contents through downregulating cAMP/PKA signaling pathways. Amino Acids 2015, 47, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Garcia, E. Skin protection against UV light by dietary antioxidants. Food Funct. 2014, 5, 1994–2003. [Google Scholar] [CrossRef] [PubMed]
- Agar, N.; Young, A.R. Melanogenesis: A photoprotective response to DNA damage? Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2005, 571, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Schallreuter, K.U.; Wazir, U.; Kothari, S.; Gibbons, N.C.; Moore, J.; Wood, J.M. Human phenylalanine hydroxylase is activated by H2O2: A novel mechanism for increasing the L-tyrosine supply for melanogenesis in melanocytes. Biochem. Biophys. Res. Commun. 2004, 322, 88–92. [Google Scholar] [CrossRef]
- Draelos, Z.D. Skin lightening preparations and the hydroquinone controversy. Dermatol. Ther. 2007, 20, 308–313. [Google Scholar] [CrossRef]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef]
- Byun, E.B.; Song, H.Y.; Mushtaq, S.; Kim, H.M.; Kang, J.A.; Yang, M.S.; Sung, N.Y.; Jang, B.S.; Byun, E.H. Gamma-Irradiated luteolin inhibits 3-Isobutyl-1-Methylxanthine-induced melanogenesis through the regulation of CREB/MITF, PI3K/Akt, and ERK pathways in B16BL6 melanoma cells. J. Med. Food. 2017, 20, 812–819. [Google Scholar] [CrossRef]
- Dillon, S.L.; Shapter, F.M.; Henry, R.J.; Cordeiro, G.; Izquierdo, L.; Lee, L.S. Domestication to crop improvement: Genetic resources for Sorghum and Saccharum (Andropogoneae). Ann. Bot. 2007, 100, 975–989. [Google Scholar] [CrossRef]
- Svensson, L.; Sekwati-Monang, B.; Lutz, D.L.; Schieber, A.; Ganzle, M.G. Phenolic acids and flavonoids in nonfermented and fermented red sorghum (Sorghum bicolor (L.) Moench). J. Agric. Food. Chem. 2010, 58, 9214–9220. [Google Scholar] [CrossRef]
- Sikwese, F.E.; Duodu, K.G. Antioxidant effect of a crude phenolic extract from sorghum bran in sunflower oil in the presence of ferric ions. Food Chem. 2007, 104, 324–331. [Google Scholar] [CrossRef]
- Shen, R.L.; Zhang, W.L.; Dong, J.L.; Ren, G.X.; Chen, M. Sorghum resistant starch reduces adiposity in high-fat diet-induced overweight and obese rats via mechanisms involving adipokines and intestinal flora. Food Agric. Immunol. 2015, 26, 120–130. [Google Scholar] [CrossRef]
- Kim, J.M.; Park, Y.S. Anti-diabetic effect of sorghum extract on hepatic gluconeogenesis of streptozotocin-induced diabetic rats. Nutr. Metab. 2012, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, S.H.; Chung, I.M.; Park, Y.S. Sorghum extract exerts an anti-diabetic effect by improving insulin sensitivity via PPAR-γ in mice fed a high-fat diet. Nutr. Res. Pract. 2012, 6, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Burdette, A.; Garner, P.L.; Mayer, E.P.; Hargrove, J.L.; Hartle, D.K.; Greenspan, P. Anti-inflammatory activity of select sorghum (Sorghum bicolor) brans. J. Med. Food 2010, 13, 879–887. [Google Scholar] [CrossRef]
- Wu, G.; Johnson, S.K.; Bornman, J.F.; Bennett, S.J.; Clarke, M.W.; Singh, V.; Fang, Z. Growth temperature and genotype both play important roles in sorghum grain phenolic composition. Sci. Rep. 2016, 6, 21835. [Google Scholar] [CrossRef]
