The Role of Post-Ingestive Feedback in the Development of an Enhanced Appetite for the Orosensory Properties of Glucose over Fructose in Rats
Abstract
1. Introduction
2. Experiment 1
2.1. Subjects
2.2. Surgery
2.3. Stimuli
2.4. Training Apparatus
2.5. Oral—IG Training Procedures
2.6. Brief Access Testing Apparatus
2.7. Brief Access Test Procedures
2.8. Licking Microstructure Test Procedures
2.9. Data Analyses
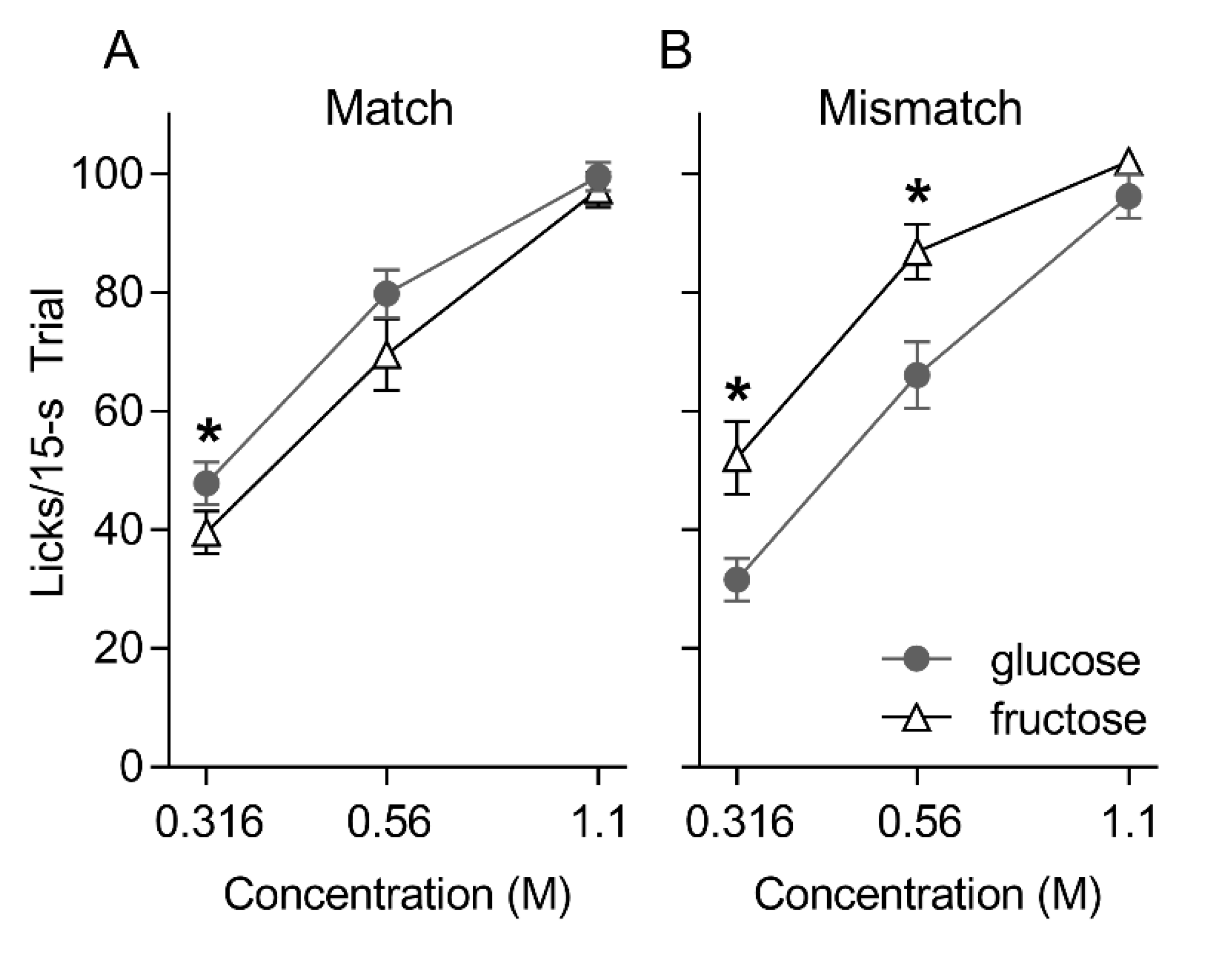
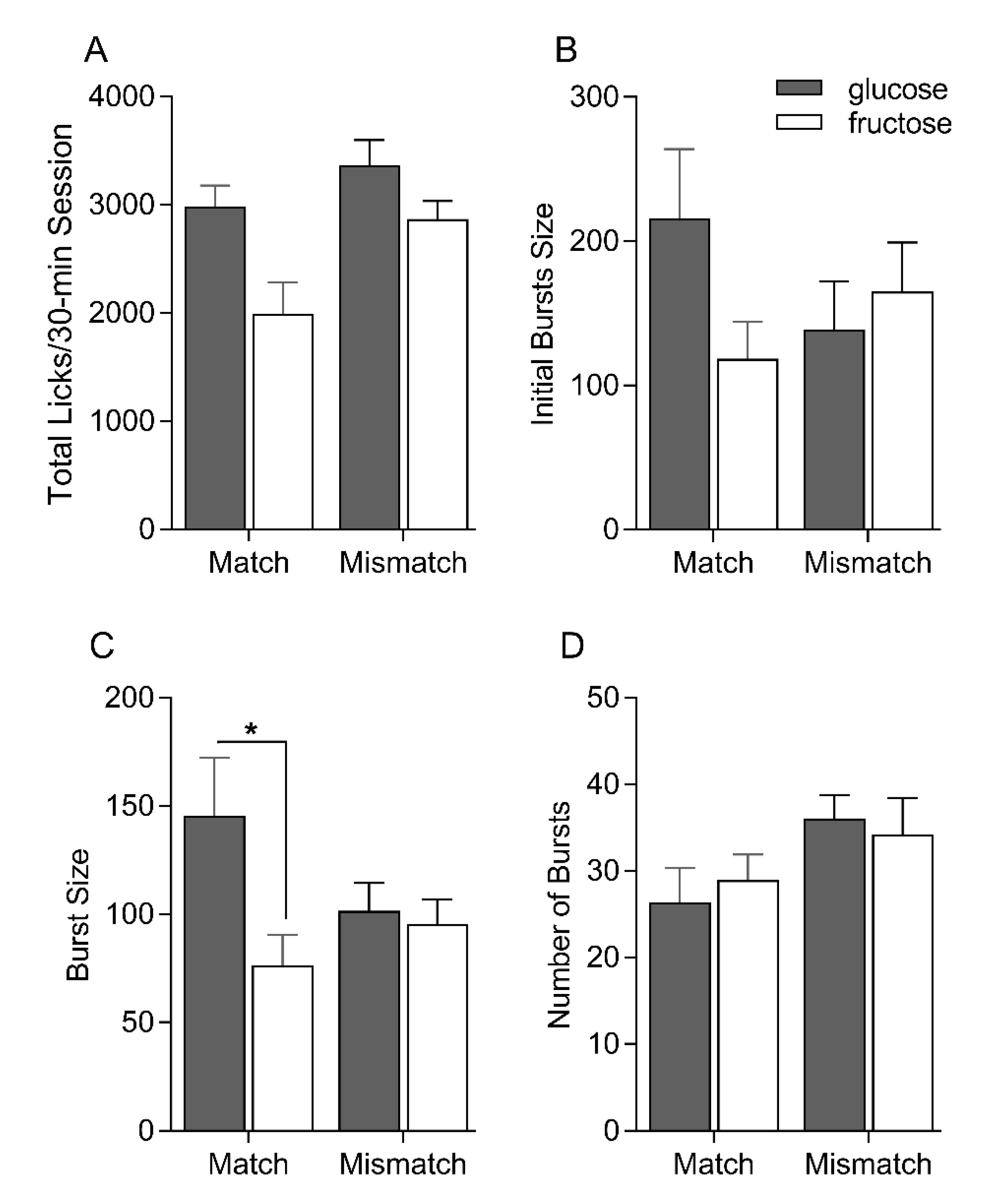
3. Results
3.1. Brief Access Taste Tests
3.2. Single-Bottle Intake Tests
4. Experiment 2
4.1. Subjects
4.2. Training and Testing Procedures
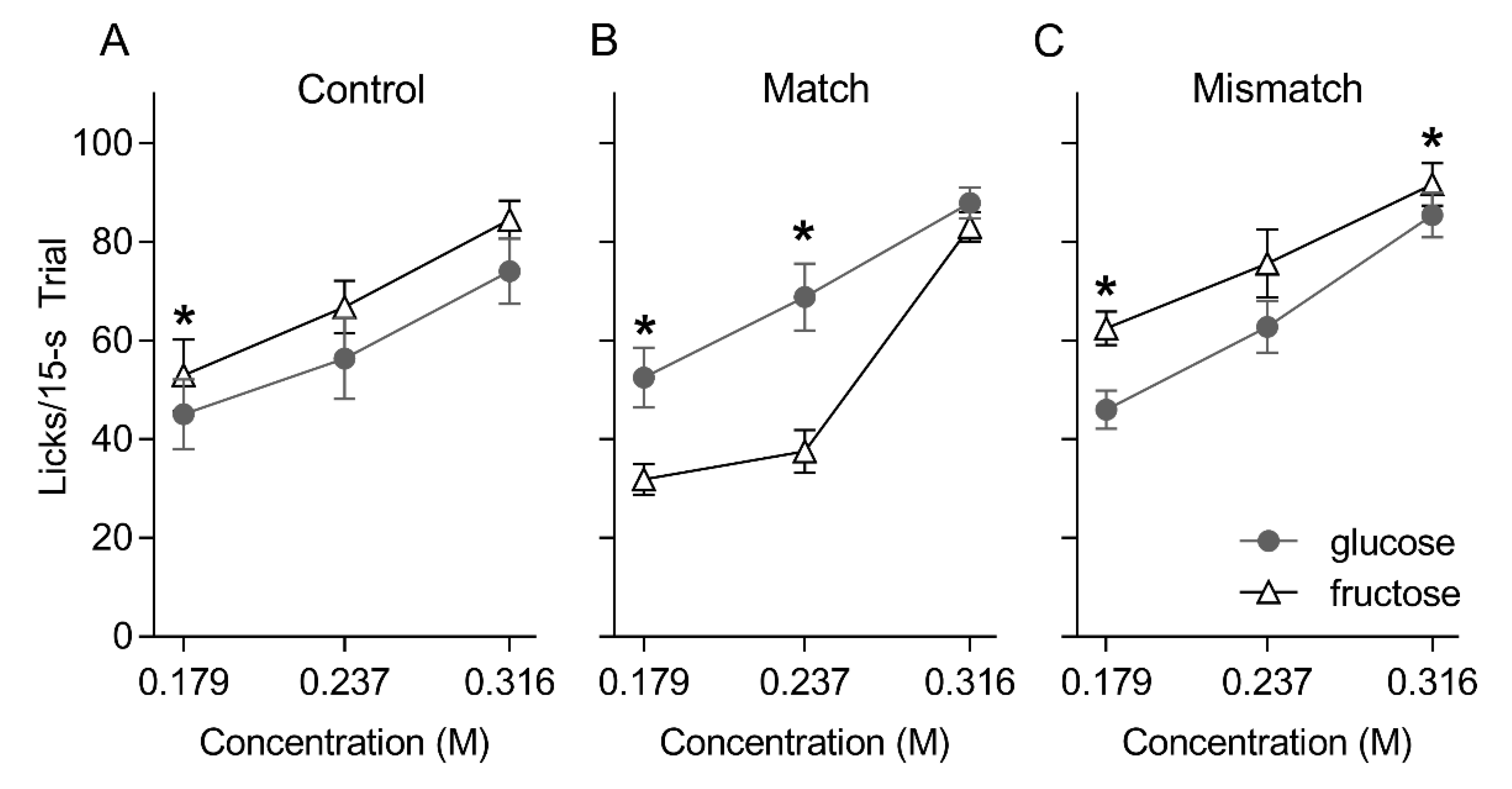
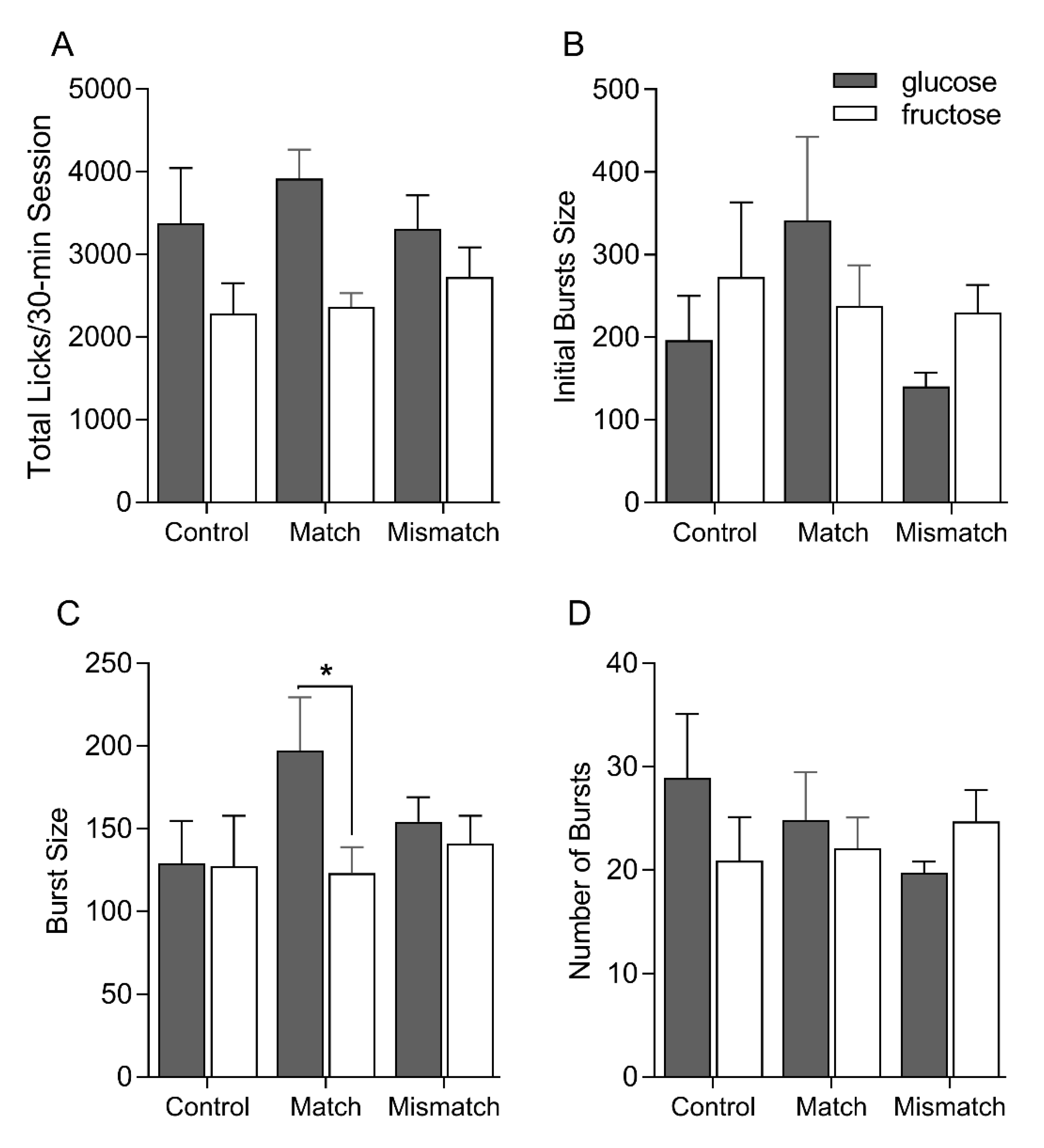
5. Results
5.1. Brief Access Taste Tests
5.2. Single-Bottle Intake Tests
6. General Discussion
7. Concluding Remarks and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Damak, S.; Rong, M.; Yasumatsu, K.; Kokrashvili, Z.; Varadarajan, V.; Zou, S. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science 2003, 301, 850–853. [Google Scholar] [CrossRef]
- Max, M.; Shanker, Y.G.; Huang, L.; Rong, M.; Liu, Z.; Campagne, F.; Weinstein, s.; Damak, s.; Margolskee, R.F. Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat. Genet. 2001, 28, 58–63. [Google Scholar] [CrossRef]
