Single Nucleotide Polymorphisms in 25-Hydroxyvitamin D3 1-Alpha-Hydroxylase (CYP27B1) Gene: The Risk of Malignant Tumors and Other Chronic Diseases
Abstract
:1. Introduction
1.1. Vitamin D in Malignancies
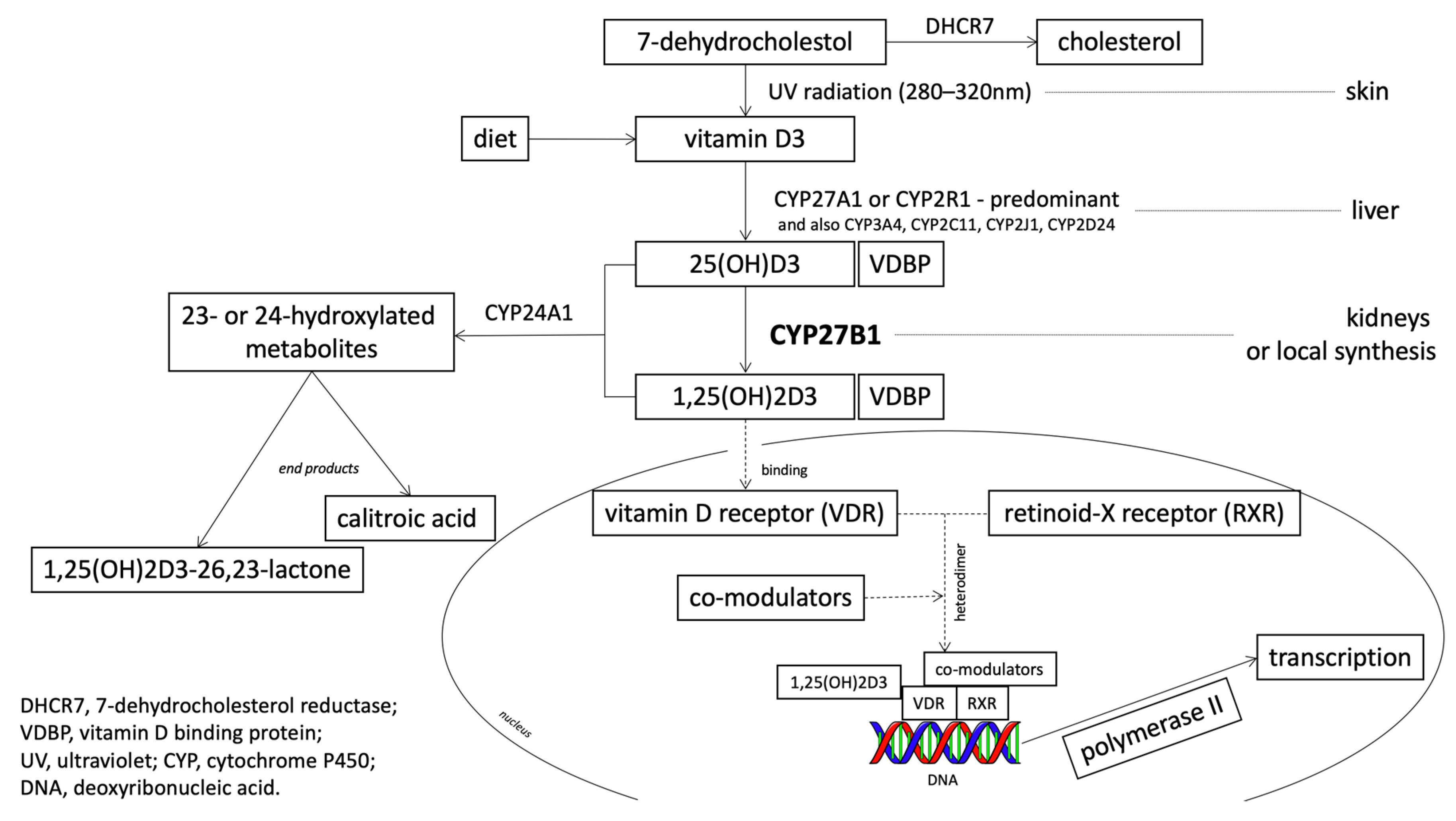
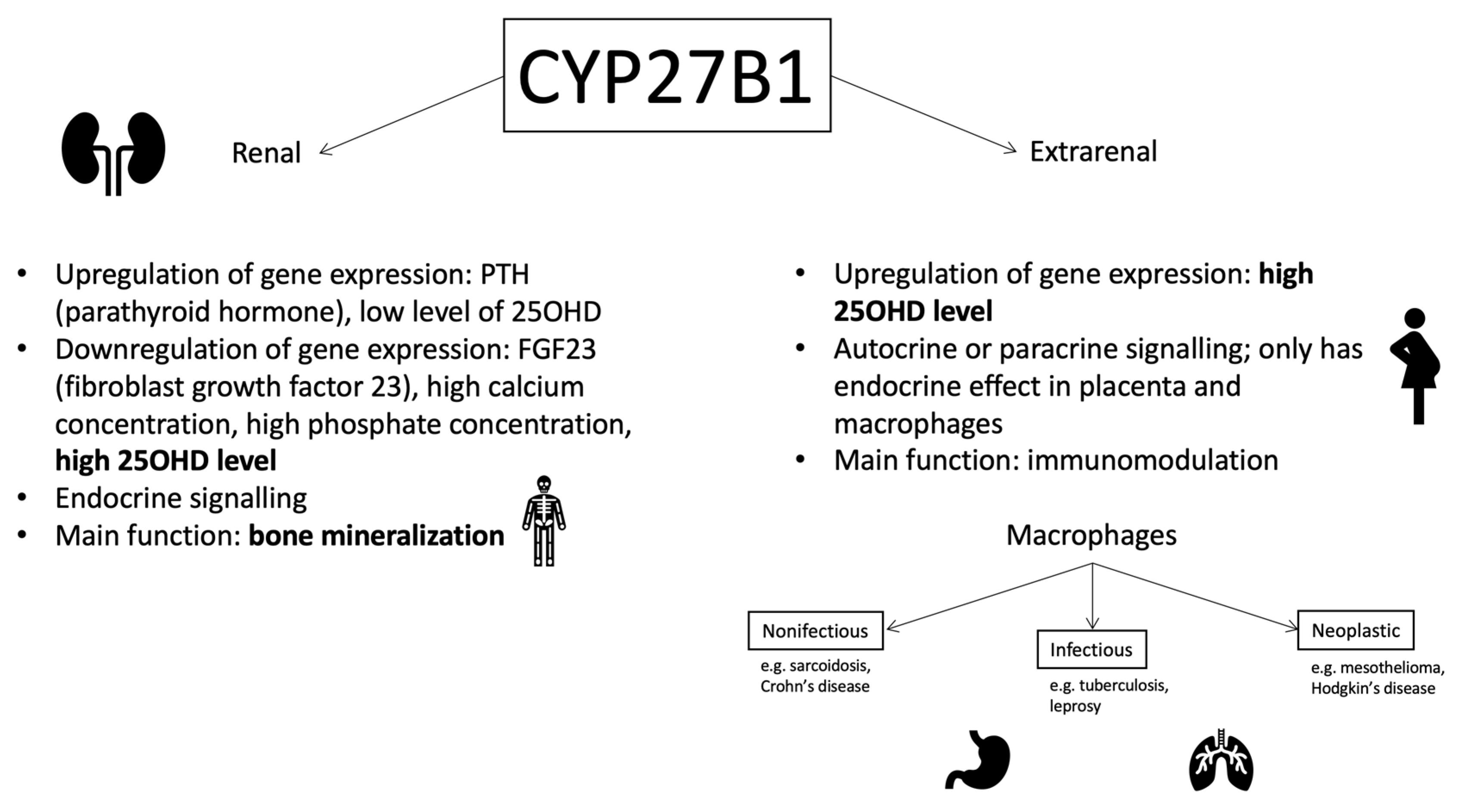
1.2. 25-Hydroxyvitamin D3 1-Alpha-Hydroxylase (CYP27B1): The Gene and the Enzyme
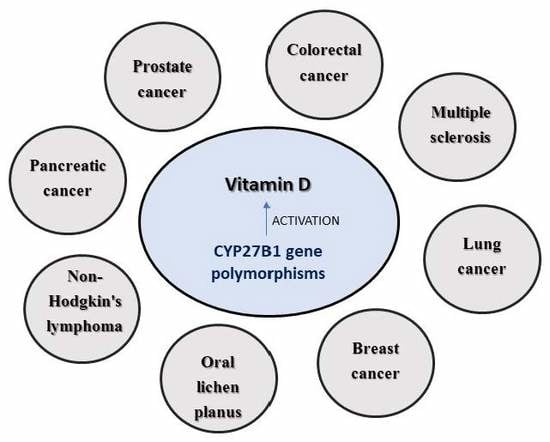
1.3. CYP27B1 and Cancer
1.4. Colorectal Cancer (CRC)
1.5. Prostate Cancer
1.6. Breast Cancer
1.7. Lung Cancer
1.8. Pancreatic Cancer
1.9. Thyroid Cancer
1.10. Liver Cancer
1.11. Non-Hodgkin’s Lymphoma
1.12. Other Diseases
1.13. Multiple Sclerosis (MS)
1.14. Vitamin-D-Dependent Rickets Type 1
1.15. Oral Lichen Planus
2. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Feldman, D.; Krishnan, A.V.; Swami, S.; Giovannucci, E.; Feldman, B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer 2014, 14, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S.; Rafison, B.; Witzel, S.; Reyes, R.E.; Shieh, A.; Chun, R.; Zavala, K.; Hewison, M.; Liu, P.T. Regulation of extrarenal CYP27B1-hydroxylase. J. Steroid Biochem. Mol. Biol. 2014, 144, 22–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, E.; Miyaura, C.; Sakagami, H.; Takeda, M.; Konno, K.; Yamazaki, T.; Yoshiki, S.; Suda, T. Differentiation of mouse myeloid leukemia cells induced by 1 alpha,25-dihydroxyvitamin D3. Proc. Natl. Acad. Sci. USA 1981, 78, 4990–4994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbour, G.L.; Coburn, J.W.; Slatopolsky, E.; Norman, A.W.; Horst, R.L. Hypercalcemia in an anephric patient with sarcoidosis: Evidence for extrarenal generation of 1,25-dihydroxyvitamin D. N. Engl. J. Med. 1981, 305, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Provvedini, D.M.; Tsoukas, C.D.; Deftos, L.J.; Manolagas, S.C. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science 1983, 221, 1181–1183. [Google Scholar] [CrossRef]
- Colston, K.; Colston, M.J.; Feldman, D. 1,25-dihydroxyvitamin D3 and malignant melanoma: The presence of receptors and inhibition of cell growth in culture. Endocrinology 1981, 108, 1083–1086. [Google Scholar] [CrossRef]
- Pereira, F.; Larriba, M.J.; Munoz, A. Vitamin D and colon cancer. Endocr. Relat. Cancer 2012, 19, R51–R71. [Google Scholar] [CrossRef] [Green Version]
- Swami, S.; Krishnan, A.V.; Wang, J.Y.; Jensen, K.; Peng, L.; Albertelli, M.A.; Feldman, D. Inhibitory effects of calcitriol on the growth of MCF-7 breast cancer xenografts in nude mice: Selective modulation of aromatase expression in vivo. Horm. Cancer 2011, 2, 190–202. [Google Scholar] [CrossRef] [Green Version]
- Swami, S.; Krishnan, A.V.; Peng, L.; Lundqvist, J.; Feldman, D. Transrepression of the estrogen receptor promoter by calcitriol in human breast cancer cells via two negative vitamin D response elements. Endocr. Relat. Cancer 2013, 20, 565–577. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, A.V.; Shinghal, R.; Raghavachari, N.; Brooks, J.D.; Peehl, D.M.; Feldman, D. Analysis of vitamin D-regulated gene expression in LNCaP human prostate cancer cells using cDNA microarrays. Prostate 2004, 59, 243–251. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Peehl, D.M.; Navone, N.M.; Feldman, D. 1α,25-dihydroxyvitamin D3 inhibits prostate cancer cell growth by androgen-dependent and androgen-independent mechanisms. Endocrinology 2000, 141, 2548–2556. [Google Scholar] [CrossRef] [PubMed]
- Tuohimaa, P.; Lyakhovich, A.; Aksenov, N.; Pennanen, P.; Syvälä, H.; Lou, Y.R.; Ahonen, M.; Hasan, T.; Pasanen, P.; Bläuer, M.; et al. Vitamin D and prostate cancer. J. Steroid Biochem. Mol. Biol. 2001, 76, 125–134. [Google Scholar] [CrossRef]
- Jenkinson, C. The vitamin D metabolome: An update on analysis and function. Cell Biochem. Funct. 2019, 37, 408–423. [Google Scholar] [CrossRef] [PubMed]
- Morrison, N.A.; Qi, J.C.; Tokita, A.; Kelly, P.J.; Crofts, L.; Nguyen, T.V.; Sambrook, P.N.; Eisman, J.A. Prediction of bone density from vitamin D receptor alleles. Nature 1994, 367, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Morrison, N.A.; Yeoman, R.; Kelly, P.J.; Eisman, J.A. Contribution of trans-acting factor alleles to normal physiological variability: Vitamin D receptor gene polymorphism and circulating osteocalcin. Proc. Natl. Acad. Sci. USA 1992, 89, 6665–6669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jolliffe, D.A.; Walton, R.T.; Griffiths, C.J.; Martineau, A.R. Single nucleotide polymorphisms in the vitamin D pathway associating with circulating concentrations of vitamin D metabolites and non-skeletal health outcomes: Review of genetic association studies. J. Steroid Biochem. Mol. Biol. 2016, 164, 18–29. [Google Scholar] [CrossRef]
