An Acute, Placebo-Controlled, Single-Blind, Crossover, Dose-Response, Exploratory Study to Assess the Effects of New Zealand Pine Bark Extract (Enzogenol®) on Glycaemic Responses in Healthy Participants
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Study Design
2.3. Treatments
2.4. Parameters of Glycaemic Response during the OGTT
2.5. Statistical Analysis
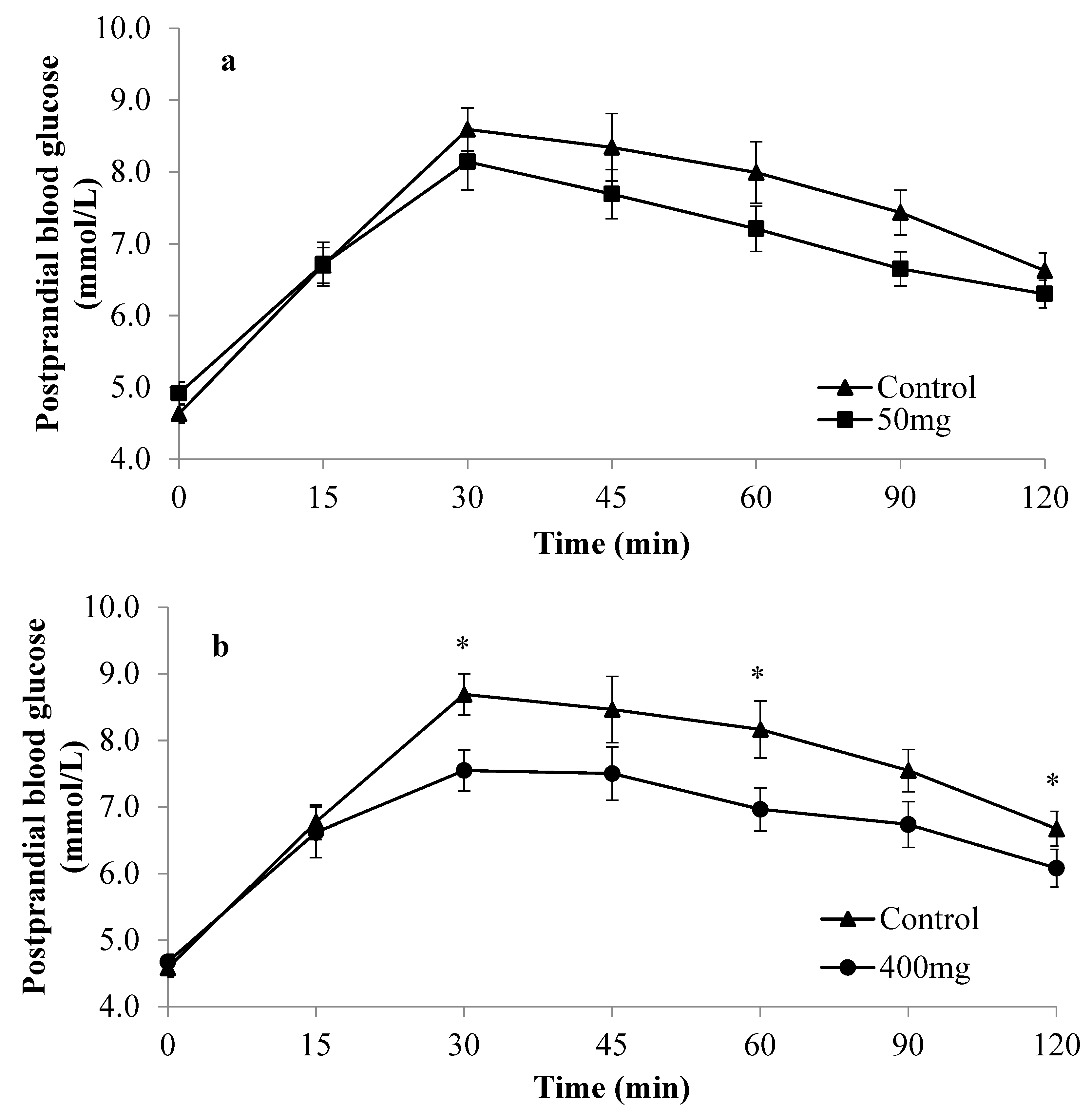
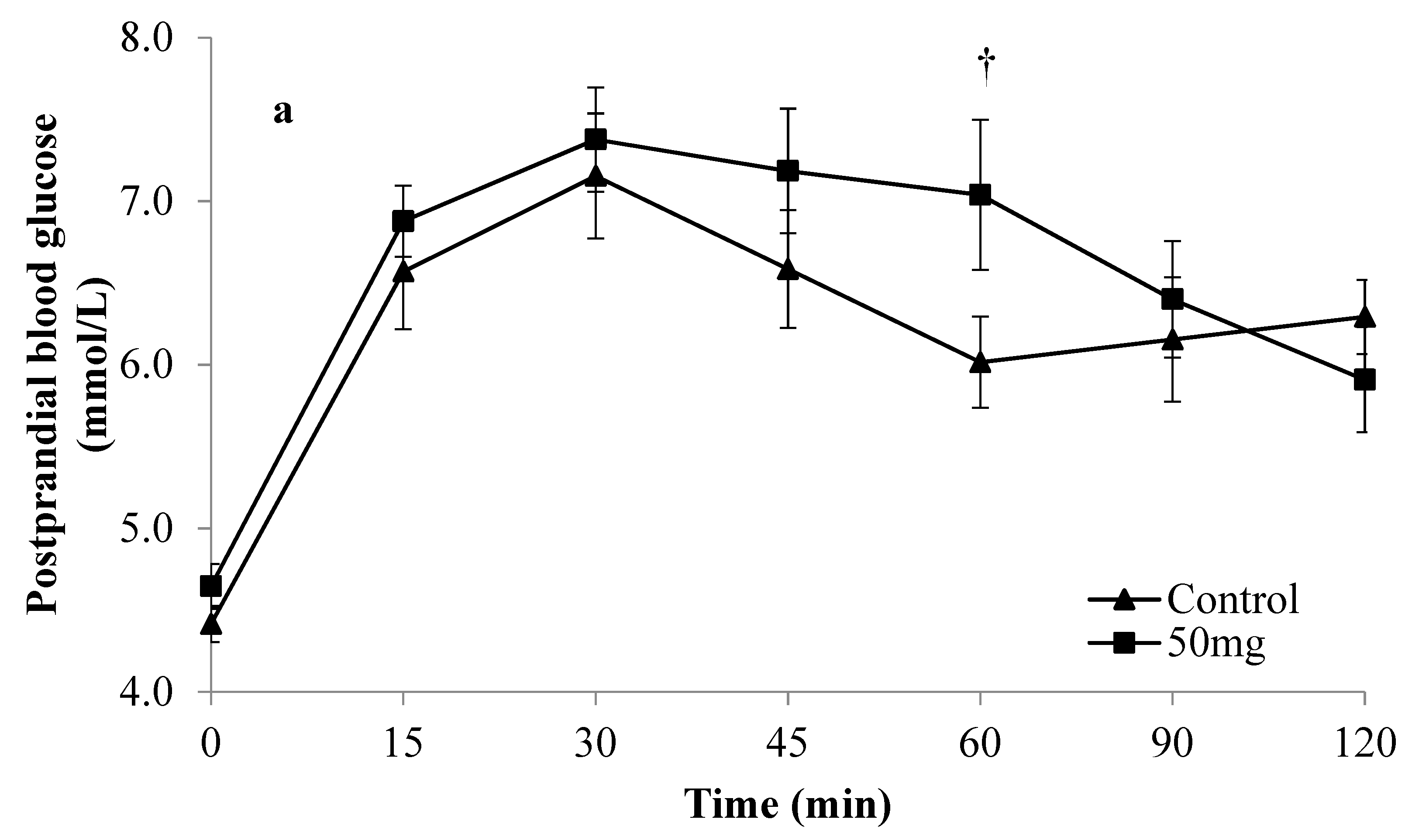
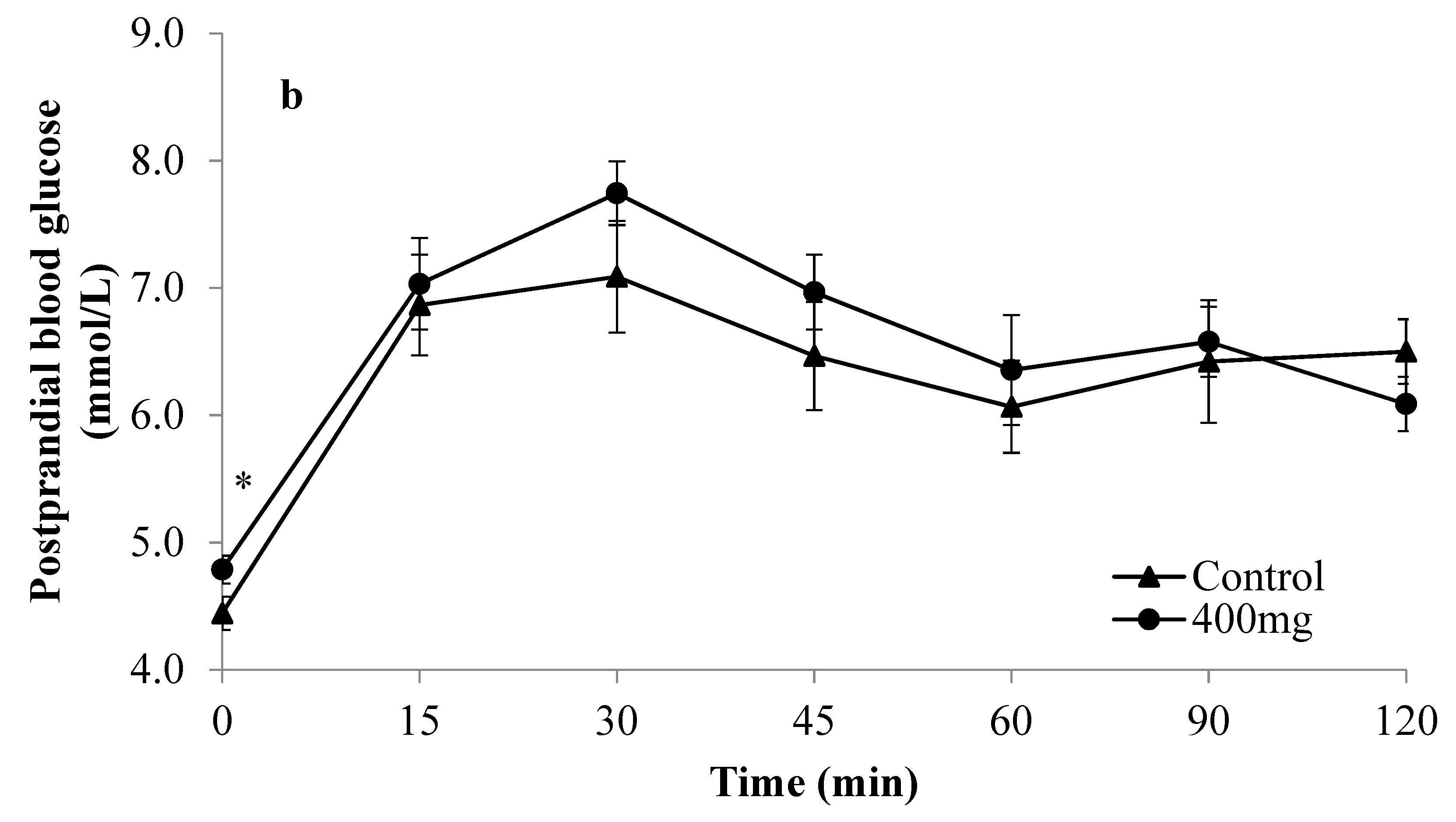
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stumvoll, M.; Goldstein, B.J.; van Haeften, T.W. Type 2 diabetes: Principles of pathogenesis and therapy. Lancet 2005, 365, 1333–1346. [Google Scholar] [CrossRef]
- Zimmet, P.; Alberti, K.; Shaw, J. Global and societal implications of the diabetes epidemic. Nature 2001, 414, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Litwak, L.; Goh, S.Y.; Hussein, Z.; Malek, R.; Prusty, V.; Khamseh, M.E. Prevalence of diabetes complications in people with type 2 diabetes mellitus and its association with baseline characteristics in the multinational Achieve study. Diabetol. Metabol. Syndr. 2013, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Gregg, E.W.; Sattar, N.; Ali, M.K. The changing face of diabetes complications. Lancet Diabetes Endocrinol. 2016, 4, 537–547. [Google Scholar] [CrossRef]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Gupta, C.; Prakash, D. Phytonutrients as therapeutic agents. J. Complement. Integr. Med. 2014, 11, 151–169. [Google Scholar] [CrossRef]
- Dias, T.R.; Alves, M.G.; Casal, S.; Oliveira, P.F.; Silva, B.M. Promising potential of dietary (poly)phenolic compounds in the prevention and treatment of diabetes mellitus. Curr. Med. Chem. 2017, 24, 334–354. [Google Scholar]
- Adisakwattana, S.; Jiphimai, P.; Prutanopajai, P.; Chanathong, B.; Sapwarobol, S.; Ariyapitipan, T. Evaluation of alpha-glucosidase, alpha-amylase and protein glycation inhibitory activities of edible plants. Int. J. Food Sci. Nutr. 2010, 61, 295–305. [Google Scholar] [CrossRef]
- Kim, Y.; Keogh, J.B.; Clifton, P.M. Polyphenols and glycemic control. Nutrients 2016, 8, 17. [Google Scholar] [CrossRef]
- Tahrani, A.A.; Barnett, A.H.; Bailey, C.J. Pharmacology and therapeutic implications of current drugs for type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2016, 12, 566–592. [Google Scholar] [CrossRef]
