Glycolate is a Novel Marker of Vitamin B2 Deficiency Involved in Gut Microbe Metabolism in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Vitamin Measurement
2.3. Glycolate Oxidase Activity Assay
2.4. Metabolome Analysis by Capillary Electrophoresis Electrospray Ionization Time-of-Flight Mass Spectrometry
2.5. Statistical Analyses
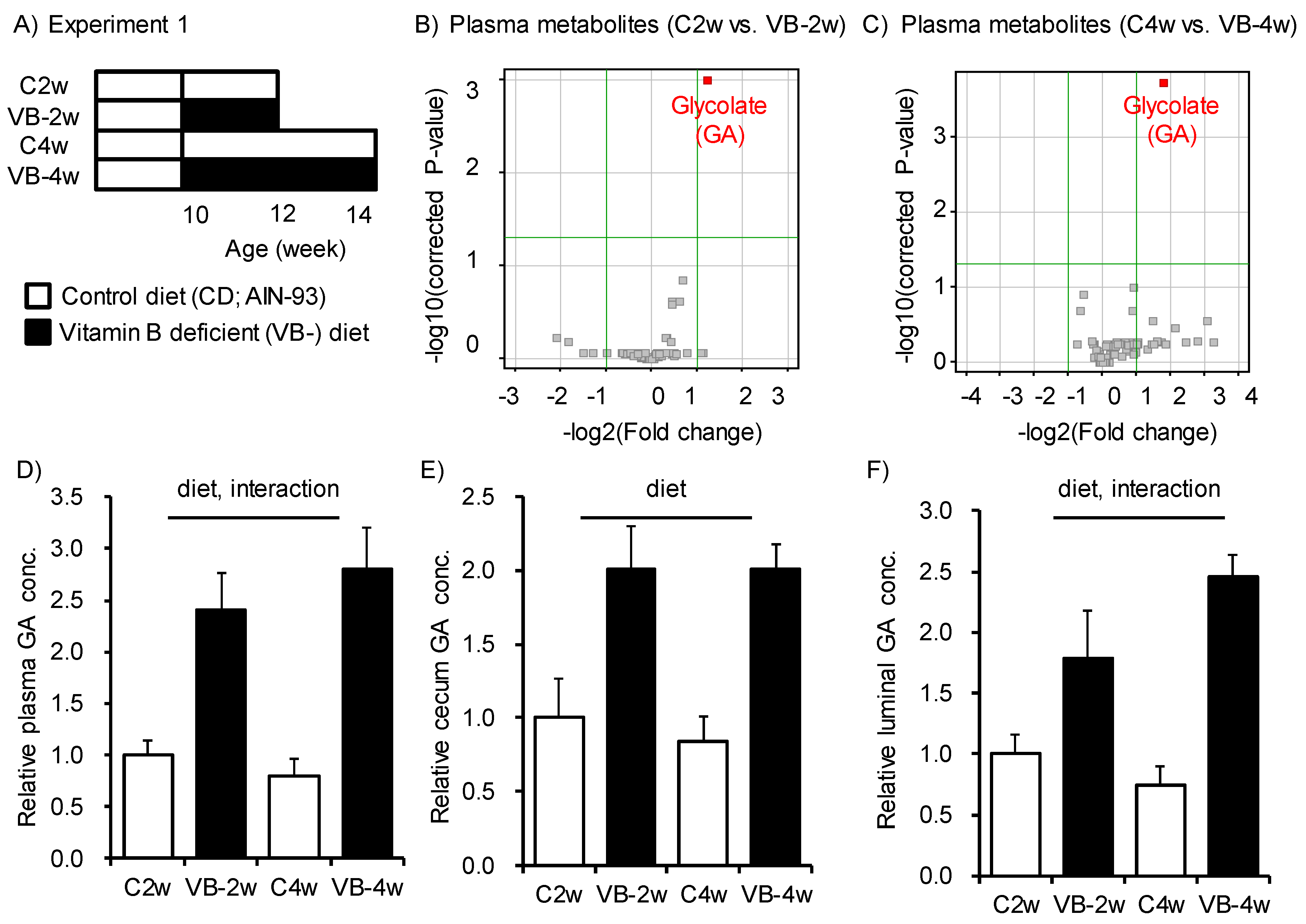
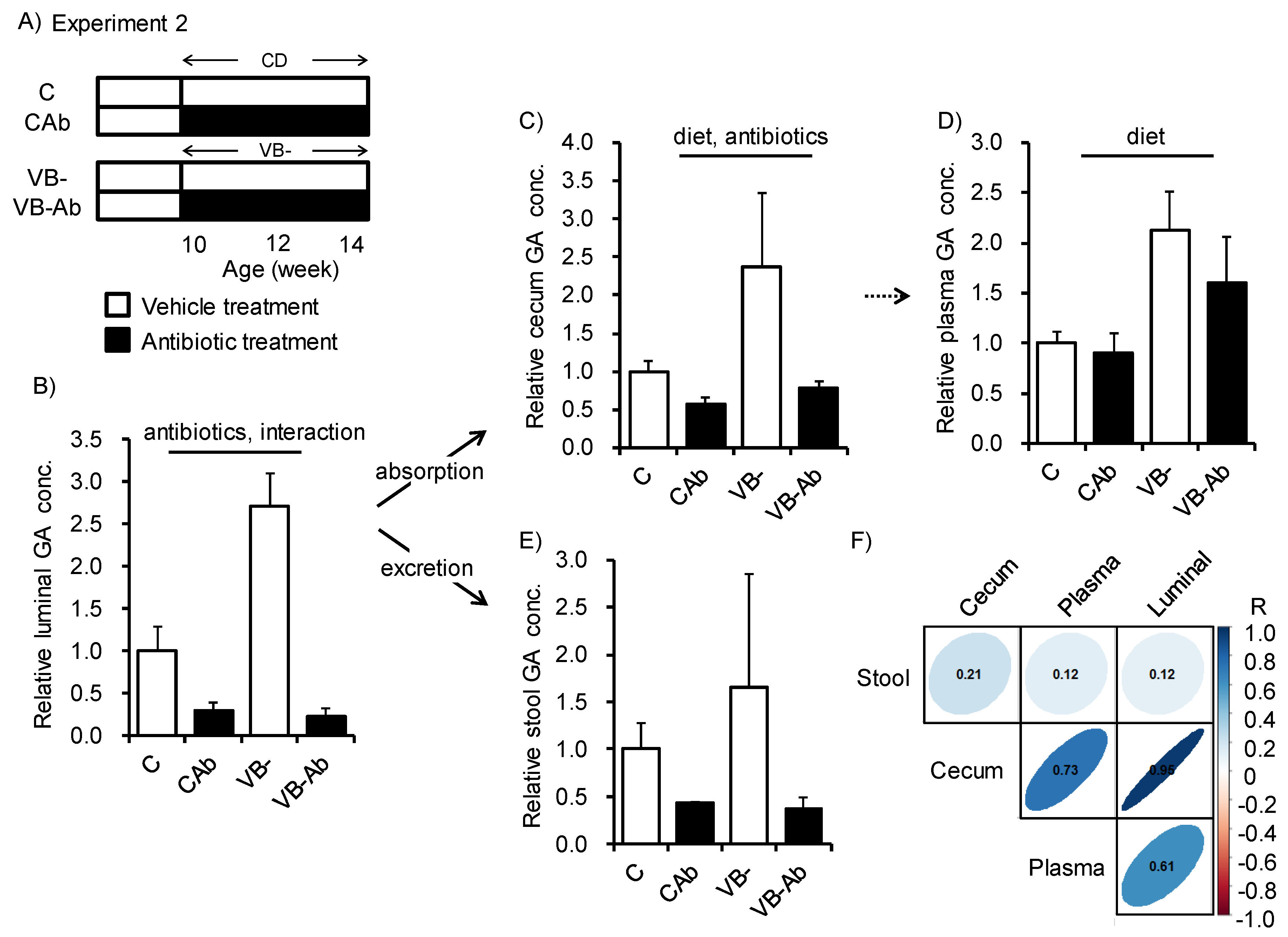
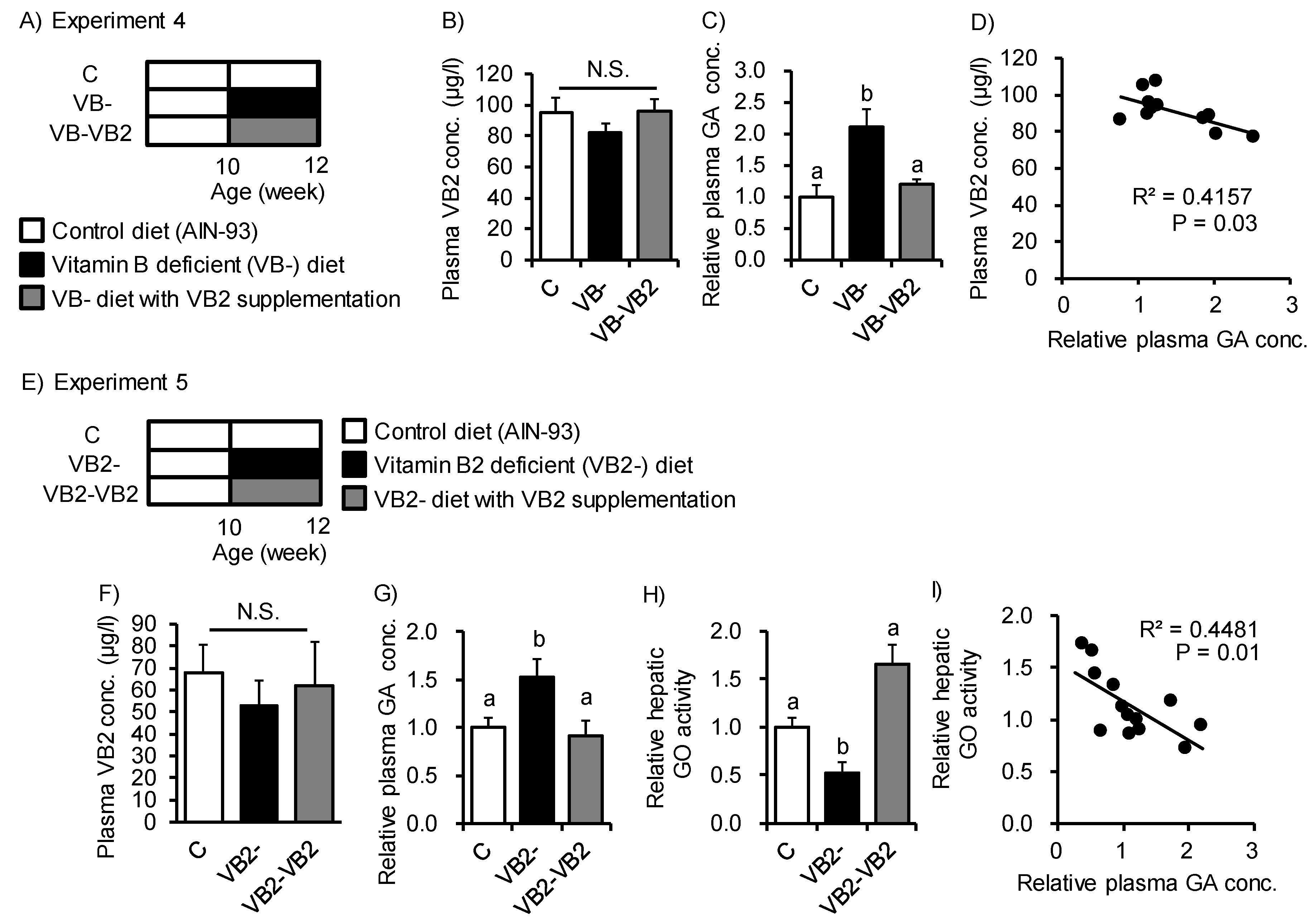
3. Results
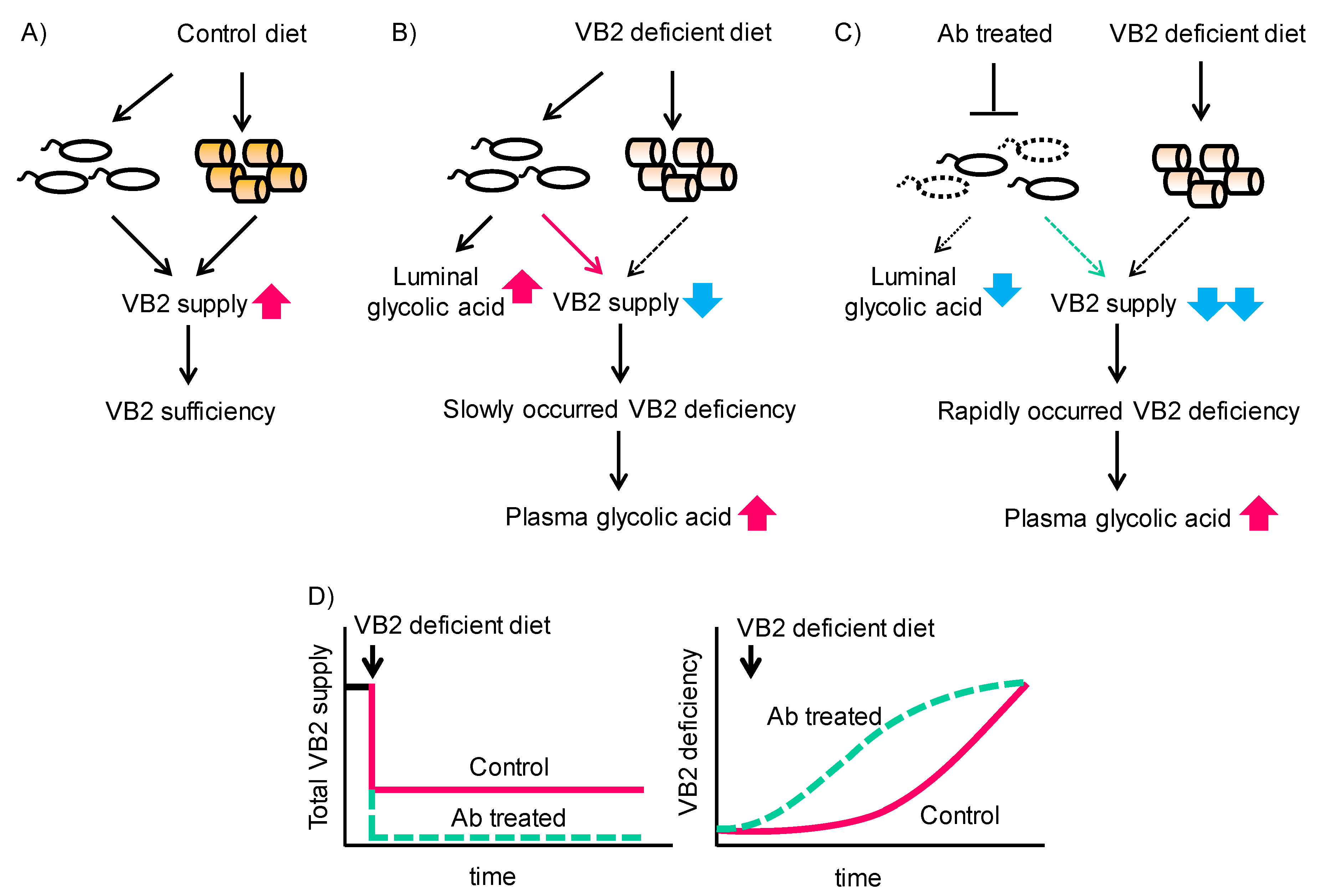
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sender, R.; Fuchs, S.; Milo, R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell 2016, 164, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Stiemsma, L.T.; Michels, K.B. The Role of the Microbiome in the Developmental Origins of Health and Disease. Pediatrics 2018, 141, e20172437. [Google Scholar] [CrossRef] [PubMed]
- Wostmann, B.S.; Larkin, C.; Moriarty, A.; Bruckner-Kardoss, E. Dietary intake, energy metabolism, and excretory losses of adult male germfree Wistar rats. Lab. Anim. Sci. 1983, 33, 46–50. [Google Scholar] [PubMed]
- Schroeder, B.O.; Backhed, F. Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 2016, 22, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Backhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef]
- Davie, J.R. Inhibition of histone deacetylase activity by butyrate. J. Nutr. 2003, 133, 2485S–2493S. [Google Scholar] [CrossRef]
- Kawamata, Y.; Fujii, R.; Hosoya, M.; Harada, M.; Yoshida, H.; Miwa, M.; Fukusumi, S.; Habata, Y.; Itoh, T.; Shintani, Y.; et al. A G protein-coupled receptor responsive to bile acids. J. Biol. Chem. 2003, 278, 9435–9440. [Google Scholar] [CrossRef]