- Deeb, K.K.; Trump, D.L.; Johnson, C.S. Vitamin D signalling pathways in cancer: Potential for anticancer therapeutics. Nat. Rev. Cancer 2007, 7, 684–700. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Millen, A.E.; Bodnar, L.M. Vitamin D assessment in population-based studies: A review of the issues. Am. J. Clin. Nutr. 2008, 87, 1102S–1105S. [Google Scholar] [CrossRef] [Green Version]
- Muindi, J.R.; Adjei, A.A.; Wu, Z.R.; Olson, I.; Huang, H.; Groman, A.; Tian, L.; Singh, P.K.; Sucheston, L.E.; Johnson, C.S.; et al. Serum Vitamin D metabolites in colorectal cancer patients receiving cholecalciferol supplementation: Correlation with polymorphisms in the vitamin D genes. Horm. Cancer 2012, 4, 242–250. [Google Scholar] [CrossRef] [Green Version]
- Fu, G.K.; Portale, A.A.; Miller, W.L. Complete structure of the human gene for the vitamin D 1-alpha-hydroxylase, P450cl-alpha. DNA Cell Biol. 1997, 16, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Kitanaka, S.; Takeyama, K.; Murayama, A.; Sato, T.; Okumura, K.; Nogami, M.; Hasegawa, Y.; Niimi, H.; Yanagisawa, J.; Tanaka, T.; et al. Inactivating mutations in the 25-hydroxyvitamin D3 1alpha-hydroxylase gene in patients with pseudovitamin D-deficiency rickets. N. Engl. J. Med. 1998, 338, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.F.; Zhu, X.H.; Pei, Y.L.; Jackson, D.M.; Holick, M.F. Molecular cloning, characterization, and promoter analysis of the human 25-hydroxyvitamin D3-1alpha-hydroxylase gene. Proc. Natl. Acad. Sci. USA 1999, 96, 6988–6993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawashima, H.; Kurokawa, K. Unique hormonal regulation of vitamin D metabolism in the mammalian kidney. Miner. Electrolyte Metab. 1983, 9, 227–235. [Google Scholar] [PubMed]
- Kawashima, H.; Torikai, S.; Kurokawa, K. Calcitonin selectively stimulates 25-hydroxyvitamin D3-1 alpha-hydroxylase in the proximal straight tubule of the rat kidney. Nature 1981, 291, 327–329. [Google Scholar] [CrossRef]
- Adams, J.S.; Hewison, M. Extrarenal expression of the 25-hydroxyvitamin D-1-hydroxylase. Arch. Biochem. Biophys. 2012, 523, 95–102. [Google Scholar] [CrossRef]
- Christakos, S.; DeLuca, H.F. Minireview: Vitamin D: Is there a role in extraskeletal health? Endocrinology 2011, 152, 2930–2936. [Google Scholar] [CrossRef]
- Cross, H.S.; Petra Bareis, P.; Hofer, H.; Bischof, M.G.; Bajna, E.; Kriwanek, S.; Bonner, E.; Peterlik, M. 25-Hydroxyvitamin D3-1α-hydroxylase and vitamin D receptor gene expression in human colonic mucosa is elevated during early cancerogenesis. Steroids 2001, 66, 287–292. [Google Scholar] [CrossRef]
- Grant, W.B.; Garland, C.F. A critical review of studies on vitamin D in relation to colorectal cancer. Nutr. Cancer 2004, 48, 115–123. [Google Scholar] [CrossRef]
- Giovannucci, E. The epidemiology of vitamin D and colorectal cancer: Recent findings. Curr. Opin. Gastroenterol. 2006, 22, 24–29. [Google Scholar] [CrossRef]
- Wactawski-Wende, J.; Kotchen, J.M.; Anderson, G.L.; Assaf, A.R.; Brunner, R.L.; O’Sullivan, M.J.; Margolis, K.L.; Ockene, J.K.; Phillips, L.; Women’s Health Initiative Investigators; et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N. Engl. J. Med. 2006, 354, 684–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Giovannucci, E. Calcium, vitamin D and colorectal cancer chemoprevention. Best Pract. Res. Clin. Gastroenterol. 2011, 25, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Tangpricha, V.; Flanagan, J.N.; Whitlatch, L.W.; Tseng, C.C.; Chen, T.C.; Holt, P.R.; Lipkin, M.S.; Holick, M.F. 25-hydroxyvitamin D-1alpha-hydroxylase in normal and malignant colon tissue. Lancet 2001, 357, 1673–1674. [Google Scholar] [CrossRef]
