Potential Risk of Higenamine Misuse in Sports: Evaluation of Lotus Plumule Extract Products and a Human Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Extraction Procedure
2.2.1. MAE Process
2.2.2. DOE Study for MAE
2.2.3. Comparison with Other Extraction Methods
2.3. Quantitative Determination of Lotus Plumule Products
2.3.1. Chromatographic Conditions and Validation
2.3.2. Preparation of Lotus Plumule Products
2.4. Human Study
2.4.1. Enrollment Criteria and Dosage Regimen
2.4.2. Urine Sample Preparation and Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Statistical Analysis and Model Fitting for Lotus Plumule Extraction Using MAE
| YHigenamine = 782.51 + 91.38X1 + 14.86X2 + 295.64X3 – 152.04X12 − 142.16X22 − 135.41X32 |
| YLiensinine = 650.95 + 117.63X1 + 25.34X2 + 315.79X3 − 174.69X12 − 139.55 X22 |
| YDauricine = 1893.87 + 897.97X3 |
| YIsoliensinine = 60.41 + 18.91X1 + 39.73X3 |
| YNeferine = 2214.44 + 273.85X1 + 75.08X2 + 749.94X3 − 477.12X12 – 405.71X22 – 408.73X32 |
3.2. Verification of Optimized MAE Condition
3.3. Comparison of MAE with Other Extraction Methods