- Dotson, C.D.; Spector, A.C. Behavioral Discrimination between Sucrose and Other Natural Sweeteners in Mice: Implications for the Neural Coding of T1R Ligands. J. Neurosci. 2007, 27, 11242–11253. [Google Scholar] [CrossRef]
- Treesukosol, Y.; Spector, A.C. Orosensory detection of sucrose, maltose, and glucose is severely impaired in mice lacking T1R2 or T1R3, but Polycose sensitivity remains relatively normal. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R218–R235. [Google Scholar] [CrossRef]
- Zhao, G.Q.; Zhang, Y.; Hoon, M.A.; Chandrashekar, J.; Erlenbach, I.; Ryba, N.J.; Zuker, C. The receptors for mammalian sweet and umami taste. Cell 2003, 115, 255–266. [Google Scholar] [CrossRef]
- Li, X.; Staszewski, L.; Xu, H.; Durick, K.; Zoller, M.; Adler, E. Human receptors for sweet and umami taste. Proc. Natl. Acad. Sci. USA 2002, 99, 4692–4696. [Google Scholar] [CrossRef]
- Nelson, G.; Hoon, M.A.; Chandrashekar, J.; Zhang, Y.; Ryba, N.J.; Zuker, C.S. Mammalian sweet taste receptors. Cell 2001, 106, 381–390. [Google Scholar] [CrossRef]
