Mild to Moderate Iodine Deficiency and Inadequate Iodine Intake in Lactating Women in the Inland Area of Norway
Abstract
:1. Introduction
2. Materials and Methods
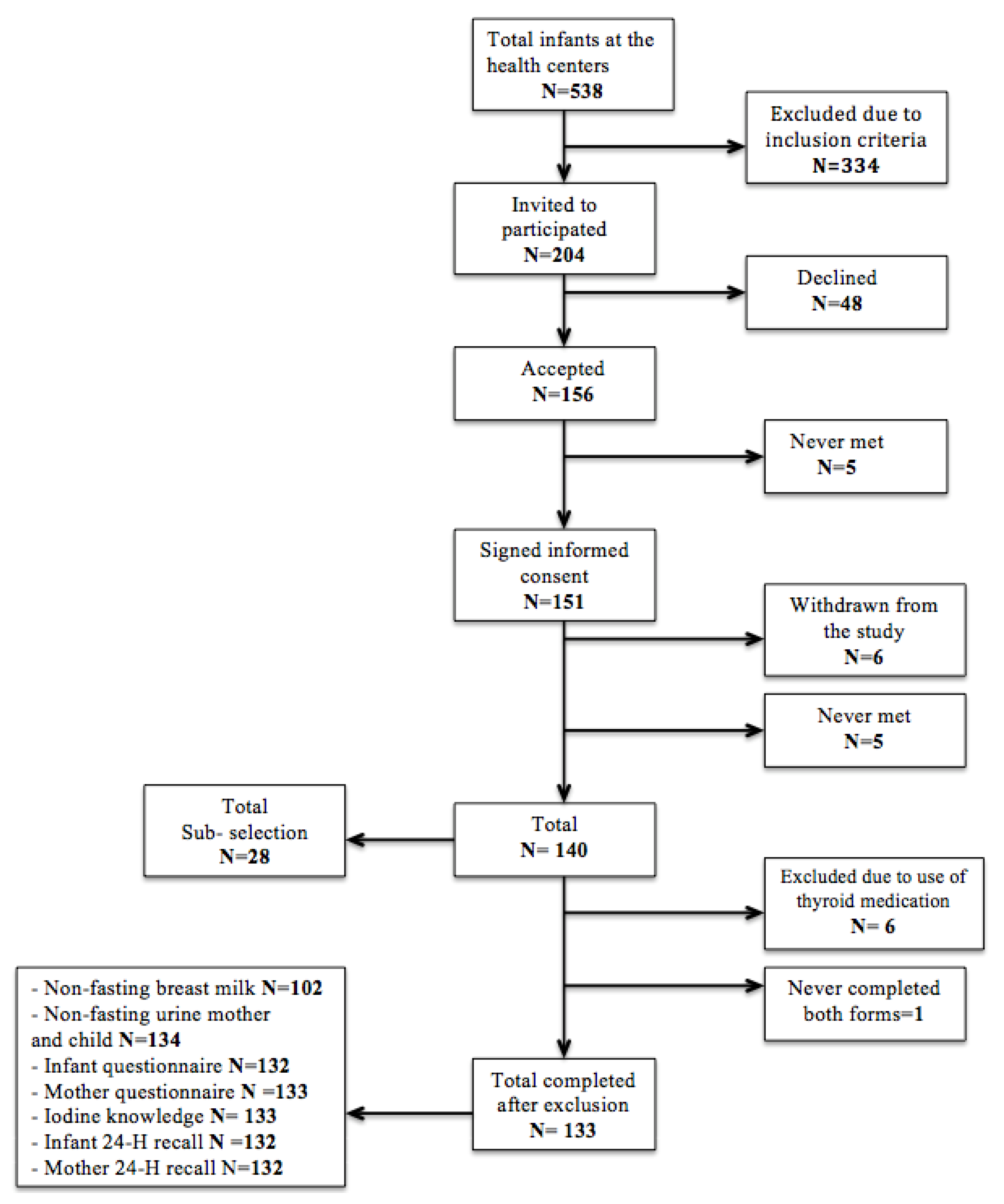
2.1. Study Population
2.2. Study Design
2.3. Etichs
2.4. Collection of Human Milk and Urine Samples
2.5. Chemical Analysis
2.6. Iodine Intake From Food and Supplements
2.7. Assessment of Iodine Knowledge
2.8. Definitions
2.9. Statistical Analysis
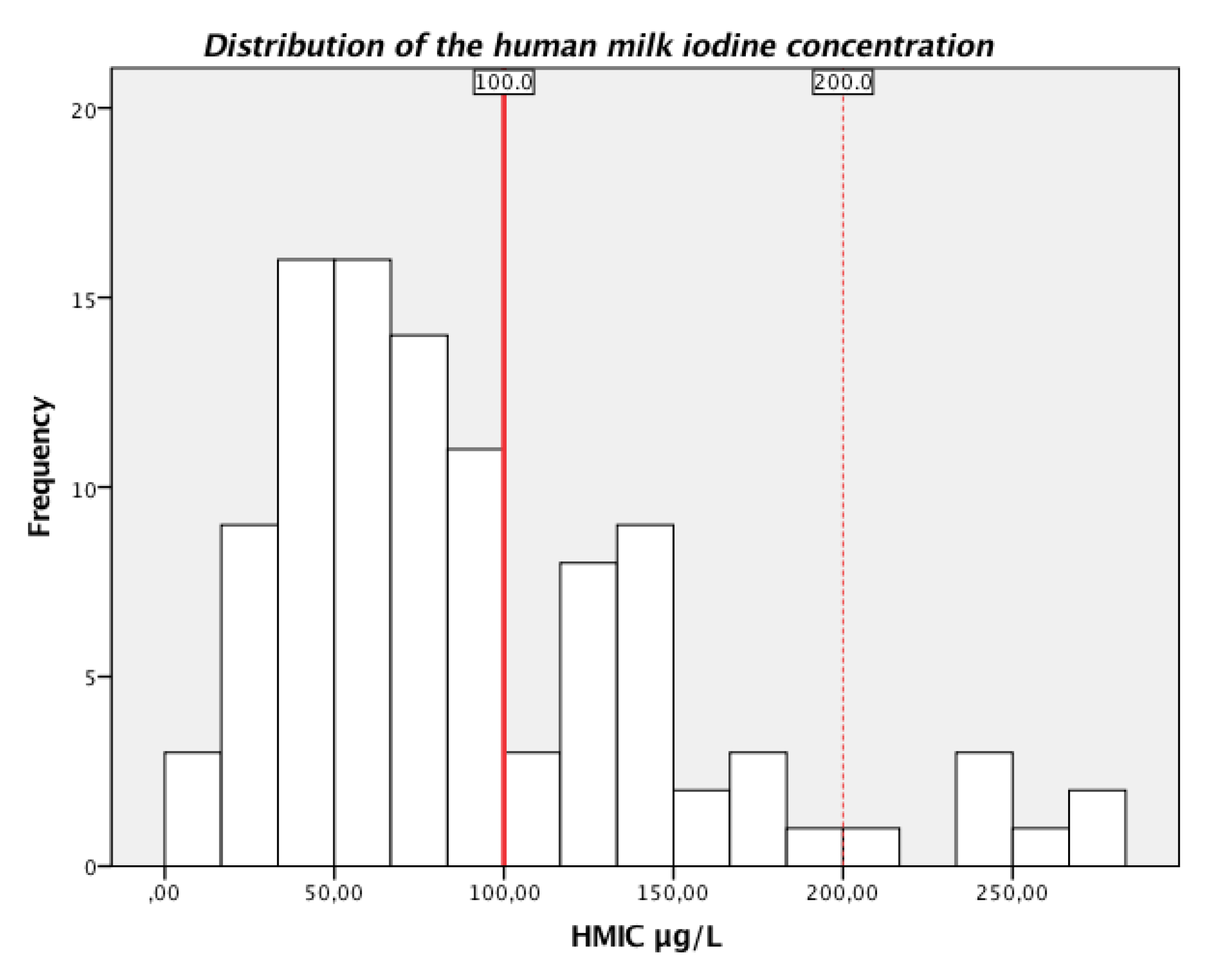
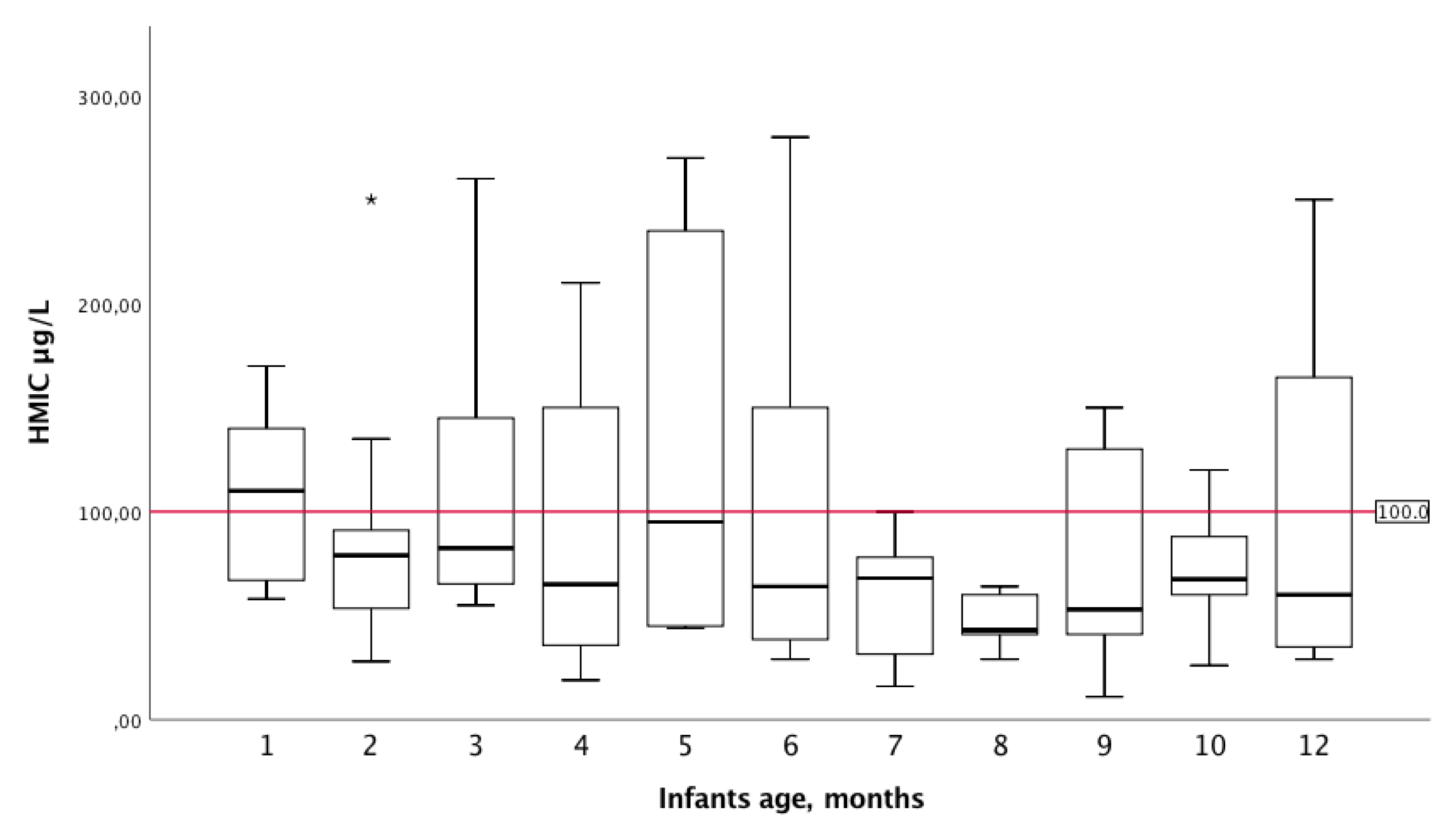
3. Results
3.1. Participant Characteristics
3.2. Calculated Habitual and 24-h Iodine Intake
3.3. Iodine Knowledge Scores and Association with HMIC and Iodine Intake
3.4. Predictors of Human Milk iodine concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zimmermann, M. The role of iodine in human growth and development. Semin. Cell Dev. Boil. 2011, 22, 645–652. [Google Scholar] [CrossRef]