- Kállay, E.; Bises, G.; Bajna, E.; Bieglmayer, C.; Gerdenitsch, W.; Steffan, I.; Kato, S.; Armbrecht, H.J.; Cross, H.S. Colon-specific regulation of vitamin D hydroxylases - a possible approach for tumor prevention. Carcinogenesis 2005, 26, 1581–1589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, L.M.; Ulrich, C.M.; Hsu, L.; Duggan, D.J.; Benitez, D.S.; White, E.; Slattery, M.L.; Farin, F.M.; Makar, K.W.; Carlson, C.S.; et al. Vitamin D related genes, CYP24A1 and CYP27B1, and colon cancer risk. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 2540–2548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bises, G.; Kállay, E.; Weiland, T.; Wrba, F.; Wenzl, E.; Bonner, E.; Kriwanek, S.; Obrist, P.; Cross, H.S. 25-hydroxyvitamin D3-1alpha-hydroxylase expression in normal and malignant human colon. J. Histochem. Cytochem. 2004, 52, 985–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gacche, R.N. Compensatory angiogenesis and tumor refractoriness. Oncogenesis 2015, 4, e153. [Google Scholar] [CrossRef] [Green Version]
- Moan, J.; Porojnicu, A.; Lagunova, Z.; Berg, J.P.; Dahlback, A. Colon cancer: Prognosis for different latitudes, age groups and seasons in Norway. J. Photochem. Photobiol. 2007, 89, 148–155. [Google Scholar] [CrossRef]
- Wang, T.J.; Zhang, F.; Richards, J.B.; Kestenbaum, B.; van Meurs, J.B.; Berry, D.; Kiel, D.P.; Streeten, E.A.; Ohlsson, C.; Koller, D.L.; et al. Common genetic determinants of vitamin D insufficiency: A genome-wide association study. Lancet 2010, 376, 180–188. [Google Scholar] [CrossRef] [Green Version]
- McGrath, J.J.; Saha, S.; Burne, T.H.; Eyles, D.W. A systematic review of the association between common single nucleotide polymorphisms and 25-hydroxyvitamin D concentrations. J. Steroid Biochem. Mol. Biol. 2010, 121, 471–477. [Google Scholar] [CrossRef]
- Bu, F.X.; Armas, L.; Lappe, J.; Zhou, Y.; Gao, G.; Wang, H.W.; Recker, R.; Zhao, L.J. Comprehensive association analysis of nine candidate genes with serum 25-hydroxy vitamin D levels among healthy Caucasian subjects. Hum. Genet. 2010, 128, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Pettifor, J.M.; Bikle, D.D.; Cavaleros, M.; Zachen, D.; Kamdar, M.C.; Ross, F.P. Serum levels of free 1,25-dihydroxyvitamin D in vitamin D toxicity. Ann. Intern. Med. 1995, 122, 511–513. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012, 487, 330–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McBain, A.J.; Macfarlane, G.T. Ecological and physiological studies on large intestinal bacteria in relation to production of hydrolytic and reductive enzymes involved in formation of genotoxic metabolites. J. Med. Microbiol. 1998, 47, 407–416. [Google Scholar] [CrossRef] [Green Version]
- Lagergren, J.; Ye, W.; Ekbom, A. Intestinal cancer after cholecystectomy: Is bile involved in carcinogenesis? Gastroenterology 2001, 121, 542–547. [Google Scholar] [CrossRef]
- Meguid, R.A.; Slidell, M.B.; Wolfgang, C.L.; Chang, D.C.; Ahuja, N. Is there a difference in survival between right - versus left-sided colon cancers? Ann. Surg. Oncol. 2008, 15, 2388–2394. [Google Scholar] [CrossRef]
- Meggouh, F.; Lointier, P.; Pezet, D.; Saez, S. Evidence of 1,25-dihydroxyvitamin D3-receptors in human digestive mucosa and carcinoma tissue biopsies taken at different levels of the digestive tract, in 152 patients. J. Steroid Biochem. 1990, 36, 143–147. [Google Scholar] [CrossRef]
- Suttie, S.A.; Shaikh, I.; Mullen, R.; Amin, A.I.; Daniel, T.; Yalamarthi, S. Outcome of right- and left-sided colonic and rectal cancer following surgical resection. Colorectal Dis. 2011, 13, 884–889. [Google Scholar] [CrossRef]
- Hughes, T.A. Regulation of gene expression by alternative untranslated regions. Trends Genet. 2006, 22, 119–122. [Google Scholar] [CrossRef]
- Jacobs, E.T.; Van Pelt, C.; Forster, R.E.; Zaidi, W.; Hibler, E.A.; Galligan, M.A.; Haussler, M.R.; Jurutka, P.W. CYP24A1 and CYP27B1 polymorphisms modulate vitamin D metabolism in colon cancer cells. Cancer Res. 2013, 73, 2563–2573. [Google Scholar] [CrossRef] [Green Version]
- Meunier, B.; de Visser, S.P.; Shaik, S. Mechanism of oxidation reactions catalyzed by cytochrome p450 enzymes. Chem. Rev. 2004, 104, 3947–3980. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Li, Y.; Zhou, S.F. A bioinformatics approach for the phenotype prediction of nonsynonymous single nucleotide polymorphisms in human cytochromes P450. Drug Metab. Dispos. 2009, 37, 977–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- dbSNP. Available online: https://www.ncbi.nlm.nih.gov/snp/rs10877012 (accessed on 24 February 2020).