- Li, Y.Y.; Feng, J.; Zhang, X.L.; Cui, Y.Y. Pine bark extracts: Nutraceutical, pharmacological, and toxicological evaluation. J. Pharmacol. Exp. Ther. 2015, 353, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Frevel, M.A.E.; Pipingas, A.; Grigsby, W.J.; Frampton, C.M.; Gilchrist, N.L. Production, composition and toxicology studies of Enzogenol (R) Pinus radiata bark extract. Food Chem. Toxicol. 2012, 50, 4316–4324. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, G. Pycnogenol: A blend of procyanidins with multifaceted therapeutic applications? Fitoterapia 2010, 81, 724–736. [Google Scholar] [CrossRef]
- Schoonees, A.; Visser, J.; Musekiwa, A.; Volmink, J. Pycnogenol (R) for the treatment of chronic disorders. Cochrane Database Syst. Rev. 2012, 108. [Google Scholar] [CrossRef]
- Yang, K.Y.; Chan, C.B. Proposed mechanisms of the effects of proanthocyanidins on glucose homeostasis. Nutr. Rev. 2017, 75, 642–657. [Google Scholar] [CrossRef]
- Jerez, M.; Selga, A.; Sineiro, J.; Torres, J.L.; Nunez, M.J. A comparison between bark extracts from Pinus pinaster and Pinus radiata: Antioxidant activity and procyanidin composition. Food Chem. 2007, 100, 439–444. [Google Scholar] [CrossRef]
- Masquelier, J.; Michaud, J.; Laparra, J.; Dumon, M.C. Flavonoids and pycnogenols. Int. J. Vitamin Nutr. Res. 1979, 49, 307–311. [Google Scholar]
- Unwin, N.; Shaw, J.; Zimmet, P.; Alberti, K. Impaired glucose tolerance and impaired fasting glycaemia: The current status on definition and intervention. Diabet. Med. 2002, 19, 708–723. [Google Scholar]
- Eschwege, E.; Charles, M.A.; Simon, D.; Thibult, N.; Balkau, B. Reproducibility of the diagnosis of diabetes over a 30-month follow-up—The Paris Prospective Study. Diabetes Care 2001, 24, 1941–1944. [Google Scholar] [CrossRef]
- Ahren, B. Insulin secretion and insulin sensitivity in relation to fasting glucose in healthy subjects. Diabetes Care 2007, 30, 644–648. [Google Scholar] [CrossRef]