- Azizi, F.; Smyth, P. Breastfeeding and maternal and infant iodine nutrition. Clin. Endocrinol. 2009, 70, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Dold, S.; Cherkaoui, M.; Jia, Q.; Kusić, Z.; Quirino, A.; Luis, T.O.S.; Vandea, E.; Zimmermann, M.; Aboussad, A.; Jukić, T.; et al. Breast Milk Iodine Concentration Is a More Accurate Biomarker of Iodine Status Than Urinary Iodine Concentration in Exclusively Breastfeeding Women. J. Nutr. 2017, 147, 528–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurberg, P.; Pedersen, I.; Carlé, A.; Andersen, S.; Knudsen, N.; Ovesen, L.; Rasmussen, L.B. The U-Shaped Curve of Iodine Intake and Thyroid Disorders. In Comprehensive Handbook of Iodine; Preedy, V.R., Burrow, G.N., Watson, R., Eds.; Academic Press: Cambridge, MA, USA, 2009; pp. 449–455. [Google Scholar]
- Velasco, I.; Bath, S.; Rayman, M.P. Iodine as Essential Nutrient during the First 1000 Days of Life. Nutrients 2018, 10, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmermann, M. Iodine Deficiency. Endocr. Rev. 2009, 30, 376–408. [Google Scholar] [CrossRef] [Green Version]
- Moog, N.K.; Entringer, S.; Heim, C.; Wadhwa, P.D.; Kathmann, N.; Buss, C. Influence of maternal thyroid hormones during gestation on fetal brain development. Neuroscience 2015, 342, 68–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The EUthyroid Consortium. The Krakow Declaration on Iodine: Tasks and Responsibilities for Prevention Programs Targeting Iodine Deficiency Disorders. Eur. Thyroid. J. 2018, 7, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Frey, H.; Rosenlund, B.; Try, K.; Theodorsen, L. Urinary Excretion of Iodine in Norway. In Iodine Deficiency in Europe; Delange, F., Ed.; Plenum Press: New York, NY, USA, 1993; pp. 297–300. [Google Scholar]
- Nyström, H.F.; Brantsæter, A.L.; Erlund, I.; Gunnarsdottir, I.; Hulthen, L.; Laurberg, P.; Mattisson, I.; Rasmussen, L.B.; Virtanen, S.; Meltzer, H.M. Iodine status in the Nordic countries-past and present. Food Nutr. Res. 2016, 60, 31969. [Google Scholar] [CrossRef] [PubMed]
- Henjum, S.; Abel, M.H.; Meltzer, H.M.; Dahl, L.; Alexander, J.; Torheim, L.E.; Brantsæter, A.L. Er inntaket av jod i befolkningen tilstrekkelig? Tidsskr. Den Nor. Legeforening 2019, 139, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, J.H. Iodine status in europe in 2014. Eur. Thyroid. J. 2014, 3, 3–6. [Google Scholar] [CrossRef] [Green Version]
- Lande, B.; Helleve, A. Amming Og Spedbarns Kosthold. Landsomfattende Undersøkelse 2013 [Breastfeeding and Infant Diet. A National Survey 2013, in Norwegian]; Helsedirektoratet [Norwegian Directorate of Health]: Oslo, Norway, 2014; ISBN 978-82-8081-342-8.
