Bioactive Factors in Human Breast Milk Attenuate Intestinal Inflammation during Early Life
Abstract
1. Introduction
2. Methods
Literature Search
3. Microbiome and Microbial Factors
3.1. Microbiome and Probiotics
3.2. Human Milk Oligosaccharides and Glycans
4. Immunological Factors: Immunoglobulins and Immunological Proteins
4.1. Immunoglobulins
4.2. Cytokines and Growth Factors
4.3. Lactoferrin, Lactadherin, and Lysozyme
5. Metabolic Factors
5.1. Adipokines
5.2. Antioxidants and Anti-Proteases
5.3. Dietary Fatty Acids
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eldelman, A.I.; Schandler, R.J. Breastfeeding and the use of human milk. Pediatrics 2012, 129, e827–e841. [Google Scholar] [CrossRef]
- Underwood, M.A. Human milk for the premature infant. Pediatr. Clin. North Am. 2013, 60, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Schanler, R.J.; Shulman, R.J.; Lau, C. Feeding strategies for premature infants: beneficial outcomes of feeding fortified human milk versus preterm formula. Pediatrics 1999, 103, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Cortez, J.; Makker, K.; Kraemer, D.F.; Neu, J.; Sharma, R.; Hudak, M.L. Maternal milk feedings reduce sepsis, necrotizing enterocolitis and improve outcomes of premature infants. J. Perinatol. 2018, 38, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Sisk, P.M.; A Lovelady, C.; Dillard, R.G.; Gruber, K.; O’Shea, T.M. Early human milk feeding is associated with a lower risk of necrotizing enterocolitis in very low birth weight infants. J. Perinatol. 2007, 27, 428–433. [Google Scholar] [CrossRef]
- Meinzen-Derr, J.; Poindexter, B.; Wrage, L.; Morrow, A.L.; Stoll, B.; Donovan, E.F. Role of human milk in extremely low birth weight infants’ risk of necrotizing enterocolitis or death. J. Perinatol. 2009, 29, 57–62. [Google Scholar] [CrossRef]
- Hermansson, H.; Kumar, H.; Collado, M.C.; Salminen, S.; Isolauri, E.; Rautava, S. Breast Milk Microbiota Is Shaped by Mode of Delivery and Intrapartum Antibiotic Exposure. Front. Nutr. 2019, 6, 4. [Google Scholar] [CrossRef]
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.M.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.J.; Bittinger, K.; et al. Association Between Breast Milk Bacterial Communities and Establishment and Development of the Infant Gut Microbiome. JAMA Pediatr. 2017, 171, 647–654. [Google Scholar] [CrossRef]
- Arrieta, M.-C.; Stiemsma, L.T.; Dimitriu, P.A.; Thorson, L.; Russell, S.; Yurist-Doutsch, S.; Kuzeljevic, B.; Gold, M.J.; Britton, H.; Lefebvre, D.L.; et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Transl. Med. 2015, 7, 307ra152. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature. 2009, 457, 480–484. [Google Scholar] [CrossRef]
- Fujimura, K.E.; Sitarik, A.R.; Havstad, S.; Lin, D.L.; LeVan, S.; Fadrosh, D.; Panzer, A.R.; LaMere, B.; Rackaityte, E.; Lukacs, N.W.; et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat. Med. 2016, 22, 1187–1191. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Zabetakis, I. Invited review: The anti-inflammatory properties of dairy lipids. J. Dairy Sci. 2017, 100, 4197–4212. [Google Scholar] [CrossRef] [PubMed]
- Quin, C.; Gibson, D. Dietary Fatty Acids and Host–Microbial Crosstalk in Neonatal Enteric Infection. Nutr. 2019, 11, 2064. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef] [PubMed]
- Kuban, K.C.; O’Shea, T.M.; Allred, E.N.; Fichorova, R.N.; Heeren, T.; Paneth, N.; Hirtz, D.; Dammann, O.; Leviton, A.; ELGAN Study Investigators. The breadth and type of systemic inflammation and the risk of adverse neurological outcomes in extremely low gestation newborns. Pediatr. Neurol. 2015, 52, 42–48. [Google Scholar] [CrossRef]