- Piche, M.E.; Lemieux, S.; Perusse, L.; Weisnagel, S.J. High normal 2-h plasma glucose is associated with insulin sensitivity and secretion that may predispose to type 2 diabetes. Diabetologia 2005, 48, 732–740. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gerstein, H.C.; Santaguida, P.; Raina, P.; Morrison, K.M.; Balion, C.; Hunt, D.; Yazdi, H.; Booker, L. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: A systematic overview and meta-analysis of prospective studies. Diabetes Res. Clin. Practice 2007, 78, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Tschritter, O.; Fritsche, A.; Shirkavand, F.; Machicao, F.; Haring, H.; Stumvoll, M. Assessing the shape of the glucose curve during an oral glucose tolerance test. Diabetes Care 2003, 26, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Michaliszyn, S.F.; Nasr, A.; Lee, S.; Tfayli, H.; Hannon, T.; Hughan, K.S.; Bacha, F.; Arslanian, S. The shape of the glucose response curve during an oral glucose tolerance test heralds biomarkers of Type 2 diabetes risk in obese youth. Diabetes Care 2016, 39, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Kaga, H.; Tamura, Y.; Takeno, K.; Kakehi, S.; Someya, Y.; Suzuki, R.; Kadowaki, S.; Sugimoto, D.; Furukawa, Y.; Funayama, T.; et al. The shape of the glucose response curve during an oral glucose tolerance test was associated with muscle insulin sensitivity and visceral fat accumulation in non-obese healthy men. Diabetes 2018, 67. [Google Scholar] [CrossRef]
- Tura, A.; Morbiducci, U.; Sbrignadello, S.; Winhofer, Y.; Pacini, G.; Kautzky-Willer, A. Shape of glucose, insulin, C-peptide curves during a 3-h oral glucose tolerance test: Any relationship with the degree of glucose tolerance? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R941–R948. [Google Scholar] [CrossRef]
- Kanauchi, M.; Kimura, K.; Kanauchi, K.; Saito, Y. Beta-cell function and insulin sensitivity contribute to the shape of plasma glucose curve during an oral glucose tolerance test in non-diabetic individuals. Int. J. Clin. Prac. 2005, 59, 427–432. [Google Scholar] [CrossRef]
- Trujillo-Arriaga, H.M.; Roman-Ramos, R. Fitting and evaluating the glucose curve during a quasi continuous sampled oral glucose tolerance test. Comput. Biol. Med. 2008, 38, 185–195. [Google Scholar] [CrossRef]
- Hollenbeck, C.; Reaven, G.M. Variations in insulin stimulated glucose uptake in healthy individuals with normal glucose tolerance. J. Clin. Endocrinol. Metabol. 1987, 64, 1169–1173. [Google Scholar] [CrossRef]
