Exercise-Induced Muscle Damage and Cardiac Stress During a Marathon Could be Associated with Dietary Intake During the Week Before the Race
Abstract
1. Introduction
2. Material and Methods
2.1. Participants
2.2. Experimental Procedures
2.3. Blood Sample Analysis
2.4. Anthropometric Measures
2.5. Dietary Assessment
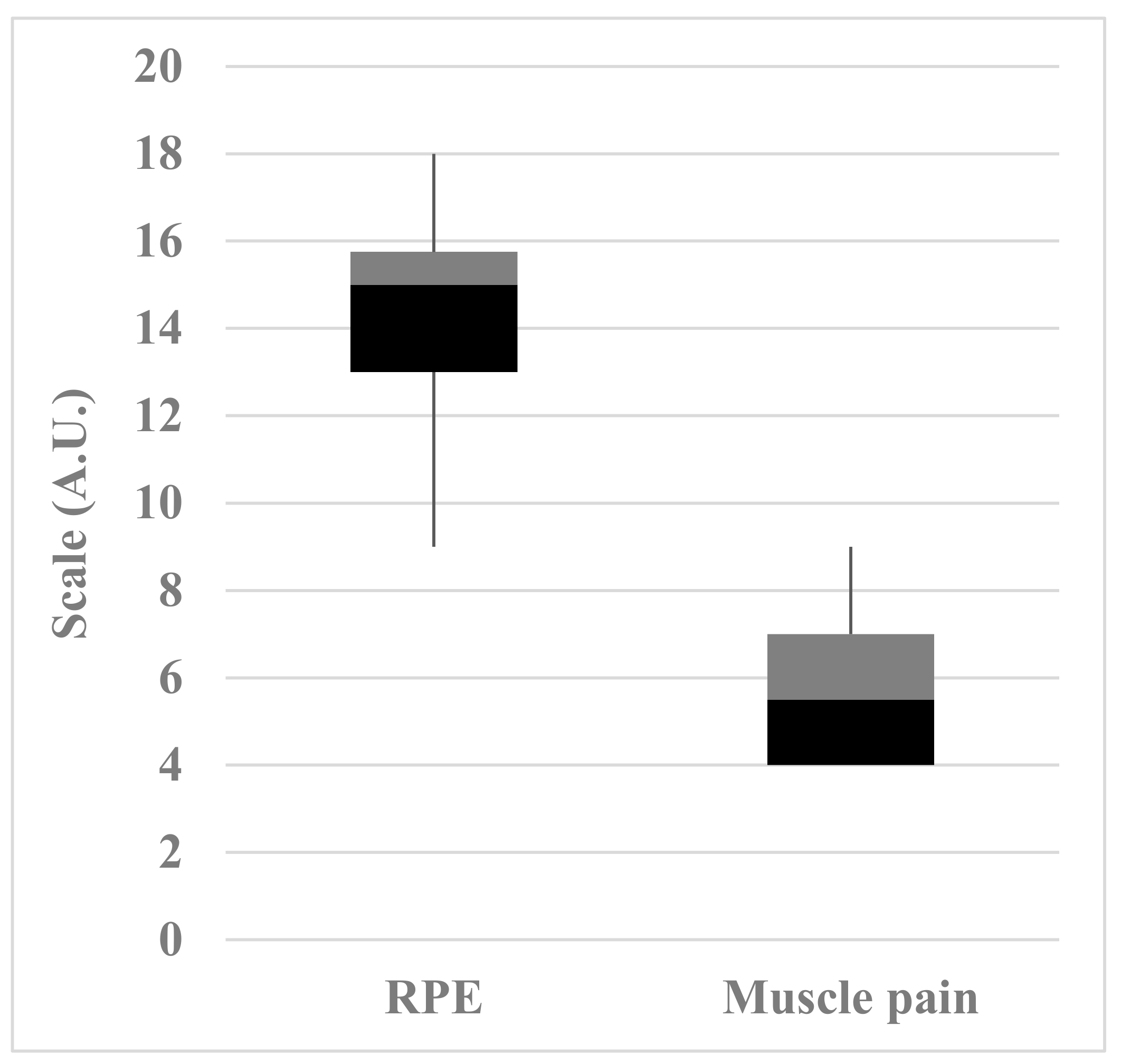
2.6. Rating of Perceived Exertion (RPE) and Muscle Pain Intensity
2.7. Statistical Analysis
3. Results
4. Discussion
4.1. Strengths, Limitations, and Future Research
4.2. Practical Applications
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Del Coso, J.; Fernandez de Velasco, D.; Abian-Vicen, J.; Salinero, J.J.; Gonzalez-Millan, C.; Areces, F.; Ruiz, D.; Gallo, C.; Calleja-Gonzalez, J.; Perez-Gonzalez, B. Running Pace Decrease during a Marathon is Positively Related to Blood Markers of Muscle Damage. PLoS ONE 2013, 8, e57602. [Google Scholar] [CrossRef]
- Rapoport, B.I. Metabolic Factors Limiting Performance in Marathon Runners. PLoS Comput. Boil. 2010, 6, e1000960. [Google Scholar] [CrossRef]
- Howatson, G.; McHugh, M.P.; Hill, J.A.; Brouner, J.; Jewell, A.P.; van Someren, K.A.; Shave, R.E.; Howatson, S.A. Influence of Tart Cherry Juice on Indices of Recovery Following Marathon Running. Scand. J. Med. Sci. Sports 2010, 20, 843–852. [Google Scholar] [CrossRef]
- Knechtle, B.; Nikolaidis, P.T. Physiology and Pathophysiology in Ultra-Marathon Running. Front. Physiol. 2018, 9, 634. [Google Scholar] [CrossRef]
- Herrmann, M.; Scharhag, J.; Miclea, M.; Urhausen, A.; Herrmann, W.; Kindermann, W. Post-Race Kinetics of Cardiac Troponin T and I and N-Terminal Pro-Brain Natriuretic Peptide in Marathon Runners. Clin. Chem. 2003, 49, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Kratz, A.; Lewandrowski, K.B.; Siegel, A.J.; Chun, K.Y.; Flood, J.G.; Van Cott, E.M.; Lee-Lewandrowski, E. Effect of Marathon Running on Hematologic and Biochemical Laboratory Parameters, Including Cardiac Markers. Am. J. Clin. Pathol. 2002, 118, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Neilan, T.G.; Januzzi, J.L.; Lee-Lewandrowski, E.; Ton-Nu, T.-T.; Yoerger, D.M.; Jassal, D.S.; Lewandrowski, K.B.; Siegel, A.J.; Marshall, J.E.; Douglas, P.S.; et al. Myocardial Injury and Ventricular Dysfunction Related to Training Levels Among Nonelite Participants in the Boston Marathon. Circulation 2006, 114, 2325–2333. [Google Scholar] [CrossRef] [PubMed]
