Lactobacillus Rhamnosus GG Affects the BDNF System in Brain Samples of Wistar Rats with Pepsin-Trypsin-Digested Gliadin (PTG)-Induced Enteropathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Gliadin Digest
2.2. Animals and Experimental Design
2.3. Western Immunoblotting
2.4. PCR
2.5. Statistical Analysis
3. Results
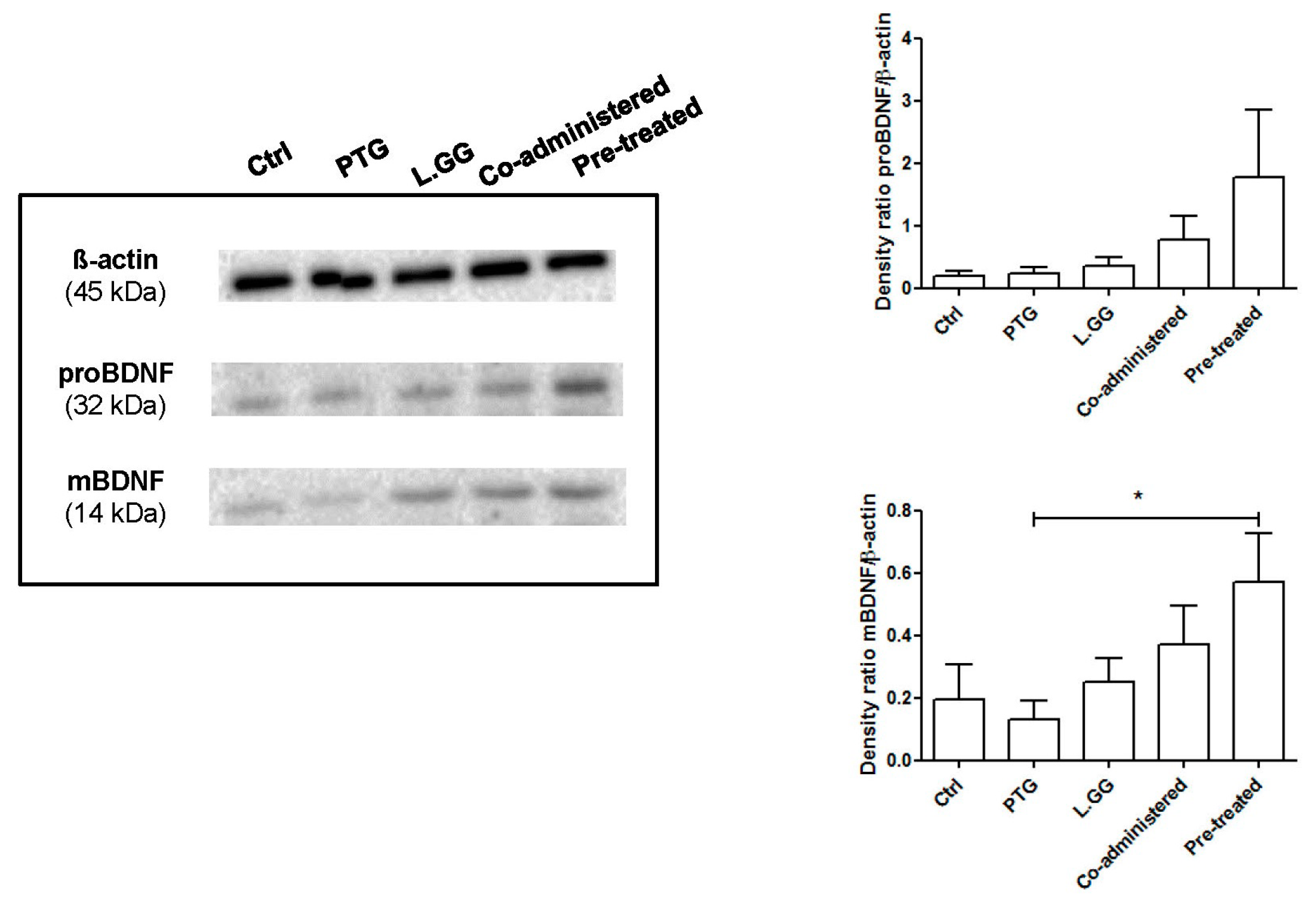
3.1. BDNF Analysis
3.2. TrkB and p75NTR Analysis
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Balakireva, A.V.; Zamyatnin, A.A. Properties of Gluten Intolerance: Gluten Structure, Evolution, Pathogenicity and Detoxification Capabilities. Nutrients 2016, 8, 644. [Google Scholar] [CrossRef] [PubMed]
- Slim, M.; Rico-Villademoros, F.; Calandre, E.P. Psychiatric Comorbidity in Children and Adults with Gluten-Related Disorders: A Narrative Review. Nutrients 2018, 10, 875. [Google Scholar] [CrossRef] [PubMed]
- Campagna, G.; Pesce, M.; Tatangelo, R.; Rizzuto, A.; La Fratta, I.; Grilli, A. The progression of coeliac disease: Its neurological and psychiatric implications. Nutr. Res. Rev. 2017, 30, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, F.; Rossetti, AC.; Racagni, G.; Gass, P.; Riva, M.A.; Molteni, R. Brain-derived neurotrophic factor: A bridge between inflammation and neuroplasticity. Front. Cell. Neurosci. 2014, 8, 430. [Google Scholar] [CrossRef] [PubMed]
- Arévalo, J.C.; Wu, SH. Neurotrophin signaling: Many exciting surprises! Cell. Mol. Life Sci. 2006, 63, 1523–1537. [Google Scholar] [CrossRef]
- Lommatzsch, M.; Braun, A.; Mannsfeldt, A.; Botchkarev, V.A.; Botchkareva, N.V.; Paus, R.; Fischer, A.; Lewin, G.R.; Renz, H. Abundant production of brain-derived neurotrophic factor by adult visceral epithelia. Implications for paracrine and target-derived Neurotrophic functions. Am. J. Pathol. 1999, 155, 1183–1193. [Google Scholar] [CrossRef]
- Grider, J.R.; Piland, B.E.; Gulick, M.A.; Qiao, L.Y. Brain-derived neurotrophic factor augments peristalsis by augmenting 5-HT and calcitonin gene-related peptide release. Gastroenterology 2006, 130, 771–780. [Google Scholar] [CrossRef]
- Boesmans, W.; Gomes, P.; Janssens, J.; Tack, J.; Vanden Berghe, P. Brain-derived neurotrophic factor amplifies neurotransmitter responses and promotes synaptic communication in the enteric nervous system. Gut 2008, 57, 314–322. [Google Scholar] [CrossRef]
- Yu, Y.B.; Zhao, D.Y.; Qi, Q.Q.; Long, X.; Li, X.; Chen, F.X.; Zuo, XL. BDNF modulates intestinal barrier integrity through regulating the expression of tight junction proteins. Neurogastroenterol. Motil. 2017, 29. [Google Scholar] [CrossRef]
- Pruunsild, P.; Kazantseva, A.; Aid, T.; Palm, K.; Timmusk, T. Dissecting the human BDNF locus: Bidirectional transcription, complex splicing, and multiple promoters. Genomics 2007, 90, 397–406. [Google Scholar] [CrossRef]