- Schianca, G.P.C.; Colli, E.; Onolfo, S.; Pedrazzoli, R.; Fra, G.P.; Bartoli, E. Individuation of different metabolic phenotypes in normal glucose tolerance test. Acta Diabetol. 2010, 47, 167–172. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Ferrannini, E.; Miyazaki, Y.; Matsuda, M.; DeFronzo, R.A. Beta-cell dysfunction and glucose intolerance: Results from the San Antonio metabolism (SAM) study. Diabetologia 2004, 47, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.A.; Williams, K.; DeFronzo, R.; Stern, M. Risk of progression to type 2 diabetes based on relationship between postload plasma glucose and fasting plasma glucose. Diabetes Care 2006, 29, 1613–1618. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.A.; Lyssenko, V.; Tuomi, T.; DeFronzo, R.A.; Groop, L. The shape of plasma glucose concentration curve during OGTT predicts future risk of type 2 diabetes. Diabetes Metabol. Res. Rev. 2010, 26, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Komaki, E.; Yamaguchi, S.; Maru, I.; Kinoshita, M.; Kakehi, K.; Ohta, Y.; Tsukada, Y. Identification of anti-alpha-amylase components from olive leaf extracts. Food Sci. Technol. Res. 2003, 9, 35–39. [Google Scholar] [CrossRef]
- Boone, C.H.; Stout, J.R.; A Gordon, J.; Redd, M.J.; Church, D.D.; Oliveira, L.P.; Fukuda, D.H.; Hoffman, J.R. Acute effects of a beverage containing bitter melon extract (CARELA) on postprandial glycemia among prediabetic adults. Nutr. Diabetes 2017, 7, e2415. [Google Scholar] [CrossRef]
- Morris, C.; O’Grada, C.; Ryan, M.; Roche, H.M.; Gibney, M.J.; Gibney, E.R.; Brennan, L. Identification of Differential Responses to an Oral Glucose Tolerance Test in Healthy Adults. PLoS ONE 2013, 8, 9. [Google Scholar] [CrossRef]
- Krishnan, S.; Newman, J.W.; Hembrooke, T.A.; Keim, N.L. Variation in metabolic responses to meal challenges differing in glycemic index in healthy women: Is it meaningful? Nutr. Metabol. 2012, 9, 26. [Google Scholar] [CrossRef]
- Kerimi, A.; Nyambe-Silavwe, H.; Pyner, A.; Oladele, E.; Gauer, J.S.; Stevens, Y.; Williamson, G. Nutritional implications of olives and sugar: Attenuation of post-prandial glucose spikes in healthy volunteers by inhibition of sucrose hydrolysis and glucose transport by oleuropein. Eur. J. Nutr. 2018, 58, 1315–1330. [Google Scholar] [CrossRef]