- Abel, M.H.; Caspersen, I.H.; Meltzer, H.M.; Haugen, M.; Brandlistuen, R.; Aase, H.; Alexander, J.; Torheim, L.E.; Brantsæter, A.L. Suboptimal Maternal Iodine Intake Is Associated with Impaired Child Neurodevelopment at 3 Years of Age in the Norwegian Mother and Child Cohort Study. J. Nutr. 2017, 147, 1314–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markhus, M.W.; Dahl, L.; Moe, V.; Abel, M.H.; Brantsæter, A.L.; Øyen, J.; Meltzer, H.M.; Stormark, K.M.; Graff, I.E.; Smith, L.; et al. Maternal Iodine Status is Associated with Offspring Language Skills in Infancy and Toddlerhood. Nutrients 2018, 10, 1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abel, M.H.; Brandlistuen, R.; Caspersen, I.H.; Aase, H.; Torheim, L.E.; Meltzer, H.M.; Brantsæter, A.L. Language delay and poorer school performance in children of mothers with inadequate iodine intake in pregnancy: Results from follow-up at 8 years in the Norwegian Mother and Child Cohort Study. Eur. J. Nutr. 2018, 58, 3047–3058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NNR12 Project Group. Iodine. In Nordic Nutrition Recommendations 2012, Integrating Nutrition and Physical Activity, 5th ed.; Nordic Council of Ministers: Copenhagen, Denmark, 2014; pp. 583–590. [Google Scholar]
- Helsedirektoratet [Norwegian Directorate of Health]. Utviklingen I Norsk Kosthold 2019 [Trends in the Norwegian Diet 2019, in Norwegian]; Helsedirektoratet: Oslo, Norway, 2019.
- Dahl, L.; Johansson, L.; Julshamn, K.; Meltzer, H.M. The iodine content of Norwegian foods and diets. Public Health Nutr. 2004, 7, 569–576. [Google Scholar] [CrossRef] [Green Version]
- Carlsen, M.H.; Andersen, L.F.; Dahl, L.; Norberg, N.; Hjartåker, A. New Iodine Food Composition Database and Updated Calculations of Iodine Intake among Norwegians. Nutrients 2018, 10, 930. [Google Scholar] [CrossRef] [Green Version]
- Devold, O.; Batt, F.; Closs, K.; Backer, J. En strumaundersøkelse fra Modum. Nor. Mag. Lægevitenskap 1937, 7, 900–937. [Google Scholar]
- Schiøtz, C. Steller Norge Forsvarlig Med Sine Skolebarn Og Sin Ungdom? Nei! J.W. Cappelens Forlag: Kristiania (Oslo), Norway, 1917. [Google Scholar]
- Mattilsynet [Norwegian Food Safety Authority]. Matvaretabellen [The Norwegian food composition table]. Available online: https://www.Matportalen.No/verktoy/matvaretabellen/#tabs-1-1-anchor (accessed on 16 October 2018).
- Nerhus, I.; Markhus, M.W.; Nilsen, B.M.; Øyen, J.; Maage, A.; Ødegård, E.R.; Midtbø, L.K.; Frantzen, S.; Kogel, T.; Graff, I.E.; et al. Iodine content of six fish species, Norwegian dairy products and hen’s egg. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef] [Green Version]
- Henjum, S.; Lilleengen, A.M.; Aakre, I.; Dudareva, A.; Gjengedal, E.L.F.; Meltzer, H.M.; Brantsæter, A.L. Suboptimal Iodine Concentration in Breastmilk and Inadequate Iodine Intake among Lactating Women in Norway. Nutrients 2017, 9, 643. [Google Scholar] [CrossRef] [Green Version]
- Henjum, S.; Brantsæter, A.L.; Kurniasari, A.; Dahl, L.; Aadland, E.K.; Gjengedal, E.L.F.; Birkeland, S.; Aakre, I. Suboptimal Iodine Status and Low Iodine Knowledge in Young Norwegian Women. Nutrients 2018, 10, 941. [Google Scholar] [CrossRef] [Green Version]
- Garnweidner-Holme, L.; Aakre, I.; Lilleengen, A.M.; Brantsæter, A.L.; Henjum, S. Knowledge about Iodine in Pregnant and Lactating Women in the Oslo Area, Norway. Nutrients 2017, 9, 493. [Google Scholar] [CrossRef] [Green Version]