- Lew, J.L.; Zhao, A.; Yu, J.; Huang, L.; de Pedro, N.; Peláez, F.; Wright, S.D.; Cui, J. The farnesoid X receptor controls gene expression in a ligand- and promoter-selective fashion. J. Biol. Chem. 2004, 279, 8856–8861. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, A.; Wahlstrom, A.; Marschall, H.U. Role of Bile Acids in Metabolic Control. Trends Endocrinol. Metab. 2018, 29, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Silverman, M.; Werkman, C.H. Adaptation of the Propionic-Acid Bacteria to Vitamin B(1) Synthesis Including a Method of Assay. J. Bacteriol. 1939, 38, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Burkholder, P.R.; McVeigh, I. Synthesis of Vitamins by Intestinal Bacteria. Proc. Natl. Acad. Sci. USA 1942, 28, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Strozzi, G.P.; Mogna, L. Quantification of folic acid in human feces after administration of Bifidobacterium probiotic strains. J. Clin. Gastroenterol. 2008, 42 Pt 2 (Suppl. 3), S179–S184. [Google Scholar] [CrossRef]
- Pompei, A.; Cordisco, L.; Amaretti, A.; Zanoni, S.; Raimondi, S.; Matteuzzi, D.; Rossi, M. Administration of folate-producing bifidobacteria enhances folate status in Wistar rats. J. Nutr. 2007, 137, 2742–2746. [Google Scholar] [CrossRef]
- Hayashi, A.; Mikami, Y.; Miyamoto, K.; Kamada, N.; Sato, T.; Mizuno, S.; Naganuma, M.; Teratani, T.; Aoki, R.; Fukuda, S.; et al. Intestinal Dysbiosis and Biotin Deprivation Induce Alopecia through Overgrowth of Lactobacillus murinus in Mice. Cell Rep. 2017, 20, 1513–1524. [Google Scholar] [CrossRef]