3.4. Quantitative Determination of Lotus Plumule Products
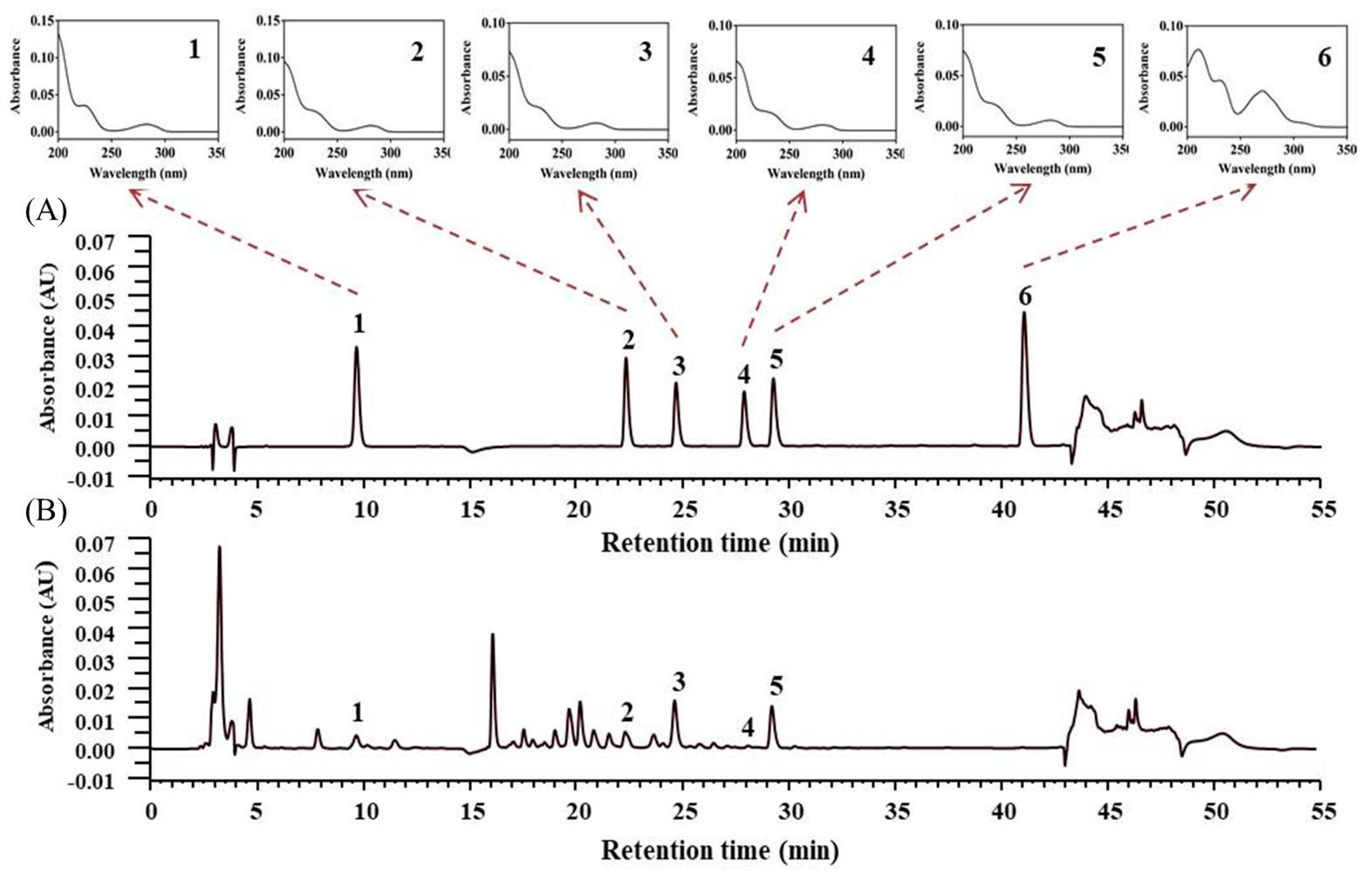
3.4.1. HPLC Validation
3.4.2. Quantitative Determination of Lotus Plumule Products
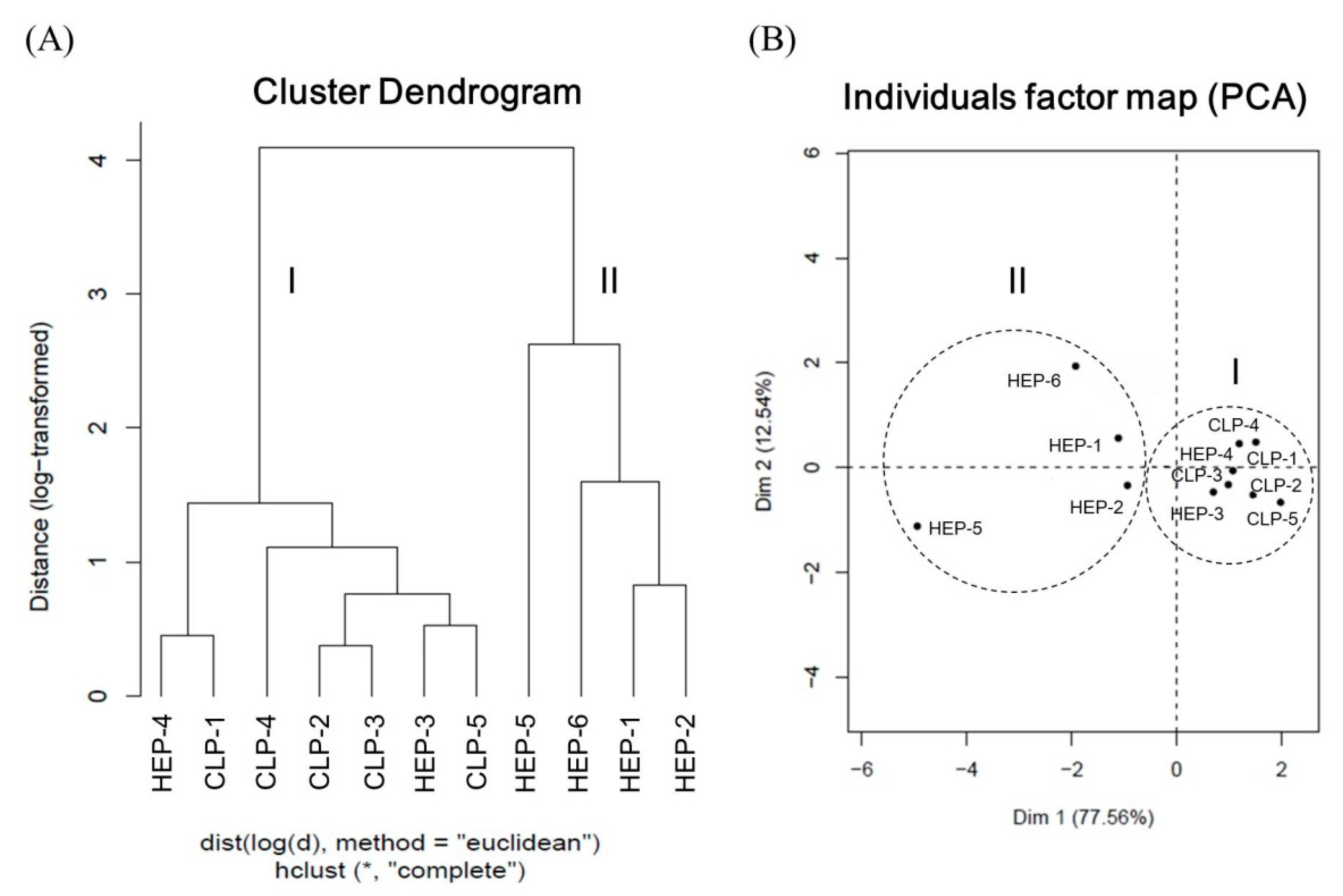
3.4.3. Quality Evaluation of Lotus Plumule Products by HCA and PCA
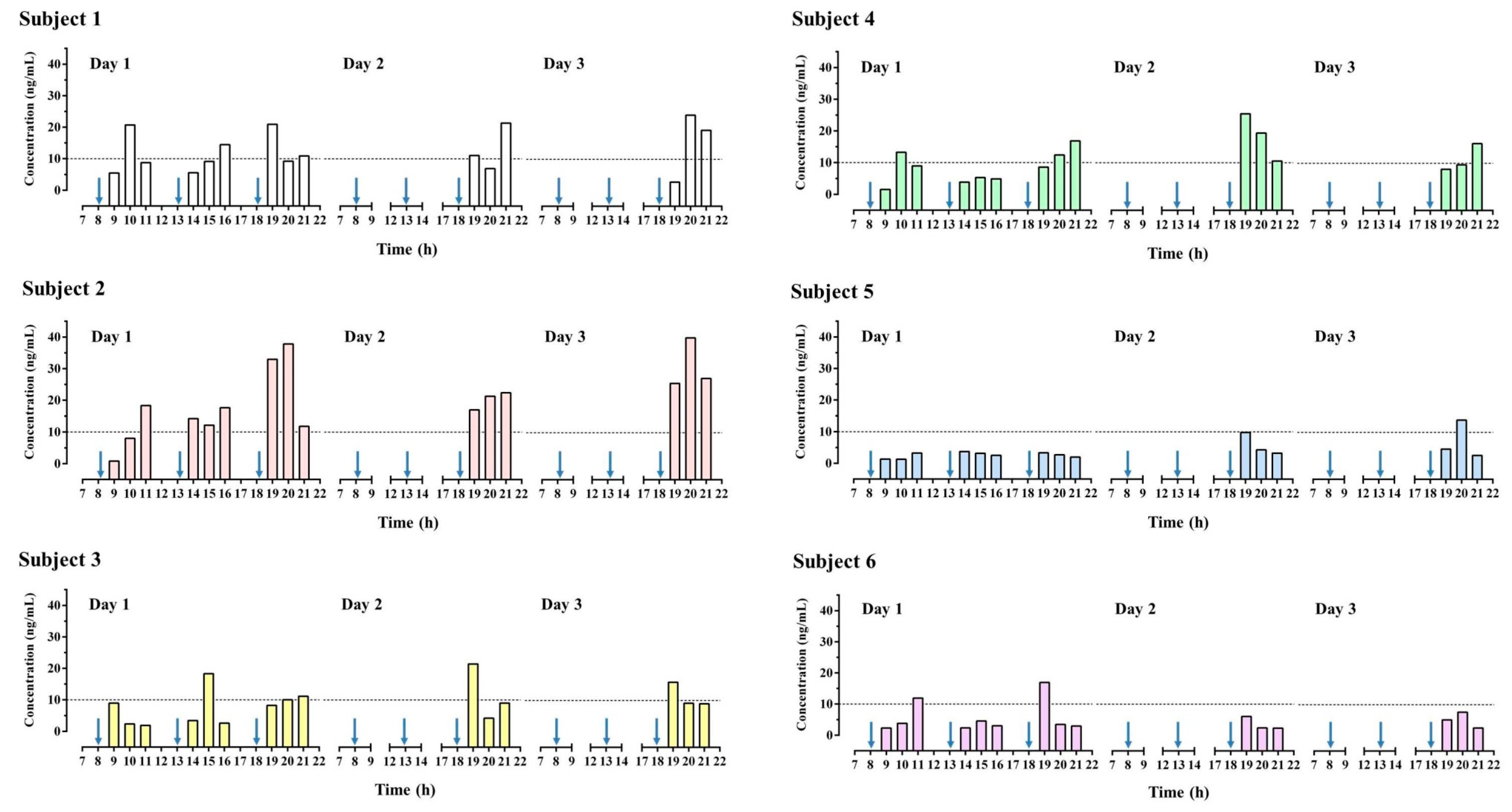
3.5. Human Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- WADA. Prohibited List. Available online: https://www.wada-ama.org/sites/default/files/wada_2019_english_prohibited_list.pdf (accessed on 10 January 2019).