- Chia, V.; Newcomb, P.A. Calcium and colorectal cancer: Some questions remain. Nutr. Rev. 2004, 62, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.M.; Ulrich, C.M.; Hsu, L.; Duggan, D.J.; Benitez, D.S.; White, E.; Slattery, M.L.; Caan, B.J.; Potter, J.D.; Peters, U. Genetic variation in calcium-sensing receptor and risk for colon cancer. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 2755–2765. [Google Scholar] [CrossRef] [Green Version]
- Feskanich, D.; Ma, J.; Fuchs, C.S.; Kirkner, G.J.; Hankinson, S.E.; Hollis, B.W.; Giovannucci, E.L. Plasma vitamin D metabolites and risk of colorectal cancer in women. Cancer Epidemiol. Biomarkers Prev. 2004, 13, 1502–1508. [Google Scholar]
- Ma, Y.; Zhang, P.; Wang, F.; Yang, J.; Liu, Z.; Qin, H. Association between vitamin D and risk of colorectal cancer: A systematic review of prospective studies. J. Clin. Oncol. 2011, 29, 3775–3782. [Google Scholar] [CrossRef]
- Wu, K.; Feskanich, D.; Fuchs, C.S.; Chan, A.T.; Willett, W.C.; Hollis, B.W.; Pollak, M.N.; Giovannucci, E. Interactions between plasma levels of 25-hydroxyvitamin D, insulin-like growth factor (IGF)-1 and C-peptide with risk of colorectal cancer. PLoS ONE 2011, 6, e28520. [Google Scholar] [CrossRef]
- Hong, S.N.; Kim, J.H.; Choe, W.H.; Lee, S.Y.; Seol, D.C.; Moon, H.W.; Hur, M.; Yun, Y.M.; Sung, I.K.; Park, H.S.; et al. Circulating vitamin D and colorectal adenoma in asymptomatic average-risk individuals who underwent first screening colonoscopy: A case–control study. Dig. Dis. Sci. 2012, 57, 753–763. [Google Scholar] [CrossRef]
- Giovannucci, E. Vitamin D and cancer incidence in the Harvard cohorts. Ann. Epidemiol. 2009, 19, 84–88. [Google Scholar] [CrossRef]
- Kühn, T.; Kaaks, R.; Teucher, B.; Hirche, F.; Dierkes, J.; Weikert, C.; Katzke, V.; Boeing, H.; Stangl, G.I.; Buijsse, B. Dietary, lifestyle, and genetic determinants of vitamin D status: A cross-sectional analysis from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Germany study. Eur. J. Nutr. 2013, 53, 731–741. [Google Scholar] [CrossRef]
- Pibiri, F.; Kittles, R.; Sandler, R.S.; Keku, T.O.; Kupfer, S.S.; Xicola, R.M.; Llor, X.; Ellis, N.A. Genetic variation in vitamin D-related genes and risk of colorectal cancer in African Americans. Cancer Causes Control 2014, 25, 561–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, M.D.; Lenz, H.J. The safety of monoclonal antibodies for treatment of colorectal cancer. Expert Opin. Drug. Saf. 2016, 15, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Grandi, N.; Raum, E.; Haug, U.; Arndt, V.; Brenner, H. Meta-analysis: Serum vitamin D and colorectal adenoma risk. Prev. Med. 2011, 53, 10–16. [Google Scholar] [CrossRef]
- Gandini, S.; Boniol, M.; Haukka, J.; Byrnes, G.; Cox, B.; Sneyd, M.J.; Mullie, P.; Autier, P. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int. J. Cancer 2011, 128, 1414–1424. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.Y.; Garland, C.F.; Gorham, E.D.; Mohr, S.B.; Giovannucci, E. Vitamin D and prevention of colorectal adenoma: A meta-analysis. Cancer Epidemiol. Biomark. Prev. 2008, 17, 2958–2969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hibler, E.A.; Sardo Molmenti, C.L.; Lance, P.; Jurutka, P.W.; Jacobs, E.T. Associations between circulating 1,25(OH)2D concentration and odds of metachronous colorectal adenoma. Cancer Causes Control 2014, 25, 809–817. [Google Scholar] [CrossRef] [Green Version]
- Hibler, E.A.; Klimentidis, Y.C.; Jurutka, P.W.; Kohler, L.N.; Lance, P.; Roe, D.J.; Thompson, P.A.; Jacobs, E.T. CYP24A1 and CYP27B1 polymorphisms, concentrations of vitamin D metabolites, and odds of colorectal adenoma recurrence. Nutr. Cancer 2015, 67, 1131–1141. [Google Scholar] [CrossRef]
- Shui, I.M.; Mucci, L.A.; Kraft, P.; Tamimi, R.M.; Lindstrom, S.; Penney, K.L.; Nimptsch, K.; Hollis, B.W.; Dupre, N.; Platz, E.A.; et al. Vitamin D-related genetic variation, plasma vitamin D, and risk of lethal prostate cancer: A prospective nested case-control study. J. Natl. Cancer Inst. 2012, 104, 690–699. [Google Scholar] [CrossRef] [Green Version]
- Bikle, D.D.; Patzek, S.; Wang, Y. Physiologic and pathophysiologic roles of extra renal CYP27B1: Case report and review. Bone Rep. 2018, 8, 255–267. [Google Scholar] [CrossRef]
- Holt, S.K.; Kwon, E.M.; Koopmeiners, J.S.; Lin, D.W.; Feng, Z.; Ostrander, E.A.; Peters, U.; Stanford, J.L. Vitamin D pathway gene variants and prostate cancer prognosis. Prostate 2010, 70, 1448–1460. [Google Scholar] [CrossRef] [Green Version]
- Albanes, D.; Mondul, A.M.; Yu, K.; Parisi, D.; Horst, R.L.; Virtamo, J.; Weinstein, S.J. Serum 25-hydroxy vitamin D and prostate cancer risk in a large nested case-control study. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1850–1860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.C.; Wang, L.; Whitlatch, L.W.; Flanagan, J.N.; Holick, M.F. Prostatic 25-hydroxyvitamin D-1alpha-hydroxylase and its implication in prostate cancer. J. Cell Biochem. 2003, 88, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Tretli, S.; Hernes, E.; Berg, J.P.; Hestvik, U.E.; Robsahm, T.E. Association between serum 25(OH)D and death from prostate cancer. Br. J. Cancer 2009, 100, 450–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, F.; Kasperzyk, J.L.; Shui, I.; Hendrickson, W.; Hollis, B.W.; Fall, K.; Ma, J.; Gaziano, J.M.; Stampfer, M.J.; Mucci, L.A.; et al. Prediagnostic plasma vitamin D metabolites and mortality among patients with prostate cancer. PLoS ONE 2011, 6, e18625. [Google Scholar] [CrossRef] [Green Version]