- WHO. Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination: A Guide for Programme Managers; World Health Organization (WHO), International Council for Control of Iodine Deficiency Disorders (ICCIDD), United Nations International Children’s Emergency Fund (UNICEF): Geneva, Switzerland, 2007. [Google Scholar]
- WHO Secretariat; Andersson, M.; De Benoist, B.; Delange, F.; Zupan, J. Prevention and control of iodine deficiency in pregnant and lactating women and in children less than 2-years-old: Conclusions and recommendations of the Technical Consultation. Public Health Nutr. 2007, 10, 1606–1611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dold, S.; Zimmermann, M.B.; Baumgartner, J.; Davaz, T.; Galetti, V.; Braegger, C.; Andersson, M. A dose-response crossover iodine balance study to determine iodine requirements in early infancy. Am. J. Clin. Nutr. 2016, 104, 620–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semba, R.D.; Delange, F. Iodine in human milk: Perspectives for infant health. Nutr. Rev. 2001, 59, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Dórea, J.G. Iodine nutrition and breast feeding. J. Trace Elem. Med. Boil. 2002, 16, 207–220. [Google Scholar] [CrossRef]
- Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Vitamin a, Vitamin k, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Butte, N.F.; Lopez-Alarcon, M.G.; Garza, C. Nutrient adequacy of exclusive breastfeeding for the term infant during the first six months of life. In Expert Consultation on the Optimal Duration of Exclusive Breastfeeding; World Health Organization: Geneva, Zwitzerland, 2002. [Google Scholar]
- Swanson, C.A.; Zimmermann, M.B.; Skeaff, S.; Pearce, E.N.; Dwyer, J.; Trumbo, P.R.; Zehaluk, C.; Andrews, K.W.; Carriquiry, A.; Caldwell, K.L.; et al. Summary of an NIH workshop to identify research needs to improve the monitoring of iodine status in the United States and to inform the DRI. J. Nutr. 2012, 142, 1175S–1185S. [Google Scholar] [CrossRef] [Green Version]
- Trumbo, P.R. Evidence Needed to Inform the Next Dietary Reference Intakes for Iodine. Adv. Nutr. 2013, 4, 718–722. [Google Scholar] [CrossRef] [Green Version]
- Delange, F.; Wolff, P.; Gnat, D.; Dramaix, M.; Pilchen, M.; Vertongen, F. Iodine deficiency during infancy and early childhood in Belgium: Does it pose a risk to brain development? Eur. J. Nucl. Med. Mol. Imaging 2001, 160, 251–254. [Google Scholar] [CrossRef]
- Dror, D.K.; Allen, L.H. Iodine in Human Milk: A Systematic Review. Adv. Nutr. 2018, 9, 347S–357S. [Google Scholar] [CrossRef] [Green Version]
- Andersen, S.L.; Møller, M.; Laurberg, P. Iodine Concentrations in Milk and in Urine during Breastfeeding Are Differently Affected by Maternal Fluid Intake. Thyroid 2014, 24, 764–772. [Google Scholar] [CrossRef] [Green Version]
- Costeira, M.J.; Oliveira, P.; Ares, S.; De Escobar, G.M.; Palha, J. Iodine Status of Pregnant Women and Their Progeny in the Minho Region of Portugal. Thyroid 2009, 19, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Helsedirektoratet [Norwegian Directorate of Health]. Gode Levevaner før Og I Svangerskapet [Advice for a Healthy Lifestyle Prior to and in Pregnancy, in Norwegian]. Available online: https://www.helsedirektoratet.no/brosjyrer/gode-levevaner-for-og-i-svangerskapet#last-ned-brosjyre-pdf (accessed on 10 April 2019).
- National Nutrition Council. Risk of Iodine Deficiency in Norway. Identification of an Acute Need for Action [in Norwegian]; Technical Report IS-0591; The Norwegian Directorate of Health: Oslo, Norway, 2016.