- McElroy, S.J.; Frey, M.R.; Torres, B.A.; Maheshwari, A. Innate and Mucosal Immunity in the Developing Gastrointestinal Tract. In Avery’s Disease of the Newborn, 10th ed.; Elsevier: Philadelphia, PA, USA, 2017; pp. 1054–1067. [Google Scholar]
- McElroy, S.J.; Weitkamp, J.-H. Innate Immunity in the Small Intestine of the Preterm Infant. NeoReviews 2011, 12, e517–e526. [Google Scholar] [CrossRef]
- Palmeira, P.; Carneiro-Sampaio, M. Immunology of breast milk. Revista da Associação Médica Brasileira 2016, 62, 584–593. [Google Scholar] [CrossRef]
- Neu, J.; Walker, W.A. Necrotizing Enterocolitis. N. Engl. J. Med. 2011, 364, 255–264. [Google Scholar] [CrossRef]
- Hackam, D.J.; Good, M.; Sodhi, C.P.; Sodhi, C.P. Mechanisms of gut barrier failure in the pathogenesis of necrotizing enterocolitis: Toll-like receptors throw the switch. Semin. Pediatr. Surg. 2013, 22, 76–82. [Google Scholar] [CrossRef]
- Maheshwari, A.; Kelly, D.R.; Nicola, T.; Ambalavanan, N.; Jain, S.K.; Murphy–Ullrich, J.; Athar, M.; Shimamura, M.; Bhandari, V.; Aprahamian, C.; et al. TGF-β2 Suppresses Macrophage Cytokine Production and Mucosal Inflammatory Responses in the Developing Intestine. Gastroenterology 2011, 140, 242–253. [Google Scholar] [CrossRef]
- Patel, R.M.; Kandefer, S.; Walsh, M.C.; Bell, E.F.; Carlo, W.A.; Laptook, A.R.; Sanchez, P.J.; Shankaran, S.; Van Meurs, K.P.; Ball, M.B.; et al. Causes and timing of death in extremely premature infants from 2000 through 2011. New Engl. J. Med. 2015, 372, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.; Cole, T. Breast milk and neonatal necrotising enterocolitis. Lancet 1990, 336, 1519–1523. [Google Scholar] [CrossRef]
- Furman, L.; Taylor, G.; Minich, N.; Hack, M. The effect of maternal milk on neonatal morbidity of very low-birth-weight infants. Arch. Pediatr. Adolesc. Med. 2003, 157, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Pammi, M.; Cope, J.; Tarr, P.I.; Warner, B.B.; Morrow, A.L.; Mai, V.; Gregory, K.E.; Kroll, J.S.; McMurtry, V.; Ferris, M.J.; et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome 2017, 5, 31. [Google Scholar] [CrossRef]
- Underwood, M.A. Probiotics and the prevention of necrotizing enterocolitis. J. Pediatr. Surg. 2019, 54, 405–412. [Google Scholar] [CrossRef]
- Dunne-Castagna, V.P.; Taft, D.H. Mother’s Touch: Milk IgA and Protection from Necrotizing Enterocolitis. Cell Host Microbe 2019, 26, 147–148. [Google Scholar] [CrossRef]
- Bode, L. Human Milk Oligosaccharides in the Prevention of Necrotizing Enterocolitis: A Journey From in vitro and in vivo Models to Mother-Infant Cohort Studies. Front. Pediatr. 2018, 6, 385. [Google Scholar] [CrossRef]
- Gopalakrishna, K.P.; Macadangdang, B.R.; Rogers, M.B.; Tometich, J.T.; Firek, B.A.; Baker, R.; Ji, J.; Burr, A.H.P.; Ma, C.; Good, M.; et al. Maternal IgA protects against the development of necrotizing enterocolitis in preterm infants. Nat. Med. 2019, 25, 1110–1115. [Google Scholar] [CrossRef]
- Hooper, L.V.; Littman, D.R.; MacPherson, A.J. Interactions Between the Microbiota and the Immune System. Sci. 2012, 336, 1268–1273. [Google Scholar] [CrossRef]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Palacio, S.D.; Montes, S.A.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Boil. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef]
- Newburg, D.; He, Y. Neonatal Gut Microbiota and Human Milk Glycans Cooperate to Attenuate Infection and Inflammation. Clin. Obstet. Gynecol. 2015, 58, 814–826. [Google Scholar] [CrossRef] [PubMed]