- Neumayr, G.; Pfister, R.; Mitterbauer, G.; Eibl, G.; Hoertnagl, H. Effect of Competitive Marathon Cycling on Plasma N-Terminal Pro-Brain Natriuretic Peptide and Cardiac Troponin T in Healthy Recreational Cyclists. Am. J. Cardiol. 2005, 96, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Vidotto, C.; Tschan, H.; Atamaniuk, J.; Pokan, R.; Bachl, N.; Müller, M.M. Responses of N-Terminal Pro-Brain Natriuretic Peptide (NT-proBNP) and Cardiac Troponin I (cTnI) to Competitive Endurance Exercise in Recreational Athletes. Int. J. Sports Med. 2005, 26, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Frassl, W.; Kowoll, R.; Katz, N.; Speth, M.; Stangl, A.; Brechtel, L.; Joscht, B.; Boldt, L.H.; Meier-Buttermilch, R.; Schlemmer, M.; et al. Cardiac markers (BNP, NT-pro-BNP, Troponin I, Troponin T, in female amateur runners before and up until three days after a marathon. Clin. Lab. 2008, 54, 81–87. [Google Scholar]
- Hewing, B.; Schattke, S.; Spethmann, S.; Sanad, W.; Schroeckh, S.; Schimke, I.; Halleck, F.; Peters, H.; Brechtel, L.; Lock, J.; et al. Cardiac and renal function in a large cohort of amateur marathon runners. Cardiovasc. Ultrasound 2015, 13, 13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Giandolini, M.; Vernillo, G.; Samozino, P.; Horvais, N.; Edwards, W.B.; Morin, J.-B.; Millet, G.Y. Fatigue associated with prolonged graded running. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 116, 1859–1873. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Millet, G.; Tarnopolsky, M.A.; International Association of Athletics Federations. Nutrition for Distance Events. J. Sports Sci. 2007, 25 (Suppl. S1), S29–S38. [Google Scholar] [CrossRef]
- Jeukendrup, A.E. Nutrition for endurance sports: Marathon, triathlon, and road cycling. J. Sports Sci. 2011, 29 (Suppl. S1), S91–S99. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, P.T.; Veniamakis, E.; Rosemann, T.; Knechtle, B. Nutrition in Ultra-Endurance: State of the Art. Nutrients 2018, 10, 1995. [Google Scholar] [CrossRef]
- Jeukendrup, A. A step towards personalized sports nutrition: Carbohydrate intake during exercise. Sports Med. 2014, 44, S25–S33. [Google Scholar] [CrossRef] [PubMed]
- Sousa, M.; Teixeira, V.H.; Soares, J. Dietary Strategies to Recover from Exercise-Induced Muscle Damage. Int. J. Food Sci. Nutr. 2014, 65, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Harty, P.S.; Cottet, M.L.; Malloy, J.K.; Kerksick, C.M. Nutritional and Supplementation Strategies to Prevent and Attenuate Exercise-Induced Muscle Damage: A Brief Review. Sports Med. Open 2019, 5, 1. [Google Scholar] [CrossRef]
- Howatson, G.; Van Someren, K.A. The prevention and treatment of exercise-induced muscle damage. Sports Med. 2008, 38, 483–503. [Google Scholar] [CrossRef]
- Owens, D.J.; Twist, C.; Cobley, J.N.; Howatson, G.; Close, G.L. Exercise-Induced Muscle Damage: What is it, what Causes it and what are the Nutritional Solutions? Eur. J. Sport. Sci. 2019, 19, 71–85. [Google Scholar] [CrossRef]