- Miki, T.; Goto, R.; Fujimoto, M.; Okada, N.; Hardt, W.D. The Bactericidal Lectin RegIIIbeta Prolongs Gut Colonization and Enteropathy in the Streptomycin Mouse Model for Salmonella Diarrhea. Cell Host Microbe 2017, 21, 195–207. [Google Scholar] [CrossRef]
- Cordonnier, C.; Le Bihan, G.; Emond-Rheault, J.G.; Garrivier, A.; Harel, J.; Jubelin, G. Vitamin B12 Uptake by the Gut Commensal Bacteria Bacteroides thetaiotaomicron Limits the Production of Shiga Toxin by Enterohemorrhagic Escherichia coli. Toxins (Basel) 2016, 8, 14. [Google Scholar] [CrossRef]
- Ducker, G.S.; Rabinowitz, J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef]
- Locasale, J.W. Serine, glycine and one-carbon units: Cancer metabolism in full circle. Nat. Rev. Cancer 2013, 13, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Macheroux, P.; Massey, V.; Thiele, D.J.; Volokita, M. Expression of spinach glycolate oxidase in Saccharomyces cerevisiae: Purification and characterization. Biochemistry 1991, 30, 4612–4619. [Google Scholar] [CrossRef] [PubMed]
- Rojas, C.M.; Senthil-Kumar, M.; Wang, K.; Ryu, C.M.; Kaundal, A.; Mysore, K.S. Glycolate oxidase modulates reactive oxygen species-mediated signal transduction during nonhost resistance in Nicotiana benthamiana and Arabidopsis. Plant Cell 2012, 24, 336–352. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, A.; Uebanso, T.; Nakahashi, M.; Shimohata, T.; Mawatari, K.; Takahashi, A. Effect of prenatal administration of low dose antibiotics on gut microbiota and body fat composition of newborn mice. J. Clin. Biochem. Nutr. 2018, 62, 155–160. [Google Scholar] [CrossRef]