- Mshvildadze, M.; Neu, J.; Shuster, J.; Theriaque, U.; Li, N.; Mai, V. Intestinal Microbial Ecology in Premature Infants Assessed with Non–Culture-Based Techniques. J. Pediatr. 2010, 156, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Hunt, K.M.; Foster, J.A.; Forney, L.J.; Schütte, U.M.E.; Beck, D.L.; Abdo, Z.; Fox, L.K.; Williams, J.E.; McGuire, M.; McGuire, M.A. Characterization of the Diversity and Temporal Stability of Bacterial Communities in Human Milk. PLoS ONE 2011, 6, e21313. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.; Langa, S.; Martin, V.; Maldonado, A.; Jiménez, E.; Martín, R.; Rodríguez, J. The human milk microbiota: Origin and potential roles in health and disease. Pharmacol. Res. 2013, 69, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fitzstevens, J.L.; Smith, K.C.; Hagadorn, J.I.; Caimano, M.J.; Matson, A.P.; Brownell, E.A. Systematic review of the human milk microbiota. Nutr. Clin. Pract. 2017, 32, 354–364. [Google Scholar] [CrossRef]
- Schwab, C.; Voney, E.; Garcia, A.R.; Vischer, M.; Lacroix, C. Characterization of the Cultivable Microbiota in Fresh and Stored Mature Human Breast Milk. Front. Microbiol. 2019, 10, 2666. [Google Scholar] [CrossRef]
- Urbaniak, C.; Angelini, M.; Gloor, G.B.; Reid, G. Human milk microbiota profiles in relation to birthing method, gestation and infant gender. Microbiome 2016, 4, 1. [Google Scholar] [CrossRef]
- Gallego, C.G.; Garcia-Mantrana, I.; Salminen, S.; Collado, M.C. The human milk microbiome and factors influencing its composition and activity. Semin. Fetal Neonatal Med. 2016, 21, 400–405. [Google Scholar] [CrossRef]
- Cabrera-Rubio, R.; Collado, M.C.; Laitinen, K.; Salminen, S.; Isolauri, E.; Mira, A.; Miras, A.; Jackson, R.N.; Goldstone, A.P.; Olbers, T.; et al. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am. J. Clin. Nutr. 2012, 96, 544–551. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic Treg Cell Homeostasis. Sci. 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nat. 2011, 469, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Embleton, N.; Berrington, J.E.; Dorling, J.; Ewer, A.K.; Juszczak, E.; Kirby, J.A.; Lamb, C.A.; Lanyon, C.; McGuire, W.; Probert, C.S.; et al. Mechanisms Affecting the gut of preterm infants in enteral feeding trials. Front. Nutr. 2017, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.A.; Arriola, J.; Gerber, C.W.; Kaveti, A.; Kalanetra, K.M.; Kananurak, A.; Bevins, C.L.; Mills, D.A.; Dvořák, B. Bifidobacterium longum subsp. infantis in experimental necrotizing enterocolitis: alterations in inflammation, innate immune response, and the microbiota. Pediatr. Res. 2014, 76, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Pang, B.; Liu, G.; Zhao, X.; Xu, X.; Jiang, C.; Yang, B.; Liu, Y.; Li, N. Lactobacillus rhamnosus from human breast milk shows therapeutic function against foodborne infection by multi-drug resistant Escherichia coli in mice. Food Funct. 2020, 11, 435–447. [Google Scholar] [CrossRef]
- Guo, S.; Guo, Y.; Ergun, A.; Lü, L.; Walker, W.A.; Ganguli, K. Secreted Metabolites of Bifidobacterium infantis and Lactobacillus acidophilus Protect Immature Human Enterocytes from IL-1β-Induced Inflammation: A Transcription Profiling Analysis. PLoS ONE 2015, 10, e0124549. [Google Scholar] [CrossRef]
- Pasparakis, M. Role of NF-κB in epithelial biology. Immunol. Rev. 2012, 246, 346–358. [Google Scholar] [CrossRef]