- Aid, T.; Kazantseva, A.; Piirsoo, M.; Palm, K.; Timmusk, T. Mouse and rat BDNF gene structure and expression revisited. J. Neurosci. Res. 2007, 85, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Takasaki, I.; Takarada, S.; Tatsumi, S.; Azegami, A.; Yasuda, M.; Fukuchi, M.; Tabuchi, A.; Kondo, T.; Tabuchi, Y.; Tsuda, M. Extracellular adenosine 5’-triphosphate elicits the expression of brain-derived neurotrophic factor exon IV mRNA in rat astrocytes. Glia 2008, 56, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Morioka, N.; Yoshida, Y.; Nakamura, Y.; Hidaka, N.; Hisaoka-Nakashima, K.; Nakata, Y. The regulation of exon-specific brain-derived neurotrophic factor mRNA expression by protein kinase C in rat cultured dorsal root ganglion neurons. Brain Res. 2013, 1509, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.W.; Chen, L. Epigenetic Regulation of BDNF Gene during Development and Diseases. Int. J. Mol. Sci. 2017, 18, 571. [Google Scholar] [CrossRef]
- Dechant, G.; Barde, Y.A. The neurotrophin receptor p75 (NTR): Novel functions and implications for diseases of the nervous system. Nat. Neurosci. 2002, 5, 1131–1136. [Google Scholar] [CrossRef]
- Lu, B.; Pang, P.T.; Woo, NH. The yin and yang of neurotrophin action. Nat. Rev. Neurosci. 2005, 6, 603–614. [Google Scholar] [CrossRef]
- Do, J.; Woo, J. From Gut to Brain: Alteration in Inflammation Markers in the Brain of Dextran Sodium Sulfate-induced Colitis Model Mice. Clin. Psychopharmacol. Neurosci. 2018, 16, 422–433. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, G.; Liu, D.R.; Wang, Y.; Yao, S.K. Increased expression of brain-derived neurotrophic factor is correlated with visceral hypersensitivity in patients with diarrhea-predominant irritable bowel syndrome. World J. Gastroenterol. 2019, 25, 269–281. [Google Scholar] [CrossRef]
- Russo, F.; Chimienti, G.; Riezzo, G.; Linsalata, M.; D’Attoma, B.; Clemente, C.; Orlando, A. Adipose Tissue-Derived Biomarkers of Intestinal Barrier Functions for the Characterization of Diarrhoea-Predominant IBS. Dis. Mark. 2018, 2018, 1827937. [Google Scholar] [CrossRef]
- Steinkamp, M.; Schulte, N.; Spaniol, U.; Pflüger, C.; Hartmann, C.; Kirsch, J.; von Boyen, G.B. Brain derived neurotrophic factor inhibits apoptosis in enteric glia during gut inflammation. Med. Sci. Monit. 2012, 18, BR117–BR122. [Google Scholar]
- Russo, F.; Chimienti, G.; Clemente, C.; Ferreri, C.; Orlando, A.; Riezzo, G. A possible role for ghrelin, leptin, brain-derived neurotrophic factor and docosahexaenoic acid in reducing the quality of life of coeliac disease patients following a gluten-free diet. Eur. J. Nutr. 2017, 56, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Margoni, D.; Michalakakou, K.; Angeli, E.; Pervanidou, P.; Kanaka-Gantenbein, C.; Chrousos, G.; Papassotiriou, I.; Roma, E. Serum brain-derived neurotrophic factor in children with coeliac disease. Eur. J. Clin. Invest. 2018, 48, e12916. [Google Scholar] [CrossRef] [PubMed]