- Ma, C.; Xiao, S.Y.; Li, Z.G.; Wang, W.; Du, L.J. Characterization of active phenolic components in the ethanolic extract of Ananas comosus L. leaves using high-performance liquid chromatography with diode array detection and tandem mass spectrometry. J. Chromatogr. A 2007, 1165, 39–44. [Google Scholar] [CrossRef]
- Steingass, C.B.; Glock, M.P.; Schweiggert, R.M.; Carle, R. Studies into the phenolic patterns of different tissues of pineapple (Ananas comosus [L.] Merr.) infructescence by HPLC-DAD-ESI-MSn and GC-MS analysis. Anal. Bioanal. Chem. 2015, 407, 6463–6479. [Google Scholar] [CrossRef]
- Wu, G.; Bennett, S.J.; Bornman, J.F.; Clarke, M.W.; Fang, Z.; Johnson, S.K. Phenolic profile and content of sorghum grains under different irrigation managements. Food Res. Int. 2017, 97, 347–355. [Google Scholar] [CrossRef]
- Kang, J.; Price, W.E.; Ashton, J.; Tapsell, L.C.; Johnson, S. Identification and characterization of phenolic compounds in hydromethanolic extracts of sorghum wholegrains by LC-ESI-MSn. Food Chem. 2016, 211, 215–226. [Google Scholar] [CrossRef]
- Kloos, D.; Lingeman, H.; Mayboroda, O.A.; Deelder, A.M.; Niessen, W.M.A.; Giera, M. Analysis of biologically-active, endogenous carboxylic acids based on chromatography-mass spectrometry. Trac-Trends Anal. Chem. 2014, 61, 17–28. [Google Scholar] [CrossRef]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2005, 579, 200–213. [Google Scholar] [CrossRef]
- Jeong, C.H.; Choi, S.G.; Heo, H.J. Analysis of nutritional components and evaluation of functional activities of Sasa borealis leaf tea. Korean J. Food Sci. Technol. 2008, 40, 586–592. [Google Scholar]
- Wang, K.H.; Lin, R.D.; Hsu, F.L.; Huang, Y.H.; Chang, H.C.; Huang, C.Y.; Lee, M.H. Cosmetic applications of selected traditional Chinese herbal medicines. J. Ethnopharmacol. 2006, 106, 353–359. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Woo, K.S.; Seo, H.I.; Lee, Y.H.; Kim, H.Y.; Ko, J.Y.; Song, S.B.; Lee, J.S.; Jung, K.Y.; Nam, M.H.; Oh, I.S.; et al. Antioxidant compounds and antioxidant activities of sweet potatoes with cultivated conditions. J. Korean Soc. Food Sci. Nutr. 2012, 41, 519–525. [Google Scholar] [CrossRef][Green Version]
- Maresca, V.; Flori, E.; Briganti, S.; Mastrofrancesco, A.; Fabbri, C.; Mileo, A.M.; Paggi, M.G.; Picardo, M. Correlation between melanogenic and catalase activity in in vitro human melanocytes: A synergic strategy against oxidative stress. Pigment Cell Melanoma Res. 2008, 21, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Park, S.A.; Park, J.; Park, C.I.; Jie, Y.J.; Hwang, Y.C.; Kim, Y.H.; Jeon, S.H.; Lee, H.M.; Ha, J.H.; Kim, K.J.; et al. Cellular antioxidant activity and whitening effects of Dendropanax morbifera leaf extracts. Microbiol. Biotechnol. Lett. 2013, 41, 407–415. [Google Scholar] [CrossRef]
- Choi, H.K.; Lim, Y.S.; Kim, Y.S.; Park, S.Y.; Lee, C.H.; Hwang, K.W.; Kwon, D.Y. Free-radical-scavenging and tyrosinase-inhibition activities of Cheonggukjang samples fermented for various times. Food Chem. 2008, 106, 564–568. [Google Scholar] [CrossRef]
- Im, D.Y.; Lee, K.I. Antioxidative and antibacterial activity and tyrosinase inhibitory activity of the extract and fractions from Taraxacum coreanum Nakai. Korean J. Med. Crop Sci. 2011, 19, 238–245. [Google Scholar] [CrossRef]