- WADA. World Anti-Doping Code 2015 with 2019 Amendments. Available online: https://www.wada-ama.org/sites/default/files/resources/files/wada_anti-doping_code_2019_english_final_revised_v1_linked.pdf (accessed on 10 July 2019).
- Bailey, R.L.; Gahche, J.J.; Lentino, C.V.; Dwyer, J.T.; Engel, J.S.; Thomas, P.R.; Betz, J.M.; Sempos, C.T.; Picciano, M.F. Dietary supplement use in the United States, 2003–2006. J. Nutr. 2010, 141, 261–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fennell, D. Determinants of supplement usage. Prev. Med. 2004, 39, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Ros, J.J.W.; Pelders, M.G.; De Smet, P.A.G.M. A case of positive doping associated with a botanical food supplement. Pharm. World Sci. 1999, 21, 44–46. [Google Scholar] [CrossRef] [PubMed]
- Avois, L.; Robinson, N.; Saudan, C.; Baume, N.; Mangin, P.; Saugy, M. Central nervous system stimulants and sport practice. Br. J. Sports Med. 2006, 40, i16–i20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selavka, C.M. Poppy seed ingestion as a contributing factor to opiate-positive urinalysis results: The Pacific perspective. J. Forensic Sci. 1991, 36, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Hermanns-Clausen, M.; Weinmann, W. Anorectic sibutramine detected in a Chinese herbal drug for weight loss. Forensic Sci. Int. 2006, 161, 221–222. [Google Scholar] [CrossRef]
- Duke, J.A. Handbook of Medicinal Herbs; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Chen, Y.; Fan, G.; Wu, H.; Wu, Y.; Mitchell, A. Separation, identification and rapid determination of liensine, isoliensinine and neferine from embryo of the seed of Nelumbo nucifera Gaertn. by liquid chromatography coupled to diode array detector and tandem mass spectrometry. J. Pharm. Biomed. Anal. 2007, 43, 99–104. [Google Scholar] [CrossRef]
- Itoh, A.; Saitoh, T.; Tani, K.; Uchigaki, M.; Sugimoto, Y.; Yamada, J.; Nakajima, H.; Ohshiro, H.; Sun, S.; Tanahashi, T. Bisbenzylisoquinoline alkaloids from Nelumbo nucifera. Chem. Pharm. Bull. 2011, 59, 947–951. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.H.; Zhang, Y.L.; Feng, X.L.; Wang, J.L.; Qian, J.Q. Effects of isoliensinine on angiotensin II-induced proliferation of porcine coronary arterial smooth muscle cells. J. Asian Nat. Prod. Res. 2006, 8, 209–216. [Google Scholar] [CrossRef]
- Chen, J.; Ma, X.; Gao, K.; Wang, Y.; Zhao, H.; Wu, H.; Wang, J.; Xie, H.; OuYang, Y.; Luo, L. The active ingredients of Jiang-Zhi-Ning: Study of the Nelumbo nucifera alkaloids and their main bioactive metabolites. Molecules 2012, 17, 9855–9867. [Google Scholar] [CrossRef] [Green Version]
- Cohen, P.A.; Travis, J.C.; Keizers, P.H.J.; Boyer, F.E.; Venhuis, B.J. The stimulant higenamine in weight loss and sports supplements. Clin. Toxicol. 2019, 57, 125–130. [Google Scholar] [CrossRef]
- WADA. Technical Document–TD2019MRPL. Minimum Required Performance Levels for Detection and Identification of Non-Threshold Substances. Available online: https://www.wada-ama.org/sites/default/files/resources/files/td2019mrpl_eng.pdf (accessed on 24 May 2019).