- Fitó, M.; Estruch, R.; Salas-Salvadó, J.; Martínez-Gonzalez, M.A.; Arós, F.; Vila, J.; Corella, D.; Diaz, O.; Sáez, G.; De La Torre, R.; et al. Effect of the Mediterranean diet on heart failure biomarkers: A randomized sample from the PREDIMED trial. Eur. J. Hear. Fail. 2014, 16, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Castell, L.M.; Stear, S.J. BJSM reviews: A-Z of supplements: Dietary supplements, sports nutrition foods and ergogenic aids for health and performance Part 1. Br. J. Sports Med. 2009, 43, 728–729. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sanz, J.M.; Sospedra, I.; Ortiz, C.M.; Baladía, E.; Gil-Izquierdo, A.; Ortiz-Moncada, R. Intended or Unintended Doping? A Review of the Presence of Doping Substances in Dietary Supplements Used in Sports. Nutrients 2017, 9, 1093. [Google Scholar] [CrossRef] [PubMed]
- James, L.J.; Stevenson, E.J.; Rumbold, P.L.S.; Hulston, C.J. Cow’s Milk as a Post-Exercise Recovery Drink: Implications for Performance and Health. Eur. J. Sport. Sci. 2019, 19, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Cockburn, E.; Bell, P.G.; Stevenson, E. Effect of Milk on Team Sport Performance after Exercise-Induced Muscle Damage. Med. Sci. Sports Exerc. 2013, 45, 1585–1592. [Google Scholar] [CrossRef]
- Cockburn, E.; Hayes, P.R.; French, D.N.; Stevenson, E.; Gibson, A.S.C. Acute milk-based protein–CHO supplementation attenuates exercise-induced muscle damage. Appl. Physiol. Nutr. Metab. 2008, 33, 775–783. [Google Scholar] [CrossRef]
- Kelley, D.S.; Adkins, Y.; Laugero, K.D. A Review of the Health Benefits of Cherries. Nutrients 2018, 10, 368. [Google Scholar] [CrossRef]
- Ammar, A.; Bailey, S.J.; Chtourou, H.; Trabelsi, K.; Turki, M.; Hökelmann, A.; Souissi, N. Effects of pomegranate supplementation on exercise performance and post-exercise recovery in healthy adults: A systematic review. Br. J. Nutr. 2018, 120, 1201–1216. [Google Scholar] [CrossRef]
- Trombold, J.R.; Barnes, J.N.; Critchley, L.; Coyle, E.F. Ellagitannin Consumption Improves Strength Recovery 2-3 d after Eccentric Exercise. Med. Sci. Sports Exerc. 2010, 42, 493–498. [Google Scholar] [CrossRef]
- Trombold, J.R.; Reinfeld, A.S.; Casler, J.R.; Coyle, E.F. The Effect of Pomegranate Juice Supplementation on Strength and Soreness after Eccentric Exercise. J. Strength Cond. Res. 2011, 25, 1782–1788. [Google Scholar] [CrossRef]
- Clifford, T.; Bell, O.; West, D.J.; Howatson, G.; Stevenson, E.J. The Effects of Beetroot Juice Supplementation on Indices of Muscle Damage Following Eccentric Exercise. Eur. J. Appl. Physiol. 2016, 116, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Clifford, T.; Allerton, D.M.; Brown, M.A.; Harper, L.; Horsburgh, S.; Keane, K.M.; Stevenson, E.J.; Howatson, G. Minimal Muscle Damage After a Marathon and no Influence of Beetroot Juice on Inflammation and Recovery. Appl. Phys. Nutr. Metab. 2016, 42, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Guigoz, Y. Dietary Proteins in Humans: Basic Aspects and Consumption in Switzerland. Int. J. Vitam. Nutr. Res. 2011, 81, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Philpott, J.D.; Witard, O.C.; Galloway, S.D.R. Applications of Omega-3 Polyunsaturated Fatty Acid Supplementation for Sport Performance. Res. Sports Med. 2019, 27, 219–237. [Google Scholar] [CrossRef]