- Mai, T.T.; Thu, N.N.; Tien, P.G.; Van Chuyen, N. Alpha-glucosidase inhibitory and antioxidant activities of Vietnamese edible plants and their relationships with polyphenol contents. J. Nutr. Sci. Vitaminol. 2007, 53, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Gillbro, J.M.; Olsson, M.J. The melanogenesis and mechanisms of skin-lightening agents–existing and new approaches. Int. J. Cosmet. Sci. 2011, 33, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Ahn, S.M.; Chang, H.K.; Cho, N.S.; Joo, K.M.; Lee, B.G.; Chang, I.S.; Hwang, J.S. Influence of N-glycan processing disruption on tyrosinase and melanin synthesis in HM3KO melanoma cells. Exp. Dermatol. 2007, 16, 110–117. [Google Scholar] [CrossRef]
- Ando, H.; Kondoh, H.; Ichihashi, M.; Hearing, V.J. Approaches to identify inhibitors of melanin biosynthesis via the quality control of tyrosinase. J. Invest. Dermatol. 2007, 127, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Jo, Y.H.; Liu, Q.; Ahn, J.H.; Hong, I.P.; Han, S.M.; Hwan, B.Y.; Lee, M.K. Optimization of extraction condition of bee pollen using response surface methodology: Correlation between anti-melanogenesis, antioxidant activity, and phenolic content. Molecules 2015, 20, 19764–19774. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Chung, Y.H.; Park, J.K.; Kim, S.J.; Jung, D.H.; Choi, H.G. Inhibitory effects of Papenfusiella kuromo ethanol extracts on melanin formation in B16F10 murine melanoma cells. J. Korean Beauty Soc. 2019, 25, 156–161. [Google Scholar]
- Han, E.B.; Chang, B.Y.; Kim, D.S.; Cho, H.K.; Kim, S.Y. Melanogenesis inhibitory effect of aerial part of Pueraria thunbergiana in vitro and in vivo. Arch. Dermatol. Res. 2015, 307, 57–72. [Google Scholar] [CrossRef]
- Huang, H.C.; Chang, S.J.; Wu, C.Y.; Ke, H.J.; Chang, T.M. [6]-Shogaol inhibits α-MSH-induced melanogenesis through the acceleration of ERK and PI3K/Akt-mediated MITF degradation. Biomed. Res. Int. 2014, 2014, 842569. [Google Scholar] [CrossRef]
- Choi, M.H.; Jo, H.G.; Yang, J.; Ki, S.; Shin, H.J. Antioxidative and anti-melanogenic activities of bamboo stems (Phyllostachys nigra variety henosis) via PKA/CREB-mediated MITF downregulation in B16F10 melanoma cells. Int. J. Mol. Sci. 2018, 19, 409. [Google Scholar] [CrossRef]
- Yoo, D.H.; Joo, D.H.; Lee, S.Y.; Lee, J.Y. Antioxidant effect of Nelumbo nucifera G. leaf extract and inhibition of MITF, TRP-1, TRP-2, and tyrosinase expression in a B16F10 melanoma cell line. Korean J. Life Sci. 2015, 25, 1115–1123. [Google Scholar] [CrossRef]
- Weindl, G.; Schäfer-Korting, M.; Schaller, M.; Korting, H.C. Peroxisome proliferator-activated receptors and their ligands. Drugs 2005, 65, 1919–1934. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.S.; Kim, E.Y.; Kim, W.J.; Kim, W.K.; Kim, C.M. Antioxidant constituents from Setaria viridis. Arch. Pharm. Res. 2002, 25, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Sakai, A.; Watanabe, K.; Koketsu, M.; Akuzawa, K.; Yamada, R.; Li, Z.; Sadanari, H.; Matsubara, K.; Murayama, T. Anti-human cytomegalovirus activity of constituents from Sasa albo-marginata (Kumazasa in Japan). Antivir. Chem. Chemother. 