- Lommatzsch, M.; Zingler, D.; Schuhbaeck, K.; Schloetcke, K.; Zingler, C.; Schuff-Werner, P.; Virchow, J.C. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol. Aging. 2005, 26, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Cichewicz, A.B.; Mearns, E.S.; Taylor, A.; Boulanger, T.; Gerber, M.; Leffler, D.A.; Drahos, J.; Sanders, D.S.; Thomas Craig, K.J.; Lebwohl, B. Diagnosis and Treatment Patterns in Celiac Disease. Dig. Dis. Sci. 2019, 64, 2095–2106. [Google Scholar] [CrossRef] [PubMed]
- Serena, G.; Kelly, C.P.; Fasano, A. Nondietary Therapies for Celiac Disease. Gastroenterol. Clin. North Am. 2019, 48, 145–163. [Google Scholar] [CrossRef] [PubMed]
- van den Broeck, H.C.; van Herpen, T.W.; Schuit, C.; Salentijn, E.M.; Dekking, L.; Bosch, D.; Hamer, R.J.; Smulders, M.J.; Gilissen, L.J.; van der Meer, I.M. Removing celiac disease-related gluten proteins from bread wheat while retaining technological properties: A study with Chinese Spring deletion lines. BMC Plant. Biol. 2009, 9, 41. [Google Scholar] [CrossRef]
- Yoosuf, S.; Makharia, G.K. Evolving Therapy for Celiac Disease. Front. Pediatr. 2019, 7, 193. [Google Scholar] [CrossRef]
- Girbovan, A.; Sur, G.; Samasca, G.; Lupan, I. Dysbiosis a risk factor for celiac disease. Med. Microbiol. Immunol. 2017, 206, 83–91. [Google Scholar] [CrossRef]
- Di Cagno, R.; De Angelis, M.; Auricchio, S.; Greco, L.; Clarke, C.; De Vincenzi, M.; Giovannini, C.; D’Archivio, M.; Landolfo, F.; Parrilli, G.; et al. Sourdough bread made from wheat and nontoxic flours and started with selected lactobacilli is tolerated in celiac sprue patients. Appl. Environ. Microbiol. 2004, 70, 1088–1096. [Google Scholar] [CrossRef]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef]
- Maqsood, R.; Stone, T.W. The Gut-Brain Axis, BDNF, NMDA and CNS Disorders. Neurochem. Res. 2016, 41, 2819–2835. [Google Scholar] [CrossRef] [PubMed]
- Orlando, A.; Linsalata, M.; Bianco, G.; Notarnicola, M.; D’Attoma, B.; Scavo, M.P.; Tafaro, A.; Russo, F. Lactobacillus rhamnosus GG Protects the Epithelial Barrier of Wistar Rats from the Pepsin-Trypsin-Digested Gliadin (PTG)-Induced Enteropathy. Nutrients 2018, 10, 1698. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Wang, Y.P. Gut Microbiota-brain Axis. Chin. Med. J. (Engl.) 2016, 129, 2373–2380. [Google Scholar] [CrossRef] [PubMed]
- Drago, S.; El Asmar, R.; Di Pierro, M.; Grazia Clemente, M.; Tripathi, A.; Sapone, A.; Thakar, M.; Iacono, G.; Carroccio, A.; D’Agate, C.; et al. Gliadin, zonulin and gut permeability: Effects on celiac and non celiac intestinal mucosa and intestinal cell lines. Scand. J. Gastroenterol. 2006, 41, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Wang, T.; Hu, X.; Luo, J.; Li, W.; Wu, X.; Duan, Y.; Jin, F. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience 2015, 310, 561–577. [Google Scholar] [CrossRef]
- Smiljanic, K.; Pesic, V.; Mladenovic Djordjevic, A.; Pavkovic, Z.; Brkic, M.; Ruzdijic, S.; Kanazir, S. Long-term dietary restriction differentially affects the expression of BDNF and its receptors in the cortex and hippocampus of middle-aged and aged male rats. Biogerontology 2015, 16, 71–83. [Google Scholar] [CrossRef]