- Uebanso, T.; Kano, S.; Yoshimoto, A.; Naito, C.; Shimohata, T.; Mawatari, K.; Takahashi, A. Effects of Consuming Xylitol on Gut Microbiota and Lipid Metabolism in Mice. Nutrients 2017, 9, 756. [Google Scholar] [CrossRef]
- Uebanso, T.; Ohnishi, A.; Kitayama, R.; Yoshimoto, A.; Nakahashi, M.; Shimohata, T.; Mawatari, K.; Takahashi, A. Effects of Low-Dose Non-Caloric Sweetener Consumption on Gut Microbiota in Mice. Nutrients 2017, 9, 560. [Google Scholar] [CrossRef]
- Kami, K.; Fujimori, T.; Sato, H.; Sato, M.; Yamamoto, H.; Ohashi, Y.; Sugiyama, N.; Ishihama, Y.; Onozuka, H.; Ochiai, A.; et al. Metabolomic profiling of lung and prostate tumor tissues by capillary electrophoresis time-of-flight mass spectrometry. Metabolomics 2013, 9, 444–453. [Google Scholar] [CrossRef]
- Ohashi, Y.; Hirayama, A.; Ishikawa, T.; Nakamura, S.; Shimizu, K.; Ueno, Y.; Tomita, M.; Soga, T. Depiction of metabolome changes in histidine-starved Escherichia coli by CE-TOFMS. Mol. Biosyst. 2008, 4, 135–147. [Google Scholar] [CrossRef]
- Ogawa, Y.; Hossain, R.Z.; Ogawa, T.; Yamakawa, K.; Yonou, H.; Oshiro, Y.; Hokama, S.; Morozumi, M.; Uchida, A.; Sugaya, K. Vitamin B6 deficiency augments endogenous oxalogenesis after intravenous L-hydroxyproline loading in rats. Urol. Res. 2007, 35, 15–21. [Google Scholar] [CrossRef]