- Okano, M.; Sato, M.; Kageyama, S. Determination of higenamine and coclaurine levels in human urine after the administration of a throat lozenge containing Nandina domestica fruit. Drug Test. Anal. 2017, 9, 1788–1793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.W.; Tung, C.W.; Tsai, C.C.; Wu, Y.T.; Hsu, M.C. Determination of cannabinoids in hemp nut products in Taiwan by HPLC-MS/MS coupled with chemometric analysis: Quality evaluation and a pilot human study. Drug Test. Anal. 2017, 9, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Beebe, K.R.; Pell, R.J.; Seasholtz, M.B. Chemometrics: A Practical Guide; John Wiley Publishing: New York, NY, USA, 1998; Volume 348. [Google Scholar]
- Kumar, N.; Bansal, A.; Sarma, G.S.; Rawal, R.K. Chemometrics tools used in analytical chemistry: An overview. Talanta 2014, 123, 186–199. [Google Scholar] [CrossRef]
- Camacho, J.; Picó, J.; Ferrer, A. Data understanding with PCA: Structural and variance information plots. Chemometr. Intell. Lab. Syst. 2010, 100, 48–56. [Google Scholar] [CrossRef]
- Bhuyan, D.J.; Van Vuong, Q.; Chalmers, A.C.; van Altena, I.A.; Bowyer, M.C.; Scarlett, C.J. Microwave-assisted extraction of Eucalyptus robusta leaf for the optimal yield of total phenolic compounds. Ind. Crop. Prod. 2015, 69, 290–299. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, Q.; Wang, Y.; Wang, T.; Li, X.; Ding, L.; Jiang, G. Evaluation of Soxhlet extraction, accelerated solvent extraction and microwave-assisted extraction for the determination of polychlorinated biphenyls and polybrominated diphenyl ethers in soil and fish samples. Anal. Chim. Acta 2010, 663, 43–48. [Google Scholar] [CrossRef]
- De Castro, M.D.L.; Priego-Capote, F. Soxhlet extraction: Past and present panacea. J. Chromatogr. A 2010, 1217, 2383–2389. [Google Scholar] [CrossRef]
- Zhou, H.-Y.; Liu, C.-Z. Microwave-assisted extraction of solanesol from tobacco leaves. J. Chromatogr. A 2006, 1129, 135–139. [Google Scholar] [CrossRef]
- Wang, J.; Sun, B.; Cao, Y.; Tian, Y.; Li, X. Optimisation of ultrasound-assisted extraction of phenolic compounds from wheat bran. Food Chem. 2008, 106, 804–810. [Google Scholar] [CrossRef]
- Luque-Garcia, J.L.; de Castro, L.M.D. Ultrasound: A powerful tool for leaching. Trends Anal. Chem. 2003, 22, 41–47. [Google Scholar] [CrossRef]
- Teng, H.; Choi, Y.H. Optimization of microwave-assisted extraction of bioactive alkaloid compounds from Rhizoma Coptidis (Coptis chinensis Franch.). Food Sci. Biotechnol. 2013, 22, 1–8. [Google Scholar] [CrossRef]
- Xie, D.-T.; Wang, Y.-Q.; Kang, Y.; Hu, Q.-F.; Su, N.-Y.; Huang, J.-M.; Che, C.-T.; Guo, J.-X. Microwave-assisted extraction of bioactive alkaloids from Stephania sinica. Sep. Purif. Technol. 2014, 130, 173–181. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Zhao, B.; Wang, X.; Wu, Y.; Yao, J. A comparison study on microwave-assisted extraction of Potentilla anserina L. polysaccharides with conventional method: Molecule weight and antioxidant activities evaluation. Carbohydr. Polym. 2010, 80, 84–93. [Google Scholar] [CrossRef]