- Ahn, J.; Albanes, D.; Berndt, S.I.; Peters, U.; Chatterjee, N.; Freedman, N.D.; Abnet, C.C.; Huang, W.Y.; Kibel, A.S.; Crawford, E.D.; et al. Vitamin D-related genes, serum vitamin D concentrations and prostate cancer risk. Carcinogenesis 2009, 30, 769–776. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.A.; Davey, A.; Park, S.; Hausman, D.B.; Poon, L.W.; Georgia Centenarian Study. Age race and season predict vitamin D status in African American and white octogenarians and centenarians. J. Nutr. Health Aging 2008, 12, 690–695. [Google Scholar]
- Roff, A.; Wilson, R.T. A novel SNP in a vitamin D response element of the CYP24A1 promoter reduces protein binding, transactivation, and gene expression. J. Steroid Biochem. Mol. Biol. 2008, 112, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Bonilla, C.; Hooker, S.; Mason, T.; Bock, C.H.; Kittles, R.A. Prostate cancer susceptibility loci identified on chromosome 12 in African Americans. PLoS ONE 2011, 6, e16044. [Google Scholar] [CrossRef] [Green Version]
- Reddy, S.; Shapiro, M.; Morton, R.; Brawley, O.W. Prostate cancer in black and white Americans. Cancer Metastasis Rev. 2003, 22, 83–86. [Google Scholar] [CrossRef]
- Whitman, E.J.; Pomerantz, M.; Chen, Y.; Chamberlin, M.M.; Furusato, B.; Gao, C.; Ali, A.; Ravindranath, L.; Dobi, A.; Sesterhenn, I.A.; et al. Prostate cancer risk allele specific for African descent associates with pathologic stage at prostatectomy. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Beuten, J.; Gelfond, J.A.; Franke, J.L.; Weldon, K.S.; Crandall, A.C.; Johnson-Pais, T.L.; Thompson, I.M.; Leach, R.J. Single and multigenic analysis of the association between variants in 12 steroid hormone metabolism genes and risk of prostate cancer. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1869–1880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shui, I.M.; Mondul, A.M.; Lindström, S.; Tsilidis, K.K.; Travis, R.C.; Gerke, T.; Albanes, D.; Mucci, L.A.; Giovannucci, E.; Kraft, P.; et al. Circulating vitamin D, vitamin D-related genetic variation, and risk of fatal prostate cancer in the National Cancer Institute Breast and Prostate Cancer Cohort Consortium. Cancer 2015, 121, 1949–1956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, D.; Trudel, D.; Van der Kwast, T.; Nonn, L.; Giangreco, A.A.; Li, D.; Dias, A.; Cardoza, M.; Laszlo, S.; Hersey, K.; et al. Randomized clinical trial of vitamin D3 doses on prostatic vitamin D metabolite levels and ki67 labeling in prostate cancer patients. J. Clin. Endocrinol. Metab. 2013, 98, 1498–1507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, K.M.; Sandler, D.P.; Kinyamu, H.K.; Taylor, J.A.; Weinberg, C.R. Single-nucleotide polymorphisms in vitamin D-related genes may modify vitamin D-breast cancer associations. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1761–1771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Veierød, M.B.; Löf, M.; Sandin, S.; Adami, H.O.; Weiderpass, E. Prospective study of UV exposure and cancer incidence among Swedish women. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1358–1367. [Google Scholar] [CrossRef] [Green Version]
- Anderson, L.N.; Cotterchio, M.; Kirsh, V.A.; Knight, J.A. Ultraviolet sunlight exposure during adolescence and adulthood and breast cancer risk: A population-based case-control study among Ontario women. Am. J. Epidemiol. 2011, 174, 293–304. [Google Scholar] [CrossRef] [Green Version]
- Rossi, M.; McLaughlin, J.K.; Lagiou, P.; Bosetti, C.; Talamini, R.; Lipworth, L.; Giacosa, A.; Montella, M.; Franceschi, S.; Negri, E.; et al. Vitamin D intake and breast cancer risk: A case-control study in Italy. Ann. Oncol. 2009, 20, 374–378. [Google Scholar] [CrossRef]
- McCullough, M.L.; Rodriguez, C.; Diver, W.R.; Feigelson, H.S.; Stevens, V.L.; Thun, M.J.; Calle, E.E. Dairy, calcium, and vitamin D intake and postmenopausal breast cancer risk in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2898–2904. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, K.M.; Sandler, D.P.; Taylor, J.A.; Weinberg, C.R. Serum vitamin D and risk of breast cancer within five years. Environ. Health Perspect. 2017, 125, 077004. [Google Scholar] [CrossRef]
- Reimers, L.L.; Crew, K.D.; Bradshaw, P.T.; Santella, R.M.; Steck, S.E.; Sirosh, I.; Terry, M.B.; Hershman, D.L.; Shane, E.; Cremers, S.; et al. Vitamin D-related gene polymorphisms, plasma 25-hydroxyvitamin D, and breast cancer risk. Cancer Causes Control 2015, 26, 187–203. [Google Scholar] [CrossRef] [Green Version]
- McCullough, M.L.; Bostick, R.M.; Mayo, T.L. Vitamin D gene pathway polymorphisms and risk of colorectal, breast, and prostate cancer. Annu. Rev. Nutr. 2009, 29, 111–132. [Google Scholar] [CrossRef] [PubMed]
- Clendenen, T.V.; Ge, W.; Koenig, K.L.; Axelsson, T.; Liu, M.; Afanasyeva, Y.; Andersson, A.; Arslan, A.A.; Chen, Y.; Hallmans, G.; et al. Genetic polymorphisms in vitamin D metabolism and signaling genes and risk of breast cancer: A nested case-control study. PLoS ONE 2015, 10, e0140478. [Google Scholar] [CrossRef] [PubMed]
- Clemens, T.L.; Adams, J.S.; Henderson, S.L.; Holick, M.F. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet 1982, 1, 74–76. [Google Scholar] [CrossRef]
- Amend, K.; Hicks, D.; Ambrosone, C.B. Breast cancer in African-American women: Differences in tumor biology from European-American women. Cancer Res. 2006, 66, 8327–8330. [Google Scholar] [CrossRef] [Green Version]
- Ooi, L.L.; Zhou, H.; Kalak, R.; Zheng, Y.; Conigrave, A.D.; Seibel, M.J.; Dunstan, C.R. Vitamin D deficiency promotes human breast cancer growth in a murine model of bone metastasis. Cancer Res. 2010, 70, 1835–1844. [Google Scholar] [CrossRef] [Green Version]
- Yao, S.; Zirpoli, G.; Bovbjerg, D.H.; Jandorf, L.; Hong, C.C.; Zhao, H.; Sucheston, L.E.; Tang, L.; Roberts, M.; Ciupak, G.; et al. Variants in the vitamin D pathway, serum levels of vitamin D, and estrogen receptor negative breast cancer among African-American women: A case-control study. Breast Cancer Res. 2012, 14, R58. [Google Scholar] [CrossRef] [Green Version]