- Ganguli, K.; Meng, D.; Rautava, S.; Lu, L.; Walker, W.A.; Nanthakumar, N. Probiotics prevent necrotizing enterocolitis by modulating enterocyte genes that regulate innate immune-mediated inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G132–G141. [Google Scholar] [CrossRef]
- Plaza-Díaz, J.; Fontana, L.; Gil, A. Human Milk Oligosaccharides and Immune System Development. Nutr. 2018, 10, 1038. [Google Scholar] [CrossRef]
- Newburg, D.S.; Ruiz-Palacios, G.M.; Morrow, A.L. HUMAN MILK GLYCANS PROTECT INFANTS AGAINST ENTERIC PATHOGENS. Annu. Rev. Nutr. 2005, 25, 37–58. [Google Scholar] [CrossRef]
- Morrow, A.L.; Ruiz-Palacios, G.M.; Jiang, X.; Newburg, D.S. Human-Milk Glycans That Inhibit Pathogen Binding Protect Breast-feeding Infants against Infectious Diarrhea. J. Nutr. 2005, 135, 1304–1307. [Google Scholar] [CrossRef]
- Ballard, O.; Morrow, A.L. Human milk composition: nutrients and bioactive factors. Pediatr. Clin. North Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, O.; Zampini, L.; Galeazzi, T.; Padella, L.; Santoro, L.; Peila, C.; Giuliani, F.; Bertino, E.; Fabris, C.; Coppa, G.V. Preterm milk oligosaccharides during the first month of lactation. Pediatrics 2011, 128, e1520–e1531. [Google Scholar] [CrossRef] [PubMed]
- Rogier, E.; Frantz, A.L.; Bruno, M.E.C.; Kaetzel, C. Secretory IgA is concentrated in the outer layer of colonic mucus along with gut bacteria. Pathogens 2014, 3, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Cianga, P.; Medesan, C.; Richardson, J.A.; Ghetie, V.; Ward, E.S. Identification and function of neonatal Fc receptor in mammary gland of lactating mice. Eur. J. Immunol. 1999, 29, 2515–2523. [Google Scholar] [CrossRef]
- Gross, S.J.; Buckley, R.H.; Wakil, S.S.; McAllister, D.C.; David, R.J.; Faix, R.G. Elevated IgA concentration in milk produced by mothers delivered of preterm infants. J. Pediatr. 1981, 99, 389–393. [Google Scholar] [CrossRef]
- Mathur, N.B.; Dwarkadas, A.M.; Sharma, V.K.; Saha, K.; Jain, N. Anti-Infective Factors in Preterm Human Colostrum. Acta Paediatr. 1990, 79, 1039–1044. [Google Scholar] [CrossRef]
- Garofalo, R.; Chheda, S.; Mei, F.; Palkowetz, K.H.; E Rudloff, H.; Schmalstieg, F.C.; Rassin, D.K.; Goldman, A.S. Interleukin-10 in Human Milk. Pediatr. Res. 1995, 37, 444–449. [Google Scholar] [CrossRef]
- Sydora, B.C.; Tavernini, M.M.; Wessler, A.; Jewell, L.D.; Fedorak, R. Lack of interleukin-10 leads to intestinal inflammation, independent of the time at which luminal microbial colonization occurs. Inflamm. Bowel Dis. 2003, 9, 87–97. [Google Scholar] [CrossRef]
- Lepage, P.; Van De Perre, P. The Immune System of Breast Milk: Antimicrobial and Anti-inflammatory Properties. Adv. Exp. Med. Biol. 2012, 743, 121–137. [Google Scholar]
- Emami, C.N.; Chokshi, N.; Wang, J.; Hunter, C.J.; Guner, Y.; Goth, K.; Wang, L.; Grishin, A.; Ford, H.R. Role of interleukin-10 in the pathogenesis of necrotizing enterocolitis. Am. J. Surg. 2012, 203, 428–435. [Google Scholar] [CrossRef]
- Fell, J.M.; Paintin, M.; Arnaud-Battandier, F.; Beattie, R.M.; Hollis, A.; Kitching, P.; Donnet-Hughes, A.; MacDonald, T.T.; Walker-Smith, J.A. Mucosal healing and a fall in mucosal pro-inflammatory cytokine mRNA induced by a specific oral polymeric diet in paediatric Crohn’s disease. Ailm. Pharmacol. Ther. 2000, 14, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Dawod, B.; Marshall, J. Cytokines and soluble receptors in breast milk as enhancers of oral tolerance development. Front. Immunol. 