- Montmayeur, J.P.; Liberles, S.D.; Matsunami, H.; Buck, L.B. A candidate taste receptor gene near a sweet taste locus. Nat. Neurosci. 2001, 4, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Schier, L.A.; Spector, A.C. Behavioral Evidence for More than One Taste Signaling Pathway for Sugars in Rats. J. Neurosci. 2016, 36, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Schier, L.A.; Inui-Yamamoto, C.; Blonde, G.D.; Spector, A.C. T1R2+T1R3-independent chemosensory inputs contributing to behavioral discrimination of sugars in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 316, R448–R462. [Google Scholar] [CrossRef] [PubMed]
- Schier, L.A.; Spector, A.C. The Functional and Neurobiological Properties of Bad Taste. Physiol. Rev. 2019, 99, 605–663. [Google Scholar] [CrossRef]
- Ackroff, K.; Sclafani, A. Flavor preferences conditioned by sugars: Rats learn to prefer glucose over fructose. Physiol. Behav. 1991, 50, 815–824. [Google Scholar] [CrossRef]
- Sclafani, A. Conditioned food preferences. Bull. Psychon. Soc. 1991, 29, 256–260. [Google Scholar] [CrossRef]
- Sclafani, A.; Ackroff, K. Flavor preferences conditioned by nutritive and non-nutritive sweeteners in mice. Physiol. Behav. 2017, 173, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Zukerman, S.; Ackroff, K.; Sclafani, A. Post-oral appetite stimulation by sugars and nonmetabolizable sugar analogs. Am. J Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R840–R853. [Google Scholar] [CrossRef] [PubMed]