- Kudachadkar, R.; O’Regan, R.M. Aromatase inhibitors as adjuvant therapy for postmenopausal patients with early stage breast Cancer. CA Cancer J. Clin. 2005, 55, 145–163. [Google Scholar] [CrossRef] [Green Version]
- Henry, N.L.; Azzouz, F.; Desta, Z.; Li, L.; Nguyen, A.T.; Lemler, S.; Hayden, J.; Tarpinian, K.; Yakim, E.; Flockhart, D.A.; et al. Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J. Clin. Oncol. 2012, 30, 936–942. [Google Scholar] [CrossRef] [Green Version]
- Crew, K.D.; Greenlee, H.; Capodice, J.; Raptis, G.; Brafman, L.; Fuentes, D.; Sierra, A.; Hershman, D.L. Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J. Clin. Oncol. 2007, 25, 3877–3883. [Google Scholar] [CrossRef]
- Prieto-Alhambra, D.; Javaid, M.; Servitja, S.; Arden, N.; Martinez-García, M.; Diez-Perez, A.; Albanell, J.; Tusquets, I.; Nogues, X. Vitamin D threshold to prevent aromatase inhibitor-induced arthralgia: A prospective cohort study. Breast Cancer Res. Treat. 2010, 125, 869–878. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Giralt, N.; Rodríguez-Sanz, M.; Prieto-Alhambra, D.; Servitja, S.; Torres-Del Pliego, E.; Balcells, S.; Albanell, J.; Grinberg, D.; Diez-Perez, A.; Tusquets, I.; et al. Genetic determinants of aromatase inhibitor-related arthralgia: The B-ABLE cohort study. Breast Cancer Res. Treat. 2013, 140, 385–395. [Google Scholar] [CrossRef]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Groot, P.; Munden, R.F. Lung cancer epidemiology, risk factors, and prevention. Radiol. Clin. N. Am. 2012, 50, 863–876. [Google Scholar] [CrossRef] [PubMed]
- LaPar, D.J.; Nagji, A.S.; Bhamidipati, C.M.; Kozower, B.D.; Lau, C.L.; Ailawadi, G.; Jones, D.R. Seasonal variation influences outcomes following lung cancer resections. Eur. J. Cardiothorac. Surg. 2011, 40, 83–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Chen, W.; Hu, Z.B.; Xu, L.; Shu, Y.Q.; Pan, S.Y.; Dai, J.C.; Jin, G.F.; Ma, H.X.; Shen, H.B. Plasma vitamin D levels and vitamin D receptor polymorphisms are associated with survival of non-small cell lung cancer. Chin. J. Cancer Res. 2011, 23, 33–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilkkinen, A.; Knekt, P.; Heliövaara, M.; Rissanen, H.; Marniemi, J.; Hakulinen, T.; Aromaa, A. Vitamin D status and the risk of lung cancer: A cohort study in Finland. Cancer Epidemiol. Biomark. Prev. 2008, 17, 3274–3278. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Heist, R.S.; Liu, G.; Asomaning, K.; Neuberg, D.S.; Hollis, B.W.; Wain, J.C.; Lynch, T.J.; Giovannucci, E.; Su, L.; et al. Circulating 25-hydroxyvitamin D levels predict survival in early-stage non-small-cell lung cancer patients. J. Clin. Oncol. 2007, 25, 479–485. [Google Scholar] [CrossRef]
- Zhou, W.; Suk, R.; Liu, G.; Park, S.; Neuberg, D.S.; Wain, J.C.; Lynch, T.J.; Giovannucci, E.; Christiani, D.C. Vitamin D is associated with improved survival in early-stage non-small cell lung cancer patients. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2303–2309. [Google Scholar] [CrossRef] [Green Version]
- Yokomura, K.; Suda, T.; Sasaki, S.; Inui, N.; Chida, K.; Nakamura, H. Increased expression of the 25-hydroxyvitamin D(3)-1alpha-hydroxylase gene in alveolar macrophages of patients with lung cancer. J. Clin. Endocrinol. Metab. 2003, 88, 5704–5709. [Google Scholar] [CrossRef] [Green Version]
- Mio, T.; Romberger, D.J.; Thompson, A.B.; Robbins, R.A.; Heires, A.; Rennard, S.I. Cigarette smoke induces interleukin-8 release from human bronchial epithelial cells. Am. J. Respir. Crit. Care. Med. 1997, 155, 1770–1776. [Google Scholar] [CrossRef]
- Wu, X.; Cheng, J.; Yang, K. Vitamin D-related gene polymorphisms, plasma 25-hydroxy-vitamin D, cigarette smoke and non-small cell lung cancer (NSCLC) risk. Int. J. Mol. Sci. 2016, 17, 1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, J.; Xu, F.; Qu, J.; Wang, Y.; Gao, M.; Yu, H.; Qian, B. Genetic polymorphisms in the vitamin D pathway in relation to lung cancer risk and survival. Oncotarget 2015, 6, 2573–2582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gronostajski, R.M. Roles of the NFI/CTF gene family intranscription and development. Gene 2000, 249, 31–45. [Google Scholar] [CrossRef]
- Gronostajski, R.M.; Adhya, S.; Nagata, K.; Guggenheimer, R.A.; Hurwitz, J. Site-specific DNA binding of nuclear factor I: Analyses of cellular binding sites. Mol. Cell Biol. 1985, 5, 964–971. [Google Scholar] [CrossRef] [Green Version]
- Anderson, L.N.; Cotterchio, M.; Knight, J.A.; Borgida, A.; Gallinger, S.; Cleary, S.P. Genetic variants in vitamin d pathway genes and risk of pancreas cancer; results from a population based case-control study in Ontario, Canada. PLoS ONE 2013, 8, e66768. [Google Scholar] [CrossRef]
- Arem, H.; Yu, K.; Xiong, X.; Moy, K.; Freedman, N.D.; Mayne, S.T.; Albanes, D.; Arslan, A.A.; Austin, M.; Bamlet, W.R.; et al. Vitamin D metabolic pathway genes and pancreatic cancer risk. PLoS ONE 2015, 10, e0117574. [Google Scholar] [CrossRef]
- Grant, W.B. An estimate of premature cancer mortality in the U.S. due to inadequate doses of solar ultraviolet-B radiation. Cancer 2002, 94, 1867–1875. [Google Scholar] [CrossRef]
- Grant, W.B. An ecologic study of cancer mortality rates in Spain with respect to indices of solar UVB irradiance and smoking. Int. J. Cancer 2007, 120, 1123–1128. [Google Scholar] [CrossRef]
- Kinoshita, S.; Wagatsuma, Y.; Okada, M. Geographical distribution for malignant neoplasm of the pancreas in relation to selected climatic factors in Japan. Int. J. Health Geogr. 2007, 6, 34. [Google Scholar] [CrossRef] [Green Version]