- Lo, C.F.; Chen, C.M. Pharmacokinetics of higenamine in rabbits. Biopharm. Drug Dispos. 1996, 17, 791–803. [Google Scholar] [CrossRef]
- Zheng, Y.L.; Zhang, Z.; Chen, B.L.; Wand, K.; Xue, L.M.; Fang, L. Determination of Higenamine in plasma by HPLC-ECD and study on its pharmacokinetics. Chin. Pharm. J. 2004, 39, 848–850. [Google Scholar]
- Feng, S.; Jiang, J.; Hu, P.; Zhang, J.-Y.; Liu, T.; Zhao, Q.; Li, B.-L. A phase I study on pharmacokinetics and pharmacodynamics of higenamine in healthy Chinese subjects. Acta Pharmacol. Sin. 2012, 33, 1353–1358. [Google Scholar] [CrossRef] [Green Version]
- Liang, Z.; Xu, C.; Dong, L.; Fu, Y.; Wu, Q.; Zhao, J.; Ye, L.; Cai, Z.; Liu, M.; Xia, B. Involvement of UDP-glucuronosyltransferases in higenamine glucuronidation and the gender and species differences in liver. Biomed. Pharmacother. 2017, 93, 172–179. [Google Scholar] [CrossRef]
- Girard, H.; Bernard, O.; Fortier, L.-C.; Villeneuve, L.; Hao, Q.; Greenblatt, D.J.; von Moltke, L.L.; Perussed, L.; Guillemette, C. Identification of common polymorphisms in the promoter of the UGT1A9 gene: Evidence that UGT1A9 protein and activity levels are strongly genetically controlled in the liver. Pharmacogenet. Genom. 2004, 14, 501–515. [Google Scholar] [CrossRef]
- Hagenauer, B.; Salamon, A.; Thalhammer, T.; Kunert, O.; Haslinger, E.; Klingler, P.; Senderowicz, A.M.; Sausville, E.A.; Jäger, W. In vitro glucuronidation of the cyclin-dependent kinase inhibitor flavopiridol by rat and human liver microsomes: Involvement of UDP-glucuronosyltransferases 1A1 and 1A9. Drug Metab. Dispos. 2001, 29, 407–414. [Google Scholar]
- Mukherjee, P.K.; Mukherjee, D.; Maji, A.K.; Rai, S.; Heinrich, M. The sacred lotus (Nelumbo nucifera)–phytochemical and therapeutic profile. J. Pharm. Pharmacol. 2009, 61, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Smith, T.; Svetlana, P.; Yang, J.; Jin, J.-H.; Li, C.-S. Paleobiogeography of the lotus plant (Nelumbonaceae: Nelumbo) and its bearing on the paleoclimatic changes. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 399, 284–293. [Google Scholar] [CrossRef]
- Yan, K.; Wang, X.; Wang, Z.; Wang, Y.; Luan, Z.; Gao, X.; Wang, R. The risk of higenamine adverse analytical findings following oral administration of plumula nelumbinis capsules. Drug Test. Anal. 2019. [Google Scholar] [CrossRef] [PubMed]

| Code | Extraction Time (min) | Microwave Power (W) | Temperature (°C) |
|---|---|---|---|
| −1 | 5 | 500 | 60 |
| 0 | 17.5 | 1000 | 90 |
| 1 | 30 | 1500 | 120 |
| Run | Factors | Extraction Yield (μg/g) | ||||||
|---|---|---|---|---|---|---|---|---|
| X1 (min) | X2 (W) | X3 (°C) | Higenamine | Liensinine | Dauricine | Isoliensinine | Neferine | |
| 1 | 5 | 500 | 90 | 405.1 | 255.2 | 1372.8 | 15.9 | 1008.9 |
| 2 | 17.5 | 1500 | 120 | 874.7 | 856.5 | 3015.7 | 131.3 | 2424.6 |
| 3 | 17.5 | 1000 | 90 | 750.5 | 744.6 | 2450.6 | 56.7 | 2202.1 |
| 4 | 17.5 | 1000 | 90 | 762.3 | 554.9 | 2372.6 | 92.5 | 1991.5 |
| 5 | 30 | 1000 | 120 | 912.6 | 835.4 | 2413.5 | 106.7 | 2315.6 |