- Robinson, M.J.; Burd, N.A.; Breen, L.; Rerecich, T.; Yang, Y.; Hector, A.J.; Baker, S.K.; Phillips, S.M. Dose-dependent responses of myofibrillar protein synthesis with beef ingestion are enhanced with resistance exercise in middle-aged men. Appl. Physiol. Nutr. Metab. 2013, 38, 120–125. [Google Scholar] [CrossRef]
- Wilkinson, S.B.; Tarnopolsky, M.A.; Macdonald, M.J.; Macdonald, J.R.; Armstrong, D.; Phillips, S.M. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am. J. Clin. Nutr. 2007, 85, 1031–1040. [Google Scholar] [CrossRef]
- Bolling, B.W.; Chen, C.-Y.O.; McKay, D.L.; Blumberg, J.B. Tree nut phytochemicals: Composition, antioxidant capacity, bioactivity, impact factors. A systematic review of almonds, Brazils, cashews, hazelnuts, macadamias, pecans, pine nuts, pistachios and walnuts. Nutr. Res. Rev. 2011, 24, 244–275. [Google Scholar] [CrossRef]
- Mielgo-Ayuso, J.; Zourdos, M.C.; Calleja-González, J.; Urdampilleta, A.; Ostojic, S.M. Dietary intake habits and controlled training on body composition and strength in elite female volleyball players during the season. Appl. Physiol. Nutr. Metab. 2015, 40, 827–834. [Google Scholar] [CrossRef]
- Stewart, A.; Marfell-Jones, M.; Olds, T.; de Ridder, H. International Standards for Anthropometric Assessment, 3rd ed.; ISAK: Lower Hutt, New Zealand, 2011. [Google Scholar]
- European Food Safety Authority (EFSA). Guidance on the EU Menu Methodology. EFSA J. 2014, 12, 3944. [Google Scholar]
- Farrán, A.; Zamora, R.; Cervera, P. Tablas De Composición De Alimentos Del Centre D’Ensenyament Superior De Nutrició i Dietètica (CESNID); Universitat de Barcelona: Barcelona, Spain, 2004; p. 247. [Google Scholar]
- González-Gross, M.; Gutiérrez, A.; Mesa, J.L.; Ruiz-Ruiz, J.; Castillo, M.J. Nutrition in the sport practice: Adaptation of the food guide pyramid to the characteristics of athletes diet. Arch. Latinoam. Nutr. 2001, 51, 321–331. [Google Scholar]
- Mettler, S.; Mannhart, C.; Colombani, P.C. Development and validation of a food pyramid for Swiss athletes. Int. J. Sport Nutr. Exerc. Metab. 2009, 19, 504–518. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.B.; Lange, G.; Ciccone, D.S.; Liu, W.-C.; Steffener, J.; Natelson, B.H. Functional imaging of pain in patients with primary fibromyalgia. J. Rheumatol. 2004, 31, 364–378. [Google Scholar] [PubMed]