2019, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, A.; for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network; Schelonka, R.L.; Dimmitt, R.A.; Carlo, W.A.; Munoz-Hernandez, B.; Das, A.; McDonald, S.A.; Thorsen, P.; Skogstrand, K.; et al. Cytokines associated with necrotizing enterocolitis in extremely-low-birth-weight infants. Pediatr. Res. 2014, 76, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Castellote, C.; Casillas, R.; Ramírez-Santana, C.; Pérez-Cano, F.J.; Castell, M.; Moretones, M.G.; López-Sabater, M.C.; Franch, À. Premature Delivery Influences the Immunological Composition of Colostrum and Transitional and Mature Human Milk. J. Nutr. 2011, 141, 1181–1187. [Google Scholar] [CrossRef]
- Liew, F.Y.; Xu, D.; Brint, E.; O’Neill, L.A. Negative regulation of Toll-like receptor-mediated immune responses. Nat. Rev. Immunol. 2005, 5, 446–458. [Google Scholar] [CrossRef]
- Henrick, B.M.; Nag, K.; Yao, X.-D.; Drannik, A.G.; Aldrovandi, G.M.; Rosenthal, K.L. Milk Matters: Soluble Toll-Like Receptor 2 (sTLR2) in Breast Milk Significantly Inhibits HIV-1 Infection and Inflammation. PLoS ONE 2012, 7, e40138. [Google Scholar] [CrossRef]
- Coursodon, C.F.; Dvořák, B. Epidermal growth factor and necrotizing enterocolitis. Curr. Opin. Pediatr. 2012, 24, 160–164. [Google Scholar] [CrossRef]
- Halpern, M.D.; Holubec, H.; Dominguez, J.A.; Williams, C.S.; Meza, Y.G.; McWilliam, D.L.; Payne, C.M.; McCuskey, R.S.; Besselsen, D.G.; Dvorak, B. Up-regulation of IL-18 and IL-12 in the ileum of neonatal rats with necrotizing enterocolitis. Pediatr. Res. 2002, 51, 733–739. [Google Scholar] [CrossRef]
- Dvorak, B. Milk Epidermal Growth Factor and Gut Protection. J. Pediatr. 2010, 156, S31–S35. [Google Scholar] [CrossRef]
- Chatterton, D.E.; Nguyen, D.N.; Bering, S.B.; Sangild, P.T. Anti-inflammatory mechanisms of bioactive milk proteins in the intestine of newborns. Int. J. Biochem. Cell. Biol. 2013, 45, 1730–1747. [Google Scholar] [CrossRef]
- Rocourt, R.V.; Mehta, V.B.; Besner, G.E. Heparin-Binding EGF-like Growth Factor Decreases Inflammatory Cytokine Expression After Intestinal Ischemia/Reperfusion Injury. J. Surg. Res. 2007, 139, 269–273. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ozgurtas, T.; Aydin, I.; Turan, O.; Koç, E.; Hirfanoglu, I.M.; Acikel, C.H.; Akyol, M.; Erbil, M.K. Vascular endothelial growth factor, basic fibroblast growth factor, insulin-like growth factor-I and platelet-derived growth factor levels in human milk of mothers with term and preterm neonates. Cytokine 2010, 50, 192–194. [Google Scholar] [CrossRef]
- Karatepe, H.Ö.; Kilincaslan, H.; Berber, M.; Ozen, A.; Saricoban, H.E.; Ustek, D.; Kemik, A.S.; Adas, M.; Bakar, F. The effect of vascular endothelial growth factor overexpression in experimental necrotizing enterocolitis. Pediatr. Surg. Int. 2014, 30, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Buescher, E.S. Anti-inflammatory characteristics of human milk: how, where, why. Single Mol. Single Cell Seq. 2001, 501, 207–222. [Google Scholar]
- Albenzio, M.; Santillo, A.; Stolfi, I.; Manzoni, P.; Iliceto, A.; Rinaldi, M.; Magaldi, R. Lactoferrin Levels in Human Milk after Preterm and Term Delivery. Am. J. Perinatol. 2016, 33, 1085–1089. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, J.; Jenkins, P.; Vargova, M.; Bowler, U.; Juszczak, E.; King, A.; Linsell, L.; Murray, D.; Partlett, C.; Patel, M.; et al. Enteral lactoferrin supplementation for very preterm infants: a randomised placebo-controlled trial. Lancet 2019, 393, 423–433. [Google Scholar] [CrossRef]