- Laparra, J.M.; Olivares, M.; Gallina, O.; Sanz, Y. Bifidobacterium longum cect 7347 modulates immune responses in a gliadin-induced enteropathy animal model. PLoS ONE 2012, 7, e30744. [Google Scholar] [CrossRef]
- Bercik, P.; Verdu, E.F.; Foster, J.A.; Macri, J.; Potter, M.; Huang, X.; Malinowski, P.; Jackson, W.; Blennerhassett, P.; Neufeld, K.A.; et al. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology 2010, 139, 2102–2112. [Google Scholar] [CrossRef]
- Autry, A.E.; Monteggia, L.M. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol. Rev. 2012, 64, 238–258. [Google Scholar] [CrossRef]
- Simon, K.U.; Neto, E.W.; Tramontin, N.D.S.; Canteiro, P.B.; Pereira, B.D.; Zaccaron, R.P.; Silveira, P.C.L.; Muller, A.P. Intranasal insulin treatment modulates the neurotropic, inflammatory, and oxidant mechanisms in the cortex and hippocampus in a low-grade inflammation model. Peptides 2019, 123, 170175. [Google Scholar] [CrossRef]
- Heiss, C.N.; Olofsson, L.E. The role of the gut microbiota in development, function and disorders of the central nervous system and the enteric nervous system. J. Neuroendocrinol. 2019, 31, e12684. [Google Scholar] [CrossRef] [PubMed]
- Pirbaglou, M.; Katz, J.; de Souza, R.J.; Stearns, J.C.; Motamed, M.; Ritvo, P. Probiotic supplementation can positively affect anxiety and depressive symptoms: A systematic review of randomized controlled trials. Nutr. Res. 2016, 36, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, V.; Zaidi, S.Y.; Young, A.H.; Cleare, A.J.; Stone, J.M. Gut feeling: Randomized controlled trials of probiotics for the treatment of clinical depression: Systematic review and meta-analysis. Ther. Adv. Psychopharmacol. 2019, 9, 2045125319859963. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Park, S.; Paik, J.W.; Chae, S.W.; Kim, D.H.; Jeong, D.G.; Ha, E.; Kim, M.; Hong, G.; Park, S.H.; et al. Efficacy and Safety of Lactobacillus Plantarum C29-Fermented Soybean (DW2009) in Individuals with Mild Cognitive Impairment: A 12-Week, Multi-Center, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2019, 11, 305. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, Y.K.; Han, P.L. Extracellular Vesicles Derived from Lactobacillus plantarum Increase BDNF Expression in Cultured Hippocampal Neurons and Produce Antidepressant-like Effects in Mice. Exp. Neurobiol. 2019, 28, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Xu, T.; Zhang, Y.; Wang, F.; Zhao, L.; Jiang, Y.; He, F. Lactobacillus rhamnosus GG and Bifidobacterium bifidum TMC3115 Can Affect Development of Hippocampal Neurons Cultured In Vitro in a Strain-Dependent Manner. Probiotics Antimicrob. Proteins. 2019. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xiong, J.; Lim, Y.; Ruan, Y.; Huang, C.; Zhu, Y.; Zhong, J.H.; Xiao, Z.; Zhou, X.F. Upregulation of blood proBDNF and its receptors in major depression. J. Affect. Disord. 2013, 150, 776–784. [Google Scholar] [CrossRef]