- Ramos-Campo, D.J.; Ávila-Gandía, V.; Alacid, F.; Soto-Méndez, F.; Alcaraz, P.E.; López-Román, F.J.; Rubio-Arias, J. Ángel Muscle damage, physiological changes, and energy balance in ultra-endurance mountain-event athletes. Appl. Physiol. Nutr. Metab. 2016, 41, 872–878. [Google Scholar] [CrossRef]
- Twist, C.; Eston, R.G. The Effect of Exercise-Induced Muscle Damage on Perceived Exertion and Cycling Endurance Performance. Eur. J. Appl. Physiol. 2009, 105, 559–567. [Google Scholar] [CrossRef]
- Rawson, E.S.; Clarkson, P.M.; Tarnopolsky, M.A. Perspectives on Exertional Rhabdomyolysis. Sports Med. 2017, 47, 33–49. [Google Scholar] [CrossRef]
- Del Coso, J.; Salinero, J.J.; Lara, B.; Abián-Vicén, J.; Gallo-Salazar, C.; Areces, F. A comparison of the physiological demands imposed by competing in a half-marathon vs a marathon. J. Sports Med. Phys. Fit. 2017, 57, 1399–1406. [Google Scholar]
- Williamson, E. Nutritional implications for ultra-endurance walking and running events. Extrem. Physiol. Med. 2016, 5, 13. [Google Scholar] [CrossRef]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and Athletic Performance. J. Acad. Nutr. Diet. 2016, 116, 501–528. [Google Scholar] [CrossRef]
- Onywera, V.; Kiplamai, F.; Tuitoek, P.; Boit, M.; Pitsiladis, Y. Food and Macronutrient Intake of Elite Kenyan Distance Runners. Int. J. Sport Nutr. Exerc. Metab. 2004, 14, 709–719. [Google Scholar] [CrossRef]
- Beis, L.Y.; Willkomm, L.; Ross, R.; Bekele, Z.; Wolde, B.; Fudge, B.; Pitsiladis, Y.P. Food and macronutrient intake of elite Ethiopian distance runners. J. Int. Soc. Sports Nutr. 2011, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Areces, F.; Salinero, J.J.; Abian-Vicen, J.; González-Millán, C.; Ruiz-Vicente, D.; Lara, B.; Lledó, M.; Del Coso, J. The Use of Compression Stockings During a Marathon Competition to Reduce Exercise-Induced Muscle Damage: Are They Really Useful? J. Orthop. Sports Phys. Ther. 2015, 45, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Heckel, Z.; Atlasz, T.; Tekus, E.; Koszegi, T.; Laczko, J.; Vaczi, M. Monitoring Exercise-Induced Muscle Damage Indicators and Myoelectric Activity during Two Weeks of Knee Extensor Exercise Training in Young and Old Men. PLoS ONE 2019, 14, e0224866. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, P.; Wassmansdorf, R.; Salgueirosa, F.M.; Hernandez, S.G.; Nascimento, V.B.; Daros, L.B.; Wharton, L.; Osiecki, R. Time-course of changes in indirect markers of muscle damage responses following a 130-km cycling race. Rev. Braz. J. Cineantropom. Desempenho Hum. 2016, 18, 322–331. [Google Scholar] [CrossRef]
- McLennan, P.L.; Owen, A.J.; Slee, E.L.; Theiss, M.L. Myocardial function, ischaemia and n-3 polyunsaturated fatty acids: A membrane basis. J. Cardiovasc. Med. 2007, 8, S15–S18. [Google Scholar] [CrossRef]