- Bu, H.-F.; Zuo, X.-L.; Wang, X.; Ensslin, M.A.; Koti, V.; Hsueh, W.; Raymond, A.S.; Shur, B.D.; Tan, X.-D. Milk fat globule–EGF factor 8/lactadherin plays a crucial role in maintenance and repair of murine intestinal epithelium. J. Clin. Investig. 2007, 117, 3673–3683. [Google Scholar] [CrossRef]
- He, Y.; Lawlor, N.; Newburg, D. Human Milk Components Modulate Toll-Like Receptor-Mediated Inflammation. Adv. Nutr. 2016, 7, 102–111. [Google Scholar] [CrossRef]
- Lee-Huang, S.; Huang, P.L.; Sun, Y.; Huang, P.L.; Kung, H.F.; Blithe, D.L.; Chen, H.C. Lysozyme and RNases as anti-HIV components in beta-core preparations of human chorionic gonadotropin. Proc. Natl. Acad. Sci. USA 1999, 96, 2678–2681. [Google Scholar] [CrossRef]
- Weidinger, C.; Ziegler, J.F.; Letizia, M.; Schmidt, F.; Siegmund, B. Adipokines and their role in intestinal inflammation. Front. Immunol. 2018, 9, 1974. [Google Scholar] [CrossRef]
- Zulian, A.; Cancello, R.; Micheletto, G.; Gentilini, D.; Gilardini, L.; Danelli, P.; Invitti, C. Visceral adipocytes: old actors in obesity and new protagonists in Crohn’s disease? Gut 2012, 61, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Kredel, L.I.; Batra, A.; Stroh, T.; Kühl, A.A.; Zeitz, M.; Erben, U.; Siegmund, B. Adipokines from local fat cells shape the macrophage compartment of the creeping fat in Crohn’s disease. Gut. 2013, 62, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Siegmund, B.; Lehr, H.A.; Fantuzzi, G. Leptin: A pivotal mediator of intestinal inflammation in mice. Gastroenterol. 2002, 122, 2011–2025. [Google Scholar] [CrossRef] [PubMed]
- Delplanque, B.; Gibson, R.; Koletzko, B.; Lapillonne, A.; Strandvik, B. Lipid Quality in Infant Nutrition: Current Knowledge and Future Opportunities. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Valledor, A.F.; Ricote, M. Nuclear receptor signaling in macrophages. Biochem. Pharmacol. 2004, 67, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Gottrand, F. Long-Chain Polyunsaturated Fatty Acids Influence the Immune System of Infants. J. Nutr. 2008, 138, 1807S–1812S. [Google Scholar] [CrossRef]
- Ghosh, S.; DeCoffe, D.; Brown, K.; Rajendiran, E.; Estaki, M.; Dai, C.; Yip, A.; Gibson, D. Fish Oil Attenuates Omega-6 Polyunsaturated Fatty Acid-Induced Dysbiosis and Infectious Colitis but Impairs LPS Dephosphorylation Activity Causing Sepsis. PLoS ONE 2013, 8, e55468. [Google Scholar] [CrossRef]
- Hughes, D.; Pinder, A.C.; Piper, Z.; Johnson, I.; Lund, E.K. Fish oil supplementation inhibits the expression of major histocompatibility complex class II molecules and adhesion molecules on human monocytes. Am. J. Clin. Nutr. 1996, 63, 267–272. [Google Scholar] [CrossRef]
- Schmidt, E.; Pedersen, J.; Ekelund, S.; Grunnet, N.; Jersild, C.; Dyerberg, J. Cod liver oil inhibits neutrophil and monocyte chemotaxis in healthy males. Atherosclerosis 1989, 77, 53–57. [Google Scholar] [CrossRef]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef]
- Rogier, E.W.; Frantz, A.L.; Bruno, M.E.C.; Wedlund, L.; Cohen, N.A.; Stromberg, A.J.; Kaetzel, C.S. Secretory antibodies in breast milk promote long-term intestinal homeostasis by regulating the gut microbiota and host gene expression. Proc. Natl. Acad. Sci. USA 2014, 111, 3074–3079. [Google Scholar] [CrossRef] [PubMed]