- Liu, X.M.; Wei, J.P.; Tan, F.S.; Zhou, S.M.; Wurthwein, G.; Rohdewald, P. Antidiabetic effect of Pycnogenol((R)) French maritime pine bark extract in patients with diabetes type II. Life Sci. 2004, 75, 2505–2513. [Google Scholar] [CrossRef]
- Liu, X.M.; Zhou, H.J.; Rohdewald, P. French maritime pine bark extract pycnogenol dose-dependently lowers glucose in type 2 diabetic patients. Diabetes Care 2004, 27, 839. [Google Scholar] [CrossRef]
- Zibadi, S.; Rohdewald, P.J.; Park, D.; Watson, R.R. Reduction of cardiovascular risk factors in subjects with type 2 diabetes by Pycnogenol supplementation. Nutr. Res. 2008, 28, 315–320. [Google Scholar] [CrossRef]
- Wolever, T.M.S.; Jenkins, D.J.A. The use of the glycemic index in predicting the blood glucose response to mixed meals. Am. J. Clin. Nutr. 1986, 43, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.K.; Vuksan, V.; Choi, H.; Zinman, B.; Retnakaran, R. Emerging parameters of the insulin and glucose response on the oral glucose tolerance test: Reproducibility and implications for glucose homeostasis in individuals with and without diabetes. Diabetes Res. Clin. Practice 2014, 105, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Chepulis, L.; Al-Aubaidy, H.; Page, R. Effects of selected antioxidant food extracts on postprandial glucose responses in healthy individuals. Funct. Foods Health Dis. 2016, 6, 493–505. [Google Scholar] [CrossRef]
- Ministry of Health. New Zealand Primary Care Handbook 2012. Available online: https://www.health.govt.nz/system/files/documents/publications/nz-primary-care_handbook_2012.pdf (accessed on 27 May 2019).
- Bang, C.Y.; Choung, S.Y. Enzogenol improves diabetes-related metabolic change in C57BL/KsJ-db/db mice, a model of type 2 diabetes mellitus. J. Pharm. Pharmacol. 2014, 66, 875–885. [Google Scholar] [PubMed]
- Bartoli, E.; Fra, G.P.; Schianca, G.P.C. The oral glucose tolerance test (OGTT) revisited. Eur. J. Int. Med. 2011, 22, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Zhao, X.Q.; Zhou, R.R.; Gu, Y.J.; Zhu, X.H.; Tang, Z.Q.; Yuan, X.; Chen, W.; Zhang, R.; Qian, C.; et al. Delay in glucose peak time during the oral glucose tolerance test as an indicator of insulin resistance and insulin secretion in type 2 diabetes patients. J. Diabetes Investig. 2018, 9, 1288–1295. [Google Scholar] [CrossRef]