- Baum, K.; Telford, R.D.; Cunningham, R.B. Marine oil dietary supplementation reduces delayed onset muscle soreness after a 30 km run. Open Access J. Sports Med. 2013, 4, 109–115. [Google Scholar] [CrossRef]
- Cicerale, S.; Lucas, L.; Keast, R.; Keast, R. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr. Opin. Biotechnol. 2012, 23, 129–135. [Google Scholar] [CrossRef]
- Malaguti, M.; Angeloni, C.; Hrelia, S. Polyphenols in Exercise Performance and Prevention of Exercise-Induced Muscle Damage. Oxid. Med. Cell. Longev. 2013, 2013, 825928. [Google Scholar] [CrossRef]
- Esfahani, A.; Wong, J.M.W.; Truan, J.; Villa, C.R.; Mirrahimi, A.; Srichaikul, K.; Kendall, C.W.C. Health effects of mixed fruit and vegetable concentrates: A systematic review of the clinical interventions. J. Am. Coll. Nutr. 2011, 30, 285–294. [Google Scholar] [CrossRef]
- Lonnie, M.; Hooker, E.; Brunstrom, J.M.; Corfe, B.M.; Green, M.A.; Watson, A.W.; Williams, E.A.; Stevenson, E.J.; Penson, S.; Johnstone, A.M. Protein for Life: Review of Optimal Protein Intake, Sustainable Dietary Sources and the Effect on Appetite in Ageing Adults. Nutrients 2018, 10, 360. [Google Scholar] [CrossRef]
- Ribas-Agustí, A.; Díaz, I.; Sárraga, C.; García-Regueiro, J.A.; Castellari, M. Nutritional properties of organic and conventional beef meat at retail. J. Sci. Food Agric. 2019, 99, 4218–4225. [Google Scholar] [CrossRef] [PubMed]
- Markworth, J.F.; D’Souza, R.F.; Aasen, K.M.M.; Mitchell, S.M.; Durainayagam, B.R.; Sinclair, A.J.; Peake, J.M.; Egner, I.M.; Raastad, T.; Cameron-Smith, D.; et al. Arachidonic Acid Supplementation Transiently Augments the Acute Inflammatory Response to Resistance Exercise in Trained Men. J. Appl. Physiol. (1985) 2018, 125, 271–286. [Google Scholar] [CrossRef] [PubMed]
- Prisk, V.; Huard, J. Muscle injuries and repair: The role of prostaglandins and inflammation. Histol. Histopathol. 2003, 18, 1243–1256. [Google Scholar]
- Del Coso, J.; Valero, M.; Salinero, J.J.; Lara, B.; Gallo-Salazar, C.; Areces, F. Optimum Polygenic Profile to Resist Exertional Rhabdomyolysis during a Marathon. PLoS ONE 2017, 12, e0172965. [Google Scholar] [CrossRef] [PubMed]
- Banfi, G.; Colombini, A.; Lombardi, G.; Lubkowska, A. Metabolic markers in sports medicine. Adv. Clin. Chem. 2012, 56, 1–54. [Google Scholar] [PubMed]
| Mean ± SD | Median | Range | CV (%) | |
|---|---|---|---|---|
| Age (years) | 44.94 ± 8.77 | 47.50 | 24.00–62.00 | 19.5 |
| Height (cm) | 173.4 ± 8.7 | 174.8 | 150.0–185.0 | 5.0 |
| Body mass (kg) | 67.95 ± 9.08 | 70.50 | 47.00–79.50 | 13.3 |
| BMI (kg/m2) | 22.52 ± 1.79 | 22.28 | 19.27–25.93 | 7.9 |
| Experience in marathon or in long endurance events (years) | 10.9 ± 7.6 | 6.50 | 5.0–30.0 | 69.7 |
| Marathon time (min) | 239.8 ± 32.9 | 231.0 | 206.0–336.0 | 13.7 |
| Mean ± SD | Median | Range | CV (%) | Recommended Servings for Athletes * | |