- Li, X.; Knight, J.; Fargue, S.; Buchalski, B.; Guan, Z.; Inscho, E.W.; Liebow, A.; Fitzgerald, K.; Querbes, W.; Todd Lowther, W.; et al. Metabolism of (13)C5-hydroxyproline in mouse models of Primary Hyperoxaluria and its inhibition by RNAi therapeutics targeting liver glycolate oxidase and hydroxyproline dehydrogenase. Biochim. Biophys. Acta 2016, 1862, 233–239. [Google Scholar] [CrossRef]
- Martin-Higueras, C.; Luis-Lima, S.; Salido, E. Glycolate Oxidase Is a Safe and Efficient Target for Substrate Reduction Therapy in a Mouse Model of Primary Hyperoxaluria Type I. Mol. Ther. 2016, 24, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Pace, C.; Stankovich, M. Oxidation-reduction properties of glycolate oxidase. Biochemistry 1986, 25, 2516–2522. [Google Scholar] [CrossRef] [PubMed]
- Schuman, M.; Massey, V. Purification and characterization of glycolic acid oxidase from pig liver. Biochim. Biophys. Acta 1971, 227, 500–520. [Google Scholar] [CrossRef][Green Version]
- Cha, M.; Kim, E.J.; Yun, H.; Cho, B.K.; Kim, B.G. Synthesis of enantiopure (S)-2-hydroxyphenylbutanoic acid using novel hydroxy acid dehydrogenase from Enterobacter sp. BK2K. Biotechnol. Prog. 2007, 23, 606–612. [Google Scholar] [CrossRef]
- Lee, H.S.; Park, J.; Yoo, Y.J.; Yeon, Y.J. A novel d-2-hydroxy acid dehydrogenase with high substrate preference for phenylpyruvate originating from lactic acid bacteria: Structural analysis on the substrate specificity. Enzyme Microb. Technol. 2019, 125, 37–44. [Google Scholar] [CrossRef]
- Jones, J.M.; Morrell, J.C.; Gould, S.J. Identification and characterization of HAOX1, HAOX2, and HAOX3, three human peroxisomal 2-hydroxy acid oxidases. J. Biol. Chem. 2000, 275, 12590–12597. [Google Scholar] [CrossRef]