- Chung, S.T.; Ha, J.; Onuzuruike, A.U.; Kasturi, K.; Galvan-De La Cruz, M.; Bingham, B.A.; Baker, R.L.; Utumatwishima, J.N.; Mabundo, L.S.; Ricks, M.; et al. Time to glucose peak during an oral glucose tolerance test identifies prediabetes risk. Clin. Endocrinol. 2017, 87, 484–491. [Google Scholar] [CrossRef]
- Ceriello, A.; Esposito, K.; Piconi, L.; Ihnat, M.; Thorpe, J.; Testa, R.; Bonfigli, A.R.; Giugliano, D. Glucose “peak” and glucose “spike”: Impact on endothelial function and oxidative stress. Diabetes Res. Clin. Practice 2008, 82, 262–267. [Google Scholar] [CrossRef]
- Hulman, A.; Simmons, R.K.; Vistisen, D.; Tabak, A.G.; Dekker, J.M.; Alssema, M.; Rutters, F.; Koopman, A.D.M.; Solomon, T.P.J.; Kirwan, J.P.; et al. Heterogeneity in glucose response curves during an oral glucose tolerance test and associated cardiometabolic risk. Endocrine 2017, 55, 427–434. [Google Scholar] [CrossRef]
- Hulman, A.; Vistisen, D.; Glumer, C.; Bergman, M.; Witte, D.R.; Faerch, K. Glucose patterns during an oral glucose tolerance test and associations with future diabetes, cardiovascular disease and all-cause mortality rate. Diabetologia 2018, 61, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Manco, M.; Nolfe, G.; Pataky, Z.; Monti, L.; Porcellati, F.; Gabriel, R.; Mitrakou, A.; Mingrone, G. Shape of the OGTT glucose curve and risk of impaired glucose metabolism in the EGIR-RISC cohort. Metabol. Clin. Exp. 2017, 70, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Seino, Y.; Nanjo, K.; Tajima, N.; Kadowaki, T.; Kashiwagi, A.; Araki, E.; Ito, C.; Inagaki, N.; Iwamoto, Y.; Kasuga, M.; et al. Report of the Committee on the Classification and Diagnostic Criteria of Diabetes Mellitus. J. Diabetes Investig. 2010, 1, 212–228. [Google Scholar] [CrossRef] [PubMed]
- Stumvoll, M.; Fritsche, A.; Haring, H. The OGTT as test for beta cell function? Eur. J. Clin. Investig. 2001, 31, 380–381. [Google Scholar] [CrossRef] [PubMed]
- Libman, I.M.; Barinas-Mitchell, E.; Bartucci, A.; Robertson, R.; Arslanian, S. Reproducibility of the oral glucose tolerance test in overweight children. J. Clin. Endocrinol. Metabol. 2008, 93, 4231–4237. [Google Scholar] [CrossRef] [PubMed]