- Seldal, C.F. Seafood Intake and Iodine Status in Pregnant and Postpartum Norwegian Women. Master’s Thesis, Faculty of Medicine, University of Bergen, Bergen, Norway, 2012. [Google Scholar]
- Berg, V.; Nøst, T.H.; Skeie, G.; Thomassen, Y.; Berlinger, B.; Veyhe, A.S.; Jorde, R.; Odland, J.Ø.; Hansen, S. Thyroid homeostasis in mother–child pairs in relation to maternal iodine status: The MISA study. Eur. J. Clin. Nutr. 2017, 71, 1002–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahl, L.; Markhus, M.W.; Sanchez, P.V.R.; Moe, V.; Smith, L.; Meltzer, H.M.; Kjellevold, M. Iodine Deficiency in a Study Population of Norwegian Pregnant Women—Results from the Little in Norway Study (LiN). Nutrients 2018, 10, 513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henjum, S.; Aakre, I.; Lilleengen, A.M.; Garnweidner-Holme, L.; Borthne, S.; Pajalić, Z.; Blix, E.; Gjengedal, E.L.F.; Brantsæter, A.L. Suboptimal Iodine Status among Pregnant Women in the Oslo Area, Norway. Nutrients 2018, 10, 280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brantsæter, A.L.; Knutsen, H.K.; Johansen, N.C.; Nyheim, K.A.; Erlund, I.; Meltzer, H.M.; Henjum, S. Inadequate Iodine Intake in Population Groups Defined by Age, Life Stage and Vegetarian Dietary Practice in a Norwegian Convenience Sample. Nutrients 2018, 10, 230. [Google Scholar] [CrossRef] [Green Version]
- Charlton, K.; Yeatman, H.; Lucas, C.; Axford, S.; Gemming, L.; Houweling, F.; Goodfellow, A.; Ma, G. Poor Knowledge and Practices Related to Iodine Nutrition during Pregnancy and Lactation in Australian Women: Pre- and Post-Iodine Fortification. Nutrients 2012, 4, 1317–1327. [Google Scholar] [CrossRef]
- O’Kane, M.; Pourshahidi, L.K.; Farren, K.M.; Mulhern, M.S.; Strain, S.; Yeates, A. Iodine knowledge is positively associated with dietary iodine intake among women of childbearing age in the UK and Ireland. Br. J. Nutr. 2016, 116, 1728–1735. [Google Scholar] [CrossRef] [Green Version]
- McMullan, P.; Hunter, A.; McCance, D.; Woodside, J.V.; Mullan, K. Knowledge about iodine requirements during pregnancy and breastfeeding among pregnant women living in Northern Ireland. BMC Nutr. 2019, 5, 24. [Google Scholar] [CrossRef]
- Mulrine, H.M.; Skeaff, S.A.; Ferguson, E.L.; Gray, A.; Valeix, P. Breast-milk iodine concentration declines over the first 6 mo postpartum in iodine-deficient women. Am. J. Clin. Nutr. 2010, 92, 849–856. [Google Scholar] [CrossRef] [Green Version]
- Osei, J.; Andersson, M.; Van Der Reijden, O.; Dold, S.; Smuts, C.M.; Baumgartner, J. Breast-Milk Iodine Concentrations, Iodine Status, and Thyroid Function of Breastfed Infants Aged 2-4 Months and Their Mothers Residing in a South African Township. J. Clin. Res. Pediatr. Endocrinol. 2016, 8, 381–391. [Google Scholar] [CrossRef]
- Leung, A.M.; Braverman, L.E.; He, X.; Heeren, T.; Pearce, E.N. Breastmilk Iodine Concentrations Following Acute Dietary Iodine Intake. Thyroid 2012, 22, 1176–1180. [Google Scholar] [CrossRef] [Green Version]
- Laurberg, P.; Nøhr, S.B.; Pedersen, K.M.; Fuglsang, E. Iodine Nutrition in Breast-Fed Infants Is Impaired by Maternal Smoking. J. Clin. Endocrinol. Metab. 2004, 89, 181–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristic | N (%) | |
|---|---|---|
| Maternal age | Mean (SD), min–max | 31.4 (4.6) 20–42 |
| Marital status | Single | 1 (1) |
| Cohabiting | 82 (61.2) | |
| Married | 48 (35.8) | |
| In a relationship | 2 (2) | |