- Ackroff, K.; Touzani, K.; Peets, T.K.; Sclafani, A. Flavor preferences conditioned by intragastric fructose and glucose: Differences in reinforcement potency. Physiol. Behav. 2001, 72, 691–703. [Google Scholar] [CrossRef]
- Sclafani, A.; Ackroff, K. Flavor preferences conditioned by intragastric glucose but not fructose or galactose in C57BL/6J mice. Physiol. Behav. 2012, 106, 457–461. [Google Scholar] [CrossRef]
- Zhang, L.; Han, W.; Lin, C.; Li, F.; de Araujo, I.E. Sugar Metabolism Regulates Flavor Preferences and Portal Glucose Sensing. Front. Integr. Neurosci. 2018, 12, 57. [Google Scholar] [CrossRef]
- de Araujo, I.E. Circuit organization of sugar reinforcement. Physiol Behav. 2016, 164, 473–477. [Google Scholar] [CrossRef]
- Tellez, L.A.; Han, W.; Zhang, X.; Ferreira, T.L.; Perez, I.O.; Shammah-Lagnado, S.J. Separate circuitries encode the hedonic and nutritional values of sugar. Nat. Neurosci. 2016, 19, 465–470. [Google Scholar] [CrossRef]
- Ren, X.; Ferreira, J.G.; Zhou, L.; Shammah-Lagnado, S.J.; Yeckel, C.W.; de Araujo, I.E. Nutrient Selection in the Absence of Taste Receptor Signaling. J. Neurosci. 2010, 30, 8012. [Google Scholar] [CrossRef]