|---|---|---|---|---|---|
| Cereals and potatoes | 5.2 ± 1.3 | 5.1 | 2.6–8.0 | 25.0 | 6–11/day |
| Dairy products | 2.3 ± 1.2 | 2.0 | 0.0–5.0 | 54.5 | 3–4/day |
| Fruits | 2.7 ± 1.3 | 2.8 | 0.4–5.0 | 2–4/day | |
| Vegetables | 2.5 ± 2.2 | 1.9 | 0.4–10.0 | 88.0 | 3–5/day |
| Olive oil | 3.0 ± 0.9 | 3.5 | 0.00–3.50 | 30.0 | 2–4/day |
| Legumes | 0.4 ± 0.3 | 0.4 | 0.00–1.21 | 75.0 | 2–3/week or frequent (1/day) |
| Dried fruits | 1.1 ± 0.9 | 0.9 | 0.00–3.50 | 81.8 | 2–3/week or frequent (1/day) |
| Fish | 0.8 ± 0.4 | 0.9 | 0.2–1.5 | 50.0 | 2–3/day alternating between these food groups |
| Meat | 1.5 ± 1.1 | 1.2 | 0.5–5.0 | 80.0 | |
| Eggs | 0.5 ± 0.4 | 0.5 | 0.0–2.0 | 80.0 | |
| Butter and fatty meat | 0.1 ± 0.3 | 0.0 | 0.0–1.0 | 300.0 | A few times per month |
| Pastries and sweets | 1.2 ± 1.6 | 0.5 | 0.0–5.7 | 133.3 | A few times per month |
| Wine and beer | 0.4 ± 0.5 | 0.2 | 0.0–2.0 | 125.0 | A few times per month |
| Mean ± SD | Median | Range | CV (%) | |
|---|---|---|---|---|
| Energy intake | ||||
| kcal/day | 3005.7 ± 362.5 | 2881.9 | 2471.9–3807.6 | 12.1 |
| kcal/kg/day | 44.8 ± 6.2 | 45.1 | 35.1–60.2 | 13.9 |
| Carbohydrate intake | ||||
| g/day | 338.3 ± 55.1 | 333.6 | 263.5–452.5 | 16.3 |
| g/kg/day | 5.04 ± 0.89 | 4.74 | 4.07–7.09 | 17.7 |
| % TEI | 45.0 ± 4.9 | 45.4 | 34.2–55.6 | 10.9 |
| Protein intake | ||||
| g/day | 130.5 ± 24.2 | 126.9 | 90.0–172.0 | 18.6 |
| g/kg/day | 1.94 ± 0.36 | 1.95 | 1.38–2.52 | 18.6 |
| % TEI | 17.4 ± 2.6 | 17.5 | 11.9–21.8 | 15.1 |
| Fat intake | ||||
| g/day | 121.5 ± 19.8 | 117.0 | 70.0–154.5 | 16.3 |
| g/kg/day | 1.81 ± 0.35 | 1.77 | 1.34–2.73 | 19.3 |
| % TEI | 36.3 ± 3.9 | 37.4 | 25.5–41.8 | 10.8 |
| Mean ± SD | Median | Range | CV (%) | |
|---|---|---|---|---|
| Fluids (L) | 1.8 ± 0.7 | 1.6 | 0.78–3.5 | 38.8 |
| Energy (kcal) | 597.1 ± 394.9 | 519.2 | 166.0–1600.1 | 151.2 |
| Carbohydrates (g) | 141.8 ± 89.6 | 123.2 | 39.5–348.4 | 67.9 |
| Carbohydrates (g/hour) | 46.9 ± 30.6 | 39.0 | 12.4–98.8 | 65.2 |
| Protein (g) | 2.6 ± 3.1 | 1.7 | 0.3–13.3 | 118.7 |
| Lipid (g) | 1.3 ± 2.5 | 0.6 | 0.2–10.7 | 186.5 |
| Sodium (mg) | 284.0 ± 228.8 | 267.3 | 0.4–705.3 | 80.3 |
| Mean ± SD | Median | Range | CV (%) | |
|---|---|---|---|---|
| Exercise-Induced Muscle Damage (EIMD) | ||||
| CK (U/L) | 453.4 ± 268.9 | 352.5 | 168.0–1266.0 | 59.3 |
| MYO (ng/mL) | 868.8 ± 622.6 | 583.7 | 299.8–2489.0 | 71.6 |
| Exercise-Induced Cardiac Stress (EICS) | ||||
| CK-MB (U/L) | 17.45 ± 14.04 | 14.50 | 4.00–72.00 | 80.2 |
| NT-proBNP (pg/mL) | 121.8 ± 103.2 | 81.6 | 33.4–447.0 | 84.7 |
| TNI (ng/dL) | 0.05 ± 0.04 | 0.03 | 0.01–0.17 | 80.0 |
| TNT (ng/dL) | 0.03 ± 0.02 | 0.02 | 0.01–0.09 | 66.6 |
| Model | Unstandardized Coefficients | Standardized Coefficients | t | p | 95% Confidence Interval for β | |||