- Kohler, S.A.; Menotti, E.; Kuhn, L.C. Molecular cloning of mouse glycolate oxidase. High evolutionary conservation and presence of an iron-responsive element-like sequence in the mRNA. J. Biol. Chem. 1999, 274, 2401–2407. [Google Scholar] [CrossRef]
- Kun, E.; Dechary, J.M.; Pitot, H.C. The oxidation of glycolic acid by a liver enzyme. J. Biol. Chem. 1954, 210, 269–280. [Google Scholar]
- Nishijima, S.; Sugaya, K.; Hokama, S.; Oshiro, Y.; Uchida, A.; Morozumi, M.; Ogawa, Y. Effect of vitamin B6 deficiency on glyoxylate metabolism in rats with or without glyoxylate overload. Biomed. Res. 2006, 27, 93–98. [Google Scholar] [CrossRef]
- Teerajetgul, Y.; Hossain, R.Z.; Machida, N.; Sugaya, K.; Ogawa, Y. Endogenous oxalogenesis after acute intravenous loading with ethylene glycol or glycine in rats receiving standard and vitamin B6-deficient diets. Int. J. Urol. 2008, 15, 929–935. [Google Scholar] [CrossRef]
- Teerajetgul, Y.; Hossain, R.Z.; Yamakawa, K.; Morozumi, M.; Sugaya, K.; Ogawa, Y. Oxalate synthesis from hydroxypyruvate in vitamin-B6-deficient rats. Urol. Res. 2007, 35, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Frantz, S.W.; Beskitt, J.L.; Grosse, C.M.; Tallant, M.J.; Dietz, F.K.; Ballantyne, B. Pharmacokinetics of ethylene glycol. II. Tissue distribution, dose-dependent elimination, and identification of urinary metabolites following single intravenous, peroral or percutaneous doses in female Sprague-Dawley rats and CD-1 mice. Xenobiotica 1996, 26, 1195–1220. [Google Scholar] [CrossRef] [PubMed]