| Country of origin | Norway | 120 (90) |
| Other (11 different countries) | 13 (10) | |
| Pre pregnancy, BMI, kg/m2 | < 18.5 | 4 (3) |
| 18.5–24.9 | 93 (70) | |
| 25–30 | 21 (16) | |
| > 30 | 15 (11) | |
| BMI by inclusion, kg/m2 | < 18.5 | 4 (3) |
| 18.5–24.9 | 83 (62) | |
| 25–30 | 30 (23) | |
| > 30 | 16 (12) | |
| Planning new pregnancy | Yes | 54 (41) |
| Pregnant now | Yes | 2 (2) |
| Infants age, in months 1 | 1–4 | 54 (41) |
| 5–7 months | 27 (20) | |
| 8–11 months | 35 (26) | |
| 12–13 months | 17 (13) | |
| Educational level | <12 years | 6 (5) |
| 12 years | 19 (15) | |
| 1–4 years college/university | 52 (39) | |
| >4 years college/university | 56 (42) | |
| Smoking status | Never smoked | 103 (77) |
| Smoked before pregnancy | 27 (20) | |
| Occasionally during pregnancy | 0 | |
| Daily smoker during pregnancy 2 | 3 (2) | |
| Dietary supplement use | Any supplement (FFQ) | 76 (57) |
| Iodine-containing supplement past 24-h | 30 (23) | |
| Adhering to a vegetarian or vegan diet | 6 (4) | |
| Diet includes dairy | No | 9 (7) |
| Occasionally | 22 (16) | |
| Yes, daily | 103 (77) | |
| Self-reported hypothyroidism, no use of thyroid medication | 2 (2) | |
| Iodine Intakes | Median | P25 1 | P75 2 | Mean ± SD |
|---|---|---|---|---|
| Habitual iodine intake from food, µg/day | 35 | 20 | 52 | 40 ± 26 |
| Total habitual iodine intake, µg /day | 42 | 28 | 86 | 75 ± 73 |
| The 24-h iodine intake from iodine rich foods only, µg /day | 23 | 15 | 38 | 42 ± 44 |
| Total 24-h iodine intake, µg /day | 32 | 16 | 160 | 78 ± 79 |
| Dependent Variable | Predictor Variables | Unstandardized Beta | P | Standardized Beta | 95% CI Beta |
|---|---|---|---|---|---|
| HMIC µg/L, | Constant | ||||
| UIC µg/L 1 | 0.26 | < 0.001 | 0.42 | (0.16, 0.37) | |
| Breastfeeding status 2 | 19.9 | < 0.001 | 0.35 | (10.5, 29.3) | |
| Infant age, weeks | −1.17 | 0.001 | −0.31 | (−1.82, −0.52) | |
| Knowledge score 3 | 9.63 | 0.035 | 0.18 | (0.70, 18.56) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Groufh-Jacobsen, S.; Mosand, L.M.; Oma, I.; Sletten Bakken, K.; Stokke Solvik, B.; Lovise Folven Gjengedal, E.; Brantsæter, A.L.; Strand, T.A.; Henjum, S. Mild to Moderate Iodine Deficiency and Inadequate Iodine Intake in Lactating Women in the Inland Area of Norway. Nutrients 2020, 12, 630. https://doi.org/10.3390/nu12030630
Groufh-Jacobsen S, Mosand LM, Oma I, Sletten Bakken K, Stokke Solvik B, Lovise Folven Gjengedal E, Brantsæter AL, Strand TA, Henjum S. Mild to Moderate Iodine Deficiency and Inadequate Iodine Intake in Lactating Women in the Inland Area of Norway. Nutrients. 2020; 12(3):630. https://doi.org/10.3390/nu12030630
Chicago/Turabian StyleGroufh-Jacobsen, Synne, Lise Mette Mosand, Ingvild Oma, Kjersti Sletten Bakken, Beate Stokke Solvik, Elin Lovise Folven Gjengedal, Anne Lise Brantsæter, Tor Arne Strand, and Sigrun Henjum. 2020. "Mild to Moderate Iodine Deficiency and Inadequate Iodine Intake in Lactating Women in the Inland Area of Norway" Nutrients 12, no. 3: 630. https://doi.org/10.3390/nu12030630
APA StyleGroufh-Jacobsen, S., Mosand, L. M., Oma, I., Sletten Bakken, K., Stokke Solvik, B., Lovise Folven Gjengedal, E., Brantsæter, A. L., Strand, T. A., & Henjum, S. (2020). Mild to Moderate Iodine Deficiency and Inadequate Iodine Intake in Lactating Women in the Inland Area of Norway. Nutrients, 12(3), 630. https://doi.org/10.3390/nu12030630