- Karpova, N.N. Role of BDNF epigenetics in activity-dependent neuronal plasticity. Neuropharmacology 2014, 76, 709–718. [Google Scholar] [CrossRef]
- Otani, K.; Okada, M.; Yamawaki, H. Diverse distribution of tyrosine receptor kinase B isoforms in rat multiple tissues. J. Vet. Med. Sci. 2017, 79, 1516–1523. [Google Scholar] [CrossRef]
- Watson, F.L.; Porcionatto, M.A.; Bhattacharyya, A.; Stiles, C.D.; Segal, R.A. TrkA glycosylation regulates receptor localization and activity. J. Neurobiol. 1999, 39, 323–336. [Google Scholar] [CrossRef]
- Kim, H.J.; Hwang, J.J.; Behrens, M.M.; Snider, B.J.; Choi, D.W.; Koh, J.Y. TrkB mediates BDNF-induced potentiation of neuronal necrosis in cortical culture. Neurobiol. Dis. 2003, 14, 110–119. [Google Scholar] [CrossRef]




| Group | Treatment | Age (Days) | |
|---|---|---|---|
| 1 | Ctrl | Animals not treated | 10 |
| 2 | PTG | Animals previously sensitized with 1000 U IFN-γ administered intraperitoneally after birth and with a following oral administration of PTG 50 µg/day for 10 days | 10 |
| 3 | L.GG | Animals treated with an oral administration of L.GG 1 × 109 CFU for 10 days | 10 |
| 4 | Co-administered | Animals previously sensitized with 1000 U IFN-γ administered intraperitoneally after birth and with a following oral co-administration of PTG 50 µg/day and L.GG 109 CFU for 10 days | 10 |
| 5 | Pre-treated | Animals previously sensitized with 1000 U IFN-γ administered intraperitoneally after birth with a following administration of PTG for 10 days and successively treated with L.GG 109 CFU for further 10 days | 20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlando, A.; Chimienti, G.; Lezza, A.M.S.; Pesce, V.; Gigante, I.; D’Attoma, B.; Russo, F. Lactobacillus Rhamnosus GG Affects the BDNF System in Brain Samples of Wistar Rats with Pepsin-Trypsin-Digested Gliadin (PTG)-Induced Enteropathy. Nutrients 2020, 12, 629. https://doi.org/10.3390/nu12030629
Orlando A, Chimienti G, Lezza AMS, Pesce V, Gigante I, D’Attoma B, Russo F. Lactobacillus Rhamnosus GG Affects the BDNF System in Brain Samples of Wistar Rats with Pepsin-Trypsin-Digested Gliadin (PTG)-Induced Enteropathy. Nutrients. 2020; 12(3):629. https://doi.org/10.3390/nu12030629
Chicago/Turabian StyleOrlando, Antonella, Guglielmina Chimienti, Angela Maria Serena Lezza, Vito Pesce, Isabella Gigante, Benedetta D’Attoma, and Francesco Russo. 2020. "Lactobacillus Rhamnosus GG Affects the BDNF System in Brain Samples of Wistar Rats with Pepsin-Trypsin-Digested Gliadin (PTG)-Induced Enteropathy" Nutrients 12, no. 3: 629. https://doi.org/10.3390/nu12030629
APA StyleOrlando, A., Chimienti, G., Lezza, A. M. S., Pesce, V., Gigante, I., D’Attoma, B., & Russo, F. (2020). Lactobacillus Rhamnosus GG Affects the BDNF System in Brain Samples of Wistar Rats with Pepsin-Trypsin-Digested Gliadin (PTG)-Induced Enteropathy. Nutrients, 12(3), 629. https://doi.org/10.3390/nu12030629