|---|---|---|---|---|---|---|---|---|
| R2 Adjust | B | Standard Error | β | Low Limit | High Limit | |||
| CK | ||||||||
| (Constant) | 0.813 | 244.214 | 109.047 | 2.240 | 0.043 | 8.633 | 479.795 | |
| Meat | 168.156 | 31.553 | 0.643 | 5.329 | 0.000 | 99.991 | 236.322 | |
| Vegetables | −59.533 | 14.922 | −0.483 | −3.990 | 0.002 | −27.296 | −91.771 | |
| Fish | −207.809 | 92.100 | −0.272 | −2.256 | 0.042 | −406.780 | −8.838 | |
| MYO | ||||||||
| (Constant) | 0.453 | 302.879 | 204.090 | 1.484 | 0.009 | −132.128 | 737.886 | |
| Meat | 425.155 | 112.700 | 0.698 | 3.772 | 0.002 | 184.942 | 665.369 | |
| NT-proBNP | ||||||||
| (Constant) | 0.634 | 107.817 | 17.118 | 6.298 | 0.000 | 71.330 | 144.303 | |
| Butter and fatty meat | 351.993 | 69.017 | 0.796 | 5.100 | 0.000 | 204.887 | 499.098 | |
| TNI | ||||||||
| (Constant) | 0.827 | 0.147 | 0.010 | 14.826 | 0.000 | 0.125 | 0.168 | |
| Fish | −0.064 | 0.007 | −0.593 | −9.006 | 0.000 | −0.079 | −0.048 | |
| Olive Oil | −0.021 | 0.003 | −0.536 | −8.361 | 0.000 | −0.027 | −0.016 | |
| Butter and fatty meat | 0.064 | 0.010 | 0.396 | 6.698 | 0.000 | 0.043 | 0.085 | |
| TNT | ||||||||
| (Constant) | 0.697 | 0.100 | 0.013 | 7.571 | 0.000 | 0.072 | 0.129 | |
| Olive Oil | −0.009 | 0.004 | −0.415 | −2.624 | 0.021 | −0.017 | −0.002 | |
| Fish | −0.038 | 0.010 | −0.640 | −3.759 | 0.002 | −0.061 | −0.016 | |
| Pastries and sweets | −0.008 | 0.003 | −0.488 | −2.839 | 0.014 | −0.013 | −0.002 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mielgo-Ayuso, J.; Calleja-González, J.; Refoyo, I.; León-Guereño, P.; Cordova, A.; Del Coso, J. Exercise-Induced Muscle Damage and Cardiac Stress During a Marathon Could be Associated with Dietary Intake During the Week Before the Race. Nutrients 2020, 12, 316. https://doi.org/10.3390/nu12020316
Mielgo-Ayuso J, Calleja-González J, Refoyo I, León-Guereño P, Cordova A, Del Coso J. Exercise-Induced Muscle Damage and Cardiac Stress During a Marathon Could be Associated with Dietary Intake During the Week Before the Race. Nutrients. 2020; 12(2):316. https://doi.org/10.3390/nu12020316
Chicago/Turabian StyleMielgo-Ayuso, Juan, Julio Calleja-González, Ignacio Refoyo, Patxi León-Guereño, Alfredo Cordova, and Juan Del Coso. 2020. "Exercise-Induced Muscle Damage and Cardiac Stress During a Marathon Could be Associated with Dietary Intake During the Week Before the Race" Nutrients 12, no. 2: 316. https://doi.org/10.3390/nu12020316
APA StyleMielgo-Ayuso, J., Calleja-González, J., Refoyo, I., León-Guereño, P., Cordova, A., & Del Coso, J. (2020). Exercise-Induced Muscle Damage and Cardiac Stress During a Marathon Could be Associated with Dietary Intake During the Week Before the Race. Nutrients, 12(2), 316. https://doi.org/10.3390/nu12020316