- Araújo, E.D.; Gonçalves, A.K.; Cornetta, M.D.C.; Cunha, H.; Cardoso, M.L.; Morais, S.S.; Giraldo, P.C. Evaluation of the secretory immunoglobulin A levels in the colostrum and milk of mothers of term and pre-term newborns. Braz. J. Infect. Dis. 2005, 9, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Ballabio, C.; Bertino, E.; Coscia, A.; Fabris, C.; Fuggetta, D.; Molfino, S.; Testa, T.; Sgarrella, M.; Sabatino, G.; Restani, P. Immunoglobulin-A Profile in Breast Milk from Mothers Delivering Full Term and Preterm Infants. Int. J. Immunopathol. Pharmacol. 2007, 20, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Goldman, A.S.; Goldblum, R.M.; Hanson, L.Å. Anti-Inflammatory Systems in Human Milk. Adv. Exp. Med. Biol. 1990, 262, 69–76. [Google Scholar] [PubMed]
- Lewis, E.D.; Richard, C.; Larsen, B.; Field, C. The Importance of Human Milk for Immunity in Preterm Infants. Clin. Perinatol. 2017, 44, 23–47. [Google Scholar] [CrossRef] [PubMed]
- Khaleva, E.; Gridneva, Z.; Geddes, D.T.; Oddy, W.H.; Colicino, S.; Blyuss, O.; Boyle, R.J.; Warner, J.O.; Munblit, D. Transforming growth factor beta in human milk and allergic outcomes in children: A systematic review. Clin. Exp. Allergy 2019, 49, 1201–1213. [Google Scholar] [CrossRef]
- Ustundag, B. Levels of cytokines (IL-1beta, IL-2, IL-6, IL-8, TNF-alpha) and trace elements (Zn, Cu) in breast milk from mothers of preterm and term infants. Mediat. Inflamm. 2005, 2005, 331–336. [Google Scholar] [CrossRef]
- Pammi, M.; Suresh, G. Enteral lactoferrin supplementation for prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database Syst. Rev. 2017, 2017, CD007137. [Google Scholar] [CrossRef]
- Obeid, S.; Wankell, M.; Charrez, B.; Sternberg, J.; Kreuter, R.; Esmaili, S.; Ramezani-Moghadam, M.; Devine, C.; Read, S.; Bhathal, P.; et al. Adiponectin confers protection from acute colitis and restricts a B cell immune response. J. Boil. Chem. 2017, 292, 6569–6582. [Google Scholar] [CrossRef]
- Serhan, C.N.; Clish, C.B.; Brannon, J.; Colgan, S.P.; Chiang, N.; Gronert, K. Novel Functional Sets of Lipid-Derived Mediators with Antiinflammatory Actions Generated from Omega-3 Fatty Acids via Cyclooxygenase 2–Nonsteroidal Antiinflammatory Drugs and Transcellular Processing. J. Exp. Med. 2000, 192, 1197–1204. [Google Scholar] [CrossRef]
- Caplan, M.S.; Russell, T.; Xiao, Y.; Amer, M.; Kaup, S.; Jilling, T. Effect of Polyunsaturated Fatty Acid (PUFA) Supplementation on Intestinal Inflammation and Necrotizing Enterocolitis (NEC) in a Neonatal Rat Model. Pediatr. Res. 2001, 49, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Jilling, T.; Li, D.; Caplan, M.S. Polyunsaturated fatty acid supplementation alters proinflammatory gene expression and reduces the incidence of necrotizing enterocolitis in a neonatal rat model. Pediatr. Res. 2007, 61, 427–432. [Google Scholar] [CrossRef] [PubMed]
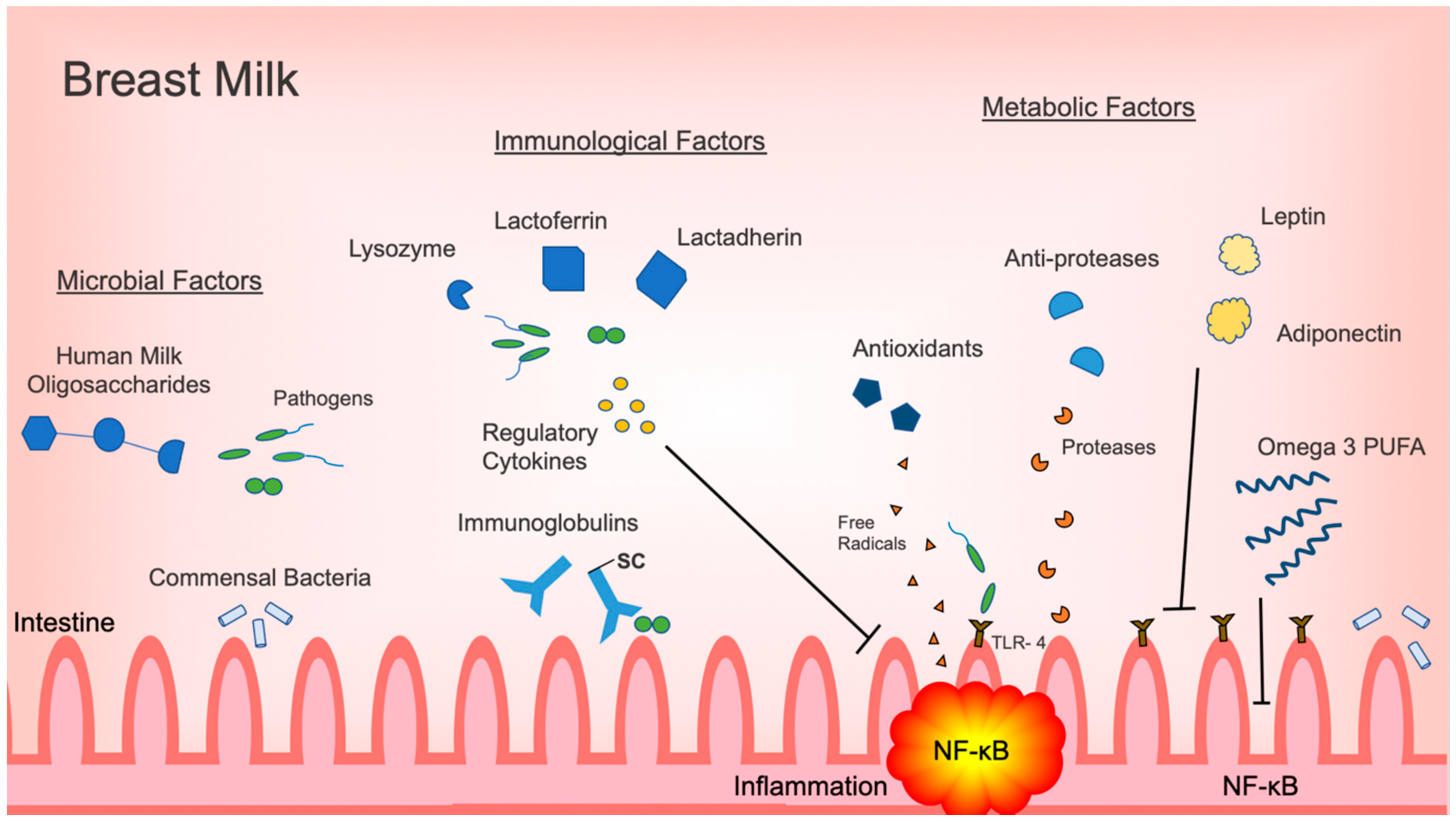
| Bioactive Components in Breast Milk | Role in Intestinal Inflammation Regulation or Prevention | Effect | References |
|---|---|---|---|
| Microbial or microbial modulating factors | |||
| Lactobacillus spp, | -Inhibit NF-κB pathway -decrease pro-inflammatory cytokines, TNF-α, IL-6 -reverse intestinal dysbiosis in bacterial intestinal infection | -decrease inflammatory response -Restore intestinal microbiome homeostasis | [41,42,43,44] |
| Bifidobacterium spp | -increase SCFA production -Decrease pro-inflammatory CK release (IL-6, CXCL-1, TNF-α, IL-23) and iNOS | -promote anti-inflammatory commensal bacteria proliferation -decrease inflammatory response | [45,46,47,48] |
| Human Milk Oligosaccharides | -regulate commensal bacteria -act as decoy receptors for pathogens -modulate immune signaling pathways, TLR3, TLR5, PAMP | -promote healthy intestinal microbiota with anti-inflammatory properties -prevent and decrease inflammatory response | [32,49,50,51,52,53] |