2008, 19, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Sale, S.; Schmid, R.; Britton, R.G.; Brown, K.; Steward, W.P.; Gescher, A.J. Flavones as colorectal cancer chemopreventive agents—phenol-O-methylation enhances efficacy. Cancer Prev. Res. 2009, 2, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Li, L.; Hu, S.Q. Molecular inhibitory mechanism of tricin on tyrosinase. Spectroc. Acta Pt A-Molec. Biomolec. Spectr. 2013, 107, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.X.; Irino, N.; Kondo, R. Melanin biosynthesis inhibitory activity of a compound isolated from young green barley (Hordeum vulgare L.) in B16 melanoma cells. J. Nat. Med. 2015, 69, 427–431. [Google Scholar] [CrossRef]
- Korkina, L.G. Phenylpropanoids as naturally occurring antioxidants: From plant defense to human health. Cell Mol. Biol. 2007, 53, 15–25. [Google Scholar]
- An, S.M.; Lee, S.I.; Choi, S.W.; Moon, S.W.; Boo, Y.C. p-Coumaric acid, a constituent of Sasa quelpaertensis Nakai, inhibits cellular melanogenesis stimulated by α-melanocyte stimulating hormone. Br. J. Dermatol. 2008, 159, 292–299. [Google Scholar] [CrossRef]





| Pearson Correlation | |||
|---|---|---|---|
| Tyrosinase Inhibitory Effect | α-Glucosidase Inhibitory Effect | ||
| L-tyrosine | L-DOPA | ||
| ABTS radical scavenging activity | 0.900 | 0.943* | 0.792 |
| DPPH radical scavenging activity | 0.718 | 0.952* | 0.937 |
| MDA inhibitory effect | 0.988 | 0.995 | 0.966* |
| No. | RT (min) | [M-H]- (m/z) | MS2 Fragments (m/z) | Proposed Compound |
|---|---|---|---|---|
| 1 | 2.38 | 625.19 | 407.11, 383.11, 221.06, 125.02, 89.02 | Unknown |
| 2 | 2.98 | 253.07 | 179.03, 161.02, 135.04 | 1-O-caffeoylglycerol |
| 3 | 3.68 | 415.10 | 253.07, 179.03, 135.04 | Dicaffeoylglycerides |
| 4 | 3.72 | 415.10 | 253.07, 179.03, 161.02, 135.04 | 1,3-O-dicaffeoylglycerol |
| 5 | 3.91 | 399.10 | 253.07, 179.03, 161.02, 135.04 | p-coumaroyl-caffeoylglycerol |
| 6 | 3.97 | 429.12 | 253.07, 193.05, 161.02, 134.03 | Feruloyl-caffeoylglycerol |
| 7 | 4.29 | 329.23 | 314.04, 299.01, 271.02 | Tricin |
| 8 | 5.55 | 315.25 | 297.24, 279.23 | Unknown |
| 9 | 5.77 | 295.22 | 277.21, 171.10 | 9-hydroxyoctadecadienoic acid (9-HODE) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, H.J.; Park, S.K.; Kang, J.Y.; Kim, J.M.; Yoo, S.K.; Heo, H.J. Anti-Melanogenic Effect of Ethanolic Extract of Sorghum bicolor on IBMX–Induced Melanogenesis in B16/F10 Melanoma Cells. Nutrients 2020, 12, 832. https://doi.org/10.3390/nu12030832
Han HJ, Park SK, Kang JY, Kim JM, Yoo SK, Heo HJ. Anti-Melanogenic Effect of Ethanolic Extract of Sorghum bicolor on IBMX–Induced Melanogenesis in B16/F10 Melanoma Cells. Nutrients. 2020; 12(3):832. https://doi.org/10.3390/nu12030832
Chicago/Turabian StyleHan, Hye Ju, Seon Kyeong Park, Jin Yong Kang, Jong Min Kim, Seul Ki Yoo, and Ho Jin Heo. 2020. "Anti-Melanogenic Effect of Ethanolic Extract of Sorghum bicolor on IBMX–Induced Melanogenesis in B16/F10 Melanoma Cells" Nutrients 12, no. 3: 832. https://doi.org/10.3390/nu12030832
APA StyleHan, H. J., Park, S. K., Kang, J. Y., Kim, J. M., Yoo, S. K., & Heo, H. J. (2020). Anti-Melanogenic Effect of Ethanolic Extract of Sorghum bicolor on IBMX–Induced Melanogenesis in B16/F10 Melanoma Cells. Nutrients, 12(3), 832. https://doi.org/10.3390/nu12030832