- Thakur, K.; Tomar, S.K.; De, S. Lactic acid bacteria as a cell factory for riboflavin production. Microb. Biotechnol. 2016, 9, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, A.; Masuda, S.; Katsura, T.; Inui, K. Identification and functional characterization of a novel human and rat riboflavin transporter, RFT1. Am. J. Physiol. Cell Physiol. 2008, 295, C632–C641. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, V.S.; Subramanya, S.B.; Rapp, L.; Marchant, J.S.; Ma, T.Y.; Said, H.M. Differential expression of human riboflavin transporters -1, -2, and -3 in polarized epithelia: A key role for hRFT-2 in intestinal riboflavin uptake. Biochim. Biophys. Acta 2011, 1808, 3016–3021. [Google Scholar] [CrossRef][Green Version]
- Subramanian, V.S.; Sabui, S.; Heskett, C.W.; Said, H.M. Sodium Butyrate Enhances Intestinal Riboflavin Uptake via Induction of Expression of Riboflavin Transporter-3 (RFVT3). Dig. Dis. Sci. 2019, 64, 84–92. [Google Scholar] [CrossRef]
- Yamamoto, S.; Inoue, K.; Ohta, K.Y.; Fukatsu, R.; Maeda, J.Y.; Yoshida, Y.; Yuasa, H. Identification and functional characterization of rat riboflavin transporter 2. J. Biochem. 2009, 145, 437–443. [Google Scholar] [CrossRef]
- Yao, Y.; Yonezawa, A.; Yoshimatsu, H.; Masuda, S.; Katsura, T.; Inui, K. Identification and comparative functional characterization of a new human riboflavin transporter hRFT3 expressed in the brain. J. Nutr. 2010, 140, 1220–1226. [Google Scholar] [CrossRef]
- Yonezawa, A.; Inui, K. Novel riboflavin transporter family RFVT/SLC52: Identification, nomenclature, functional characterization and genetic diseases of RFVT/SLC52. Mol. Aspects Med. 2013, 34, 693–701. [Google Scholar] [CrossRef]