| Immunological factors | |||
| Secretory IgA | -bind to pathogens and commensal bacteria | -prevention of typical inflammatory response, or immune exclusion -influence intestinal microbiome | [29,54] |
| IgG | -opsonization, agglutination of bacteria | -prevention of typical acute inflammatory response | [52,55,56,57] |
| IL-10 | -inhibit Th1, NK cell, macrophages | -provide immunoregulation and prevent inflammation | [18,58,59,60,61] |
| TGF- β | -inhibit differentiation of naïve T cells into Th1, Th2 cells -Stabilize FOXP3 expression | -decrease pro-inflammatory cytokine expression and inflammation -inhibit immune response and decrease inflammation | [18,60,62,63,64] |
| ILRA-1 TNFR I and II soluble TLR2 | -compete with IL-1 receptor for IL-1 -directly bind, inhibit TNF- α -decoy receptor to inhibit IL-8, TNF | -prevent pro-inflammatory cytokine expression and inflammation | [52,60,65,66,67] |
| EGF HB-EGF VEGF | -upregulate IL-10 expression -bind to bacteria -stimulate angiogenesis- | -decrease pro-inflammatory cytokine expression -prevent intestinal edema | [68,69,70,71,72,73,74] |
| Lactoferrin | -direct cytotoxicity on pathogens by forming lactoferricin -inhibit IL-1, IL-6, TNF-α, IL-8 -promote growth of probiotics | -eliminate trigger for acute inflammatory response -decrease pro-inflammatory cytokine expression and inflammation -regulate intestinal microbiome | [18,75,76,77] |
| Lactadherin | -enhance phagocytosis of apoptotic cells -blocks NF-κB pathway via TLR4 inhibition -promote healing during intestinal inflammation | -eliminate trigger for acute inflammatory response -prevent pro-inflammatory signaling and decreasing inflammatory response -limit degree of intestinal inflammation | [78,79] |
| Lysozyme | -degrades GP bacteria outer wall -kill GN bacteria with lactoferrin | -eliminate trigger for acute inflammatory response | [18,80] |
| Metabolic factors | |||
| Adiponectin | -suppress mature macrophage function | -decrease inflammatory response | [52,81] |
| Leptin | -stimulates T cells -influence polarization of macrophages to anti-inflammatory phenotype | -regulate immune response and prevent inflammation | [81,82,83,84] |
| Omega 3 PUFA | -decrease NF- κB, bind to PPAR-γ -increase proliferation of Lactobacillus and Bifidobacterium -change membrane PL concentration -inhibit leukocyte migration | -downregulate pro-inflammatory genes --promote anti-inflammatory commensal bacteria proliferation -decrease degree of inflammatory response | [13,85,86,87,88,89,90] |
| Antioxidants | -scavenge free radicals | -prevent injury and inflammation | [60] |
| Anti-proteases | -metabolize proteases produced by inflammatory cells | -prevent excessive inflammatory response | [60] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thai, J.D.; Gregory, K.E. Bioactive Factors in Human Breast Milk Attenuate Intestinal Inflammation during Early Life. Nutrients 2020, 12, 581. https://doi.org/10.3390/nu12020581
Thai JD, Gregory KE. Bioactive Factors in Human Breast Milk Attenuate Intestinal Inflammation during Early Life. Nutrients. 2020; 12(2):581. https://doi.org/10.3390/nu12020581
Chicago/Turabian StyleThai, Julie D., and Katherine E. Gregory. 2020. "Bioactive Factors in Human Breast Milk Attenuate Intestinal Inflammation during Early Life" Nutrients 12, no. 2: 581. https://doi.org/10.3390/nu12020581
APA StyleThai, J. D., & Gregory, K. E. (2020). Bioactive Factors in Human Breast Milk Attenuate Intestinal Inflammation during Early Life. Nutrients, 12(2), 581. https://doi.org/10.3390/nu12020581