- Stolzenberg-Solomon, R.Z.; Jacobs, E.J.; Arslan, A.A.; Qi, D.; Patel, A.V.; Helzlsouer, K.J.; Weinstein, S.J.; McCullough, M.L.; Purdue, M.P.; Shu, X.O.; et al. Circulating 25-hydroxyvitamin D and risk of pancreatic cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am. J. Epidemiol. 2010, 172, 81–93. [Google Scholar] [CrossRef]
- Wolpin, B.M.; Ng, K.; Bao, Y.; Kraft, P.; Stampfer, M.J.; Michaud, D.S.; Ma, J.; Buring, J.E.; Sesso, H.D.; Lee, I.M.; et al. Plasma 25-hydroxyvitamin D and risk of pancreatic cancer. Cancer Epidemiol. Biomark. Prev. 2011, 21, 82–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clinckspoor, I.; Hauben, E.; Verlinden, L.; Van den Bruel, A.; Vanwalleghem, L.; Vander Poorten, V.; Delaere, P.; Mathieu, C.; Verstuyf, A.; Decallonne, B. Altered expression of key players in vitamin D metabolism and signaling in malignant and benign thyroid tumors. J. Histochem. Cytochem. 2012, 60, 502–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khadzkou, K.; Buchwald, P.; Westin, G.; Dralle, H.; Akerström, G.; Hellman, P. 25-hydroxyvitamin D3 1alpha-hydroxylase and vitamin D receptor expression in papillary thyroid carcinoma. J. Histochem. Cytochem. 2006, 54, 355–361. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.; Fretwell, D.; Crees, Z.; Kerege, A.; Klopper, J.P. Thyroid cancer resistance to vitamin D receptor activation is associated with 24-hydroxylase levels but not the ff FokI polymorphism. Thyroid 2010, 20, 1103–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouillon, R.; Eelen, G.; Verlinden, L.; Mathieu, C.; Carmeliet, G.; Verstuyf, A. Vitamin D and cancer. J. Steroid Biochem. Mol. Biol. 2006, 102, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Penna-Martinez, M.; Ramos-Lopez, E.; Stern, J.; Hinsch, N.; Hansmann, M.L.; Selkinski, I.; Grunwald, F.; Vorlander, C.; Wahl, R.A.; Bechstein, W.O.; et al. Vitamin D receptor polymorphisms in differentiated thyroid carcinoma. Thyroid 2009, 19, 623–628. [Google Scholar] [CrossRef]
- Alter, M.J. The epidemiology of acute and chronic hepatitis C. Clin. Liver Dis. 1997, 1, 559–568. [Google Scholar] [CrossRef]
- Falleti, E.; Bitetto, D.; Fabris, C.; Cussigh, A.; Fontanini, E.; Fornasiere, E.; Fumolo, E.; Bignulin, S.; Cmet, S.; Minisini, R.; et al. Vitamin D receptor gene polymorphisms and hepatocellular carcinoma in alcoholic cirrhosis. World J. Gastroenterol. 2010, 16, 3016–3024. [Google Scholar] [CrossRef]
- Cusato, J.; Boglione, L.; De Nicolò, A.; Favata, F.; Ariaudo, A.; Mornese Pinna, S.; Guido, F.; Avataneo, V.; Cantù, M.; Carcieri, C.; et al. Vitamin D pathway gene polymorphisms and hepatocellular carcinoma in chronic hepatitis C-affected patients treated with new drugs. Cancer Chemother. Pharmacol. 2018, 81, 615–620. [Google Scholar] [CrossRef]
- Chang, E.T.; Canchola, A.J.; Cockburn, M.; Lu, Y.; Wang, S.S.; Bernstein, L.; Clarke, C.A.; Horn-Ross, P.L. Adulthood residential ultraviolet radiation, sun sensitivity, dietary vitamin D, and risk of lymphoid malignancies in the California Teachers Study. Blood 2011, 118, 1591–1599. [Google Scholar] [CrossRef] [Green Version]
- Kelly, J.L.; Drake, M.T.; Fredericksen, Z.S.; Asmann, Y.W.; Liebow, M.; Shanafelt, T.D.; Feldman, A.L.; Ansell, S.M.; Macon, W.R.; Herr, M.M.; et al. Early life sun exposure, vitamin D-related gene variants, and risk of non-Hodgkin lymphoma. Cancer Causes Control 2012, 23, 1017–1029. [Google Scholar] [CrossRef] [PubMed]
- Purdue, M.P.; Freedman, D.M.; Gapstur, S.M.; Helzlsouer, K.J.; Laden, F.; Lim, U.; Maskarinec, G.; Rothman, N.; Shu, X.O.; Stevens, V.L.; et al. Circulating 25-hydroxyvitamin D and risk of non-hodgkin lymphoma: Cohort consortium vitamin D pooling project of rarer cancers. Am. J. Epidemiol. 2010, 172, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Fichna, M.; Żurawek, M.; Januszkiewicz-Lewandowska, D.; Gryczyńska, M.; Fichna, P.; Sowiński, J.; Nowak, J. Association of the CYP27B1C(-1260)A polymorphism with autoimmune Addison’s disease. Exp. Clin. Endocrinol. Diabetes 2010, 118, 544–549. [Google Scholar] [CrossRef]
- Lange, C.M.; Bojunga, J.; Ramos-Lopez, E.; von Wagner, M.; Hassler, A.; Vermehren, J.; Herrmann, E.; Badenhoop, K.; Zeuzem, S.; Sarrazin, C. Vitamin D deficiency and a CYP27B1-1260 promoter polymorphism are associated with chronic hepatitis C and poor response to interferon-alfa based therapy. J. Hepatol. 2011, 54, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.; Cooper, J.D.; Zeitels, L.; Smyth, D.J.; Yang, J.H.; Walker, N.M.; Hyppönen, E.; Dunger, D.B.; Ramos-Lopez, E.; Badenhoop, K.; et al. Association of the vitamin D metabolism gene CYP27B1 with type 1 diabetes. Diabetes 2007, 56, 2616–2621. [Google Scholar] [CrossRef] [Green Version]
- Wilke, R.A.; Simpson, R.U.; Mukesh, B.N.; Bhupathi, S.V.; Dart, R.A.; Ghebranious, N.R.; McCarty, C.A. Genetic variation in CYP27B1 is associated with congestive heart failure in patients with hypertension. Pharmacogenomics 2009, 10, 1789–1797. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, P. Multiple sclerosis: Vitamin D and calcium as environmental determinants of prevalence. Int. J. Environ. Stud. 1974, 6, 121–129. [Google Scholar] [CrossRef]
- Beretich, B.D.; Beretich, T.M. Explaining multiple sclerosis prevalence by ultraviolet exposure: A geospatial analysis. Mult. Scler. 2009, 15, 891–898. [Google Scholar] [CrossRef]
- Holick, M.F.; Cook, S.; Suarez, G.; Rametta, M. Vitamin D deficiency and possible role in multiple sclerosis. Eur. Neurol. Rev. 2015, 10, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Marling, S.J.; Zhu, J.G.; Severson, K.S.; DeLuca, H.F. Development of experimental autoimmune encephalomyelitis (EAE) in mice requires vitamin D and the vitamin D receptor. Proc. Natl. Acad. Sci. USA 2012, 109, 8501–8504. [Google Scholar] [CrossRef] [Green Version]