- Brohall, G.; Behre, C.J.; Hulthe, J.; Wikstrand, J.; Fagerberg, B. Prevalence of diabetes and impaired glucose tolerance in 64-year-old Swedish women—Experiences of using repeated oral glucose tolerance tests. Diabetes Care 2006, 29, 363–367. [Google Scholar] [CrossRef]
- Roman, R.; Zeitler, P.S. Oral glucose tolerance testing in asymptomatic obese children: More questions than answers. J. Clin. Endocrinol. Metabol. 2008, 93, 4228–4230. [Google Scholar] [CrossRef]
- Gordon, B.A.; Fraser, S.F.; Bird, S.R.; Benson, A.C. Reproducibility of multiple repeated oral glucose tolerance tests. Diabetes Res. Clin. Practice 2011, 94, E78–E82. [Google Scholar] [CrossRef]
| Characteristics | Mean ± SEM (All) | Range (Min to Max) | Mean ± SEM (Monophasic Group) | Mean ± SEM (Complex Group) | p(Monophasic vs. Complex Shape) |
|---|---|---|---|---|---|
| N | 25 | NA | 12 | 13 | - |
| Gender (M/F) | 10/15 | NA | 3/9 | 7/6 | - |
| Age (years) | 24.8 ± 0.8 | 20–33 | 25.4 ± 1.1 | 24.2 ± 1.2 | 0.44 |
| BMI (kg/m2) | 21.2 ± 0.4 | 18.1–25.3 | 21.2 ± 0.6 | 21.2 ± 0.5 | 1.00 |
| FBG (mmol/L) | 4.4 ± 0.1 | 3.6–5.2 | 4.5 ± 0.1 | 4.3 ± 0.1 | 0.55 |
| HbA1c (mmol/mol) | 33 ± 0 | 29–38 | 33 ± 1 | 34 ± 1 | 0.24 |
| SBP (mm Hg) | 109 ± 2 | 89–133 | 111.7 ± 4.4 | 107 ± 2 | 0.30 |
| DBP (mm Hg) | 67 ± 1 | 47–77 | 65.6 ± 2.4 | 68 ± 2 | 0.45 |
| TC (mmol/L) | 4.09 ± 0.12 | 3.06–5.27 | 4.07 ± 0.13 | 4.10 ± 0.20 | 0.89 |
| TG (mmol/L) | 1.15 ± 0.13 | 0.56–3.71 | 1.30 ± 0.25 | 1.02 ± 0.11 | 0.30 |
| HDL-C (mmol/L) | 1.50 ± 0.08 | 0.98–2.49 | 1.47 ± 0.11 | 1.54 ± 0.11 | 0.67 |
| LDL-C (mmol/L) | 2.06 ± 0.09 | 1.23–2.93 | 2.01 ± 0.13 | 2.10 ± 0.12 | 0.60 |
| Non-HDL-C (mmol/L) | 2.58 ± 0.09 | 1.88–3.32 | 2.60 ± 0.13 | 2.57 ± 0.13 | 0.87 |
| TC/HDL-C ratio | 2.82 ± 0.10 | 1.8–4.1 | 2.89 ± 0.17 | 2.75 ± 0.12 | 0.49 |
| Parameters of Glycaemic Response | Monophasic Group | Complex Group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control Paired to 50 mg of Enzogenol® | 50 mg of Enzogenol® | p | Control Paired to 400 mg of Enzogenol® | 400 mg of Enzogenol® | p | Control Paired to 50 mg of Enzogenol® | 50 mg of Enzogenol® | p | Control Paired to 400 mg of Enzogenol® | 400 mg of Enzogenol® | p | |
| n | 12 | - | 11 | - | 13 | - | 9 | - | ||||
| Gender (M/F) | 3/9 | - | 3/8 | - | 7/6 | - | 5/4 | - | ||||