- Magnusdottir, S.; Ravcheev, D.; de Crecy-Lagard, V.; Thiele, I. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front. Genet. 2015, 6, 148. [Google Scholar] [CrossRef]
- Mushtaq, S.; Su, H.; Hill, M.H.; Powers, H.J. Erythrocyte pyridoxamine phosphate oxidase activity: A potential biomarker of riboflavin status? Am. J. Clin. Nutr. 2009, 90, 1151–1159. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uebanso, T.; Yoshimoto, A.; Aizawa, S.; Nakamura, M.; Masuda, R.; Shimohata, T.; Mawatari, K.; Takahashi, A. Glycolate is a Novel Marker of Vitamin B2 Deficiency Involved in Gut Microbe Metabolism in Mice. Nutrients 2020, 12, 736. https://doi.org/10.3390/nu12030736
Uebanso T, Yoshimoto A, Aizawa S, Nakamura M, Masuda R, Shimohata T, Mawatari K, Takahashi A. Glycolate is a Novel Marker of Vitamin B2 Deficiency Involved in Gut Microbe Metabolism in Mice. Nutrients. 2020; 12(3):736. https://doi.org/10.3390/nu12030736
Chicago/Turabian StyleUebanso, Takashi, Ayumi Yoshimoto, Shinta Aizawa, Maya Nakamura, Rumiko Masuda, Takaaki Shimohata, Kazuaki Mawatari, and Akira Takahashi. 2020. "Glycolate is a Novel Marker of Vitamin B2 Deficiency Involved in Gut Microbe Metabolism in Mice" Nutrients 12, no. 3: 736. https://doi.org/10.3390/nu12030736
APA StyleUebanso, T., Yoshimoto, A., Aizawa, S., Nakamura, M., Masuda, R., Shimohata, T., Mawatari, K., & Takahashi, A. (2020). Glycolate is a Novel Marker of Vitamin B2 Deficiency Involved in Gut Microbe Metabolism in Mice. Nutrients, 12(3), 736. https://doi.org/10.3390/nu12030736