- Chiuso-Minicucci, F.; Ishikawa, L.L.; Mimura, L.A.; Fraga-Silva, T.F.; França, T.G.; Zorzella-Pezavento, S.F.; Marques, C.; Ikoma, M.R.; Sartori, A. Treatment with vitamin D/MOG association suppresses experimental autoimmune encephalomyelitis. PLoS ONE 2015, 10, e0125836. [Google Scholar] [CrossRef]
- Agnello, L.; Scazzone, C.; Lo Sasso, B.; Bellia, C.; Bivona, G.; Realmuto, S.; Brighina, F.; Schillaci, R.; Ragonese, P.; Salemi, G.; et al. VDBP, CYP27B1, and 25-hydroxyvitamin D gene polymorphism analyses in a group of Sicilian multiple sclerosis patients. Biochem. Genet. 2017, 55, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Ramagopalan, S.V.; Dyment, D.A.; Cader, M.Z.; Morrison, K.M.; Disanto, G.; Morahan, J.M.; Berlanga-Taylor, A.J.; Handel, A.; De Luca, G.C.; Sadovnick, A.D.; et al. Rare variants in the CYP27B1 gene are associated with multiple sclerosis. Ann. Neuro. 2011, 70, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Alcina, A.; Fedetz, M.; Fernández, O.; Saiz, A.; Izquierdo, G.; Lucas, M.; Leyva, L.; García-León, J.A.; del Mar Abad-Grau, M.; Alloza, I.; et al. Identification of a functional variant in the KIF5A-CYP27B1-METTL1-FAM119B locus associated with multiple sclerosis. J. Med. Genet. 2013, 50, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Jiang, T.; Li, L.; Wang, Y.; Zhao, C.; Yang, J.; Ma, D.; Guan, Y.; Zhao, D.; Bao, Y.; Wang, Y.; et al. The association between genetic polymorphism rs703842 in CYP27B1 and multiple sclerosis: A meta–analysis. Medicine (Baltimore) 2016, 95, e3612. [Google Scholar] [CrossRef]
- Miller, W.L. Genetic disorders of vitamin D biosynthesis and degradation. J. Steroid Biochem. Mol. Biol. 2017, 165, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Zalewski, A.; Ma, N.S.; Legeza, B.; Renthal, N.; Flück, C.E.; Pandey, A.V. Vitamin D-dependent rickets type 1 caused by mutations in CYP27B1 affecting protein interactions with adrenodoxin. J. Clin. Endocrinol. Metab. 2016, 101, 3409–3418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demir, K.; Kattan, W.E.; Zou, M.; Durmaz, E.; BinEssa, H.; Nalbantoğlu, Ö.; Al-Rijjal, R.A.; Meyer, B.; Özkan, B.; Shi, Y. Novel CYP27B1 gene mutations in patients with vitamin D-dependent rickets type 1A. PLoS ONE 2015, 10, e0131376. [Google Scholar] [CrossRef]
- Georgakopoulou, E.A.; Achtari, M.D.; Achtaris, M.; Foukas, P.G.; Kotsinas, A. Oral lichen planus as a preneoplastic inflammatory model. J. Biomed. Biotechnol. 2012, 2012, 759626. [Google Scholar] [CrossRef]
- Carrozzo, M.; Thorpe, R. Oral lichen planus: A review. Minerva Stomatol. 2009, 58, 519–537. [Google Scholar]
- Kujundzic, B.; Zeljic, K.; Supic, G.; Magic, M.; Stanimirovic, D.; Ilic, V.; Jovanovic, B.; Magic, Z. Association of vdr, cyp27b1, cyp24a1 and mthfr gene polymorphisms with oral lichen planus risk. Clin. Oral Investig. 2016, 20, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Zeljic, K.; Supic, G.; Stamenkovic Radak, M.; Jovic, N.; Kozomara, R.; Magic, Z. Vitamin D receptor, CYP27B1 and CYP24A1 genes polymorphisms association with oral cancer risk and survival. J. Oral. Pathol. Med. 2012, 41, 779–787. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, H.F.; Zierold, C. Mechanisms and functions of vitamin D. Nutr. Rev. 1998, 56, S4–S10. [Google Scholar] [CrossRef] [PubMed]

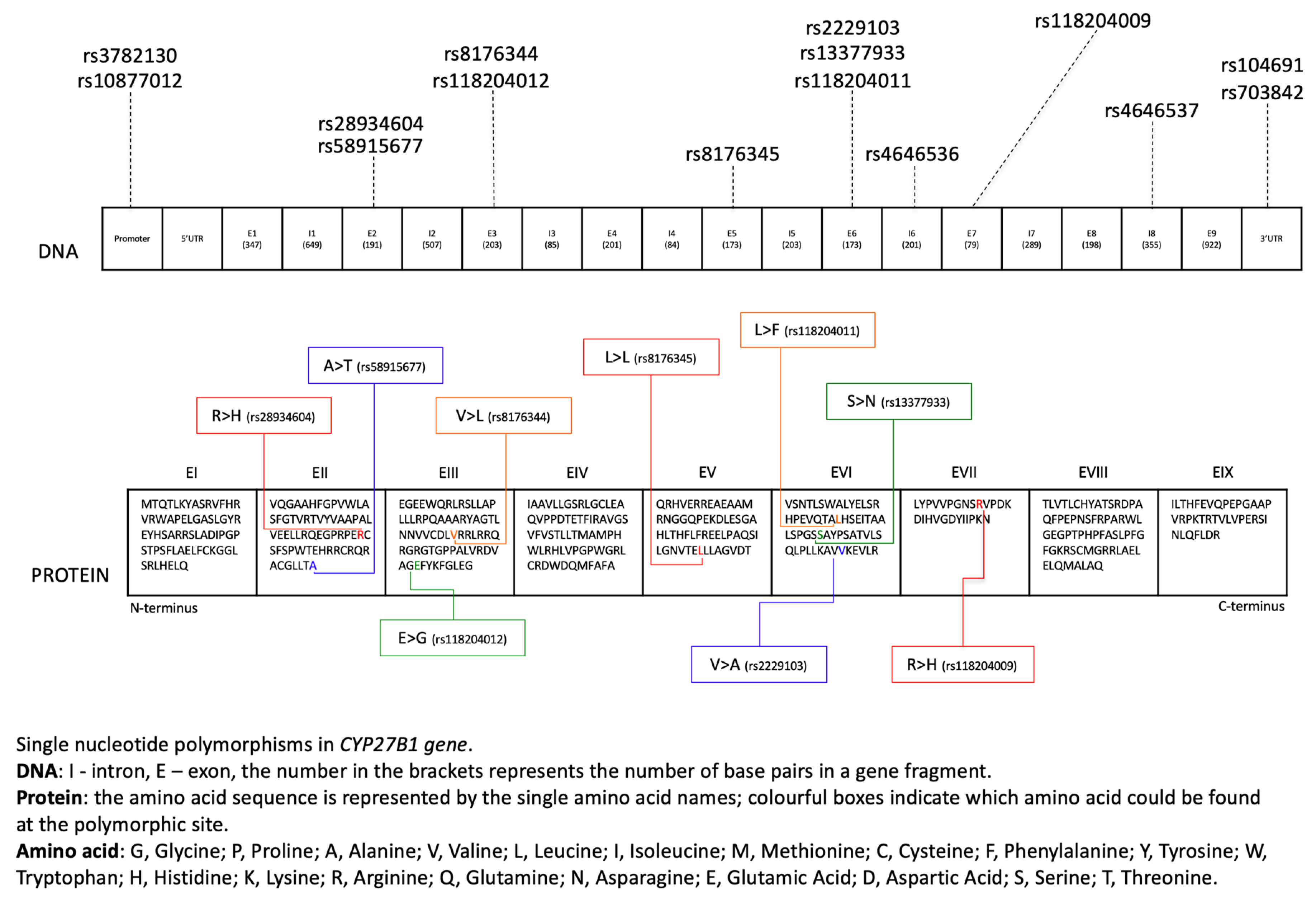
| Single Nucleotide Polymorphism | Location | Description | Frequency | Pathology | Reference |
|---|---|---|---|---|---|
| rs10877012 | 5′ of the gene/promoter region | G > T | T = 0.27826 (34940/125568, TOPMED) |
| [16,39,40,41,69,79,82,83,85,90,112,113,116,130,134,135] |
| rs4646536 | Intron 6 | A > G | G = 0.37843 (93102/246024, GnomAD) |
| [16,35,68,79,82,102,116,130,136,137,152,153] |
| rs28934604 | Exon 2 | C > T | None |
| [50] |
| rs58915677 | Exon 2 | C > T | T = 0.00020 (34/170818, GnomAD) |
| [50] |
| rs8176344 | Exon 3 | C > G | G = 0.00993 (2404/242112, GnomAD) |
| [50] |
| rs13377933 | Exon 6 | C > T | None |
| [50] |
| rs4646537 | Intron 8 | T > G | G = 0.05028 (6314/125568, TOPMED) |
| [16,62,71,79,82,93,132,137] |
| rs2229103 | Exon 6 | A > G | G = 0.00001 (2/246262, GnomAD) |
| [50,52] |
| rs1048691 | 3′ UTR | C > T | T = 0.26822 (33680/125568, TOPMED) |
| [69,76,82,83,117] |
| rs703842 | 3′ UTR | A > G | G = 0.38360 (92103/240104, GnomAD) |
| [76,79,82,116,117,132,146] |
| rs8176345 | Exon 5 | C > T | T = 0.02302 (5668/246250, GnomAD) |
| [69,76,82,83] |
| rs10877013 | N/A | C > T | T = 0.38135 (88444/231922, GnomAD) |
| [76,117] |
| rs3782130 | 5′ of the gene/promoter region | G > C | C = 0.28052 (35224/125568, TOPMED) |
| [71,79,112,113] |
| rs118204009 | Exon 7 | C > T | T = 0.00010 (25/246260, GnomAD) |
| [143,144] |
| rs118204011 | Exon 6 | G > A | None |
| [143] |
| rs118204012 | Exon 3 | T > C | C = 0.0009 (91/97256, ExAC) |
| [143] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latacz, M.; Snarska, J.; Kostyra, E.; Fiedorowicz, E.; Savelkoul, H.F.J.; Grzybowski, R.; Cieślińska, A. Single Nucleotide Polymorphisms in 25-Hydroxyvitamin D3 1-Alpha-Hydroxylase (CYP27B1) Gene: The Risk of Malignant Tumors and Other Chronic Diseases. Nutrients 2020, 12, 801. https://doi.org/10.3390/nu12030801
Latacz M, Snarska J, Kostyra E, Fiedorowicz E, Savelkoul HFJ, Grzybowski R, Cieślińska A. Single Nucleotide Polymorphisms in 25-Hydroxyvitamin D3 1-Alpha-Hydroxylase (CYP27B1) Gene: The Risk of Malignant Tumors and Other Chronic Diseases. Nutrients. 2020; 12(3):801. https://doi.org/10.3390/nu12030801
Chicago/Turabian StyleLatacz, Maria, Jadwiga Snarska, Elżbieta Kostyra, Ewa Fiedorowicz, Huub F. J. Savelkoul, Roman Grzybowski, and Anna Cieślińska. 2020. "Single Nucleotide Polymorphisms in 25-Hydroxyvitamin D3 1-Alpha-Hydroxylase (CYP27B1) Gene: The Risk of Malignant Tumors and Other Chronic Diseases" Nutrients 12, no. 3: 801. https://doi.org/10.3390/nu12030801