- Shahbandi, A.A.; Choo, E.; Dando, R. Receptor Regulation in Taste: Can Diet Influence How We Perceive Foods? J—Multidiscip. Sci. J. 2018, 1, 106–115. [Google Scholar] [CrossRef]
- Kaufman, A.; Choo, E.; Koh, A.; Dando, R. Inflammation arising from obesity reduces taste bud abundance and inhibits renewal. PLoS Biol. 2018, 16. [Google Scholar] [CrossRef] [PubMed]
- Spector, A.C.; Klumpp, P.A.; Kaplan, J.M. Analytical issues in the evaluation of food deprivation and sucrose concentration effects on the microstructure of licking behavior in the rat. Behav. Neurosci. 1998, 112, 678–694. [Google Scholar] [CrossRef] [PubMed]
- Lucas, F.; Azzara, A.V.; Sclafani, A. Flavor preferences conditioned by intragastric polycose in rats: More concentrated polycose is not always more reinforcing. Physiol. Behav. 1997, 63, 7–14. [Google Scholar] [CrossRef]
- Nie, Y.; Vigues, S.; Hobbs, J.R.; Conn, G.L.; Munger, S.D. Distinct Contributions of T1R2 and T1R3 Taste Receptor Subunits to the Detection of Sweet Stimuli. Curr. Biol. 2005, 15, 1948–1952. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, Y.; Kajiura, H.; Mochizuki, K. Differential taste responses of mouse chorda tympani and glossopharyngeal nerves to sugars and amino acids. Neurosci. Lett. 1993, 163, 197–200. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myers, K.P.; Summers, M.Y.; Geyer-Roberts, E.; Schier, L.A. The Role of Post-Ingestive Feedback in the Development of an Enhanced Appetite for the Orosensory Properties of Glucose over Fructose in Rats. Nutrients 2020, 12, 807. https://doi.org/10.3390/nu12030807
Myers KP, Summers MY, Geyer-Roberts E, Schier LA. The Role of Post-Ingestive Feedback in the Development of an Enhanced Appetite for the Orosensory Properties of Glucose over Fructose in Rats. Nutrients. 2020; 12(3):807. https://doi.org/10.3390/nu12030807
Chicago/Turabian StyleMyers, Kevin P., Megan Y. Summers, Elizabeth Geyer-Roberts, and Lindsey A. Schier. 2020. "The Role of Post-Ingestive Feedback in the Development of an Enhanced Appetite for the Orosensory Properties of Glucose over Fructose in Rats" Nutrients 12, no. 3: 807. https://doi.org/10.3390/nu12030807
APA StyleMyers, K. P., Summers, M. Y., Geyer-Roberts, E., & Schier, L. A. (2020). The Role of Post-Ingestive Feedback in the Development of an Enhanced Appetite for the Orosensory Properties of Glucose over Fructose in Rats. Nutrients, 12(3), 807. https://doi.org/10.3390/nu12030807