| Mean glucose iAUC (mmol/L·min) | 335.4 ± 34.0 † | 241.3 ± 20.2 | 0.034 * | 353.6 ± 31.5 ‡ | 249.3 ± 25.4 | 0.012 * | 222.7 ± 26.6 † | 238.4 ± 32.7 | 0.392 | 233.5 ± 36.4 ‡ | 219.1 ± 19.5 | 0.614 |
| Mean %PG | 44.9 ± 8.3 | 28.9 ± 3.8 | 0.083 | 47.5 ± 8.6 | 31.4 ± 7.9 | 0.010 * | 43.4 ± 5.9 | 28.7 ± 8.2 | 0.012* | 47.3 ± 7.2 | 27.7 ± 5.4 | 0.025 * |
| Mean time to glucose peak (min) | 35.0 ± 2.8 | 32.5 ± 2.5 | 0.504 | 35.5 ± 3.0 | 32.7 ± 4.0 | 0.441 | 28.8 ± 2.7 | 28.8 ± 4.0 | 1.000 | 28.3 ± 3.0 | 26.7 ± 4.9 | 0.729 |
| Mean glucose peak value (mmol/L) | 8.8 ± 0.3† | 8.3 ± 0.4 | 0.177 | 8.9 ± 0.3‡ | 7.9 ± 0.3 | 0.025 * | 7.7 ± 0.3 † | 8.2 ± 0.4 | 0.315 | 7.8 ± 0.3 ‡ | 8.0 ± 0.3 | 0.531 |
| Mean 2hPG (mmol/L) | 6.6 ± 0.2 | 6.3 ± 0.2 | 0.171 | 6.7 ± 0.3 | 6.1 ± 0.3 | 0.027 * | 6.3 ± 0.2 | 5.9 ± 0.3 | 0.149 | 6.5 ± 0.3 | 6.1 ± 0.2 | 0.224 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, W.X.J.; Chepulis, L.; von Hurst, P.; Gammon, C.S.; Page, R.A. An Acute, Placebo-Controlled, Single-Blind, Crossover, Dose-Response, Exploratory Study to Assess the Effects of New Zealand Pine Bark Extract (Enzogenol®) on Glycaemic Responses in Healthy Participants. Nutrients 2020, 12, 497. https://doi.org/10.3390/nu12020497
Lim WXJ, Chepulis L, von Hurst P, Gammon CS, Page RA. An Acute, Placebo-Controlled, Single-Blind, Crossover, Dose-Response, Exploratory Study to Assess the Effects of New Zealand Pine Bark Extract (Enzogenol®) on Glycaemic Responses in Healthy Participants. Nutrients. 2020; 12(2):497. https://doi.org/10.3390/nu12020497
Chicago/Turabian StyleLim, Wen Xin Janice, Lynne Chepulis, Pamela von Hurst, Cheryl S. Gammon, and Rachel A. Page. 2020. "An Acute, Placebo-Controlled, Single-Blind, Crossover, Dose-Response, Exploratory Study to Assess the Effects of New Zealand Pine Bark Extract (Enzogenol®) on Glycaemic Responses in Healthy Participants" Nutrients 12, no. 2: 497. https://doi.org/10.3390/nu12020497
APA StyleLim, W. X. J., Chepulis, L., von Hurst, P., Gammon, C. S., & Page, R. A. (2020). An Acute, Placebo-Controlled, Single-Blind, Crossover, Dose-Response, Exploratory Study to Assess the Effects of New Zealand Pine Bark Extract (Enzogenol®) on Glycaemic Responses in Healthy Participants. Nutrients, 12(2), 497. https://doi.org/10.3390/nu12020497