| 6 | 17.5 | 1000 | 90 | 935.4 | 761.9 | 3050.6 | 42.5 | 2776.5 |
| 7 | 17.5 | 500 | 120 | 677.9 | 667.0 | 2285.3 | 88.4 | 1808.9 |
| 8 | 5 | 1000 | 60 | 228.3 | 139.2 | 767.6 | 21.5 | 644.5 |
| 9 | 17.5 | 1500 | 60 | 205.4 | 136.7 | 771.3 | 20.3 | 632.4 |
| 10 | 30 | 500 | 90 | 582.3 | 536.6 | 2170.5 | 71.6 | 1611.1 |
| 11 | 5 | 1000 | 120 | 717.4 | 630.2 | 2395.1 | 88.7 | 1895.8 |
| 12 | 30 | 1000 | 60 | 122.0 | 50.8 | 529.0 | 33.6 | 458.4 |
| 13 | 30 | 1500 | 90 | 715.3 | 673.6 | 2549.0 | 84.7 | 2030.7 |
| 14 | 17.5 | 1000 | 90 | 707.8 | 684.9 | 2185.5 | 59.6 | 2169.2 |
| 15 | 5 | 1500 | 90 | 250.5 | 130.8 | 836.9 | 19.4 | 675.8 |
| 16 | 17.5 | 500 | 60 | 261.8 | 136.1 | 858.0 | 21.9 | 734.1 |
| 17 | 17.5 | 1000 | 90 | 756.5 | 757.7 | 2171.8 | 71.4 | 1933.0 |
| Factor | Optimal Conditions | ||
| Extraction time (min) | 26 | ||
| Microwave power (W) | 1046 | ||
| Temperature (°C) | 120 | ||
| Responses | Predicted Value (μg/g) | Experimental Value (μg/g, n = 3) | Residual (%) |
| Higenamine | 935.4 | 946.3 ± 86.5 | 1.2 |
| Liensinine | 967.8 | 988.6 ± 35.2 | 2.2 |
| Dauricine | 2791.8 | 2990.0 ± 148.8 | 7.1 |
| Isoliensinine | 112.9 | 115.8 ± 16.1 | 2.5 |
| Neferine | 2523.8 | 2601.5 ± 80.0 | 3.1 |
| Extraction Method | Extraction Time (min) | Extraction Yield (μg/g) | ||||
|---|---|---|---|---|---|---|
| Higenamine | Liensinine | Dauricine | Isoliensinine | Neferine | ||
| SE | 480 | 659.5 ± 40.9 a | 487.1 ± 122.1 a | 1806.0 ± 327.3 a | 85.7 ± 37.5 | 1699.0 ± 165.7 a |
| HRE | 240 | 831.5 ± 62.1 b | 590.8 ± 58.0 a | 2214.4 ± 204.0 a,b | 66.5 ± 3.6 | 2226 ± 286.0 b,c |
| UAE | 30 | 671.0 ± 74.6 a | 592.3 ± 39.1 a | 2299.0 ± 147.1 a,b | 80.8 ± 24.1 | 1962.9 ± 72.1 a,b |
| MAE | 26 | 946.3 ± 86.5 b | 988.6 ± 35.2 b | 2990.0 ± 148.8 b | 115.8 ± 16.1 | 2601.5 ± 80.0 c |
| Analytes | Linear Range (μg/mL) | R2 | LOQ (μg/mL) | LOD (μg/mL) |
|---|---|---|---|---|
| Higenamine | 0.05–25.0 | 0.9998 | 0.05 | 0.017 |
| Liensinine | 0.25–25.0 | 0.9999 | 0.25 | 0.083 |
| Dauricine | 0.25–25.0 | 0.9999 | 0.25 | 0.083 |
| Isoliensinine | 0.1–25.0 | 0.9999 | 0.1 | 0.033 |
| Neferine | 0.25–25.0 | 0.9999 | 0.25 | 0.083 |
| Nuciferine | 0.1–25.0 | 0.9997 | 0.1 | 0.033 |
| Product | Component (μg/g) | |||||
|---|---|---|---|---|---|---|
| Higenamine | Liensinine | Dauricine | Isoliensinine | Neferine | Total | |
| HEP-1 | 434.0 ± 30.4 | 517.3 ± 45.5 | 2005.3 ± 95.4 | 31.1 ± 3.1 | 1928.1 ± 80.6 | 4916.7 ± 84.2 |
| HEP-2 | 488.0 ± 26.0 | 453.3 ± 52.6 | 1275.8 ± 93.4 | 37.0 ± 1.7 | 3679.2 ± 76.1 | 5933.2 ± 203.9 |
| HEP-3 | 872.2 ± 19.6 | 649.0 ± 24.4 | 2315.3 ± 178.1 | 99.0 ± 6.3 | 5249.1 ± 115.7 | 9184.6 ± 82.8 |
| HEP-4 | 809.1 ± 48.5 | 820.5 ± 96.5 | 3012.9 ± 82.1 | 88.9 ± 5.7 | 2409.9 ± 119.3 | 7141.4 ± 183.2 |
| HEP-5 | 263.9 ± 23.7 | 114.7 ± 19.6 | 422.3 ± 28.3 | 14.5 ± 2.9 | 1029.5 ± 45.0 | 1844.0 ± 27.3 |
| HEP-6 | 343.1 ± 12.7 | 1210.6 ± 59.6 | 1211.2 ± 58.9 | 20.5 ± 1.8 | 1276.2 ± 24.4 | 4061.6 ± 145.0 |
| CLP-1 | 844.1 ± 49.9 | 726.0 ± 27.2 | 2102.7 ± 48.4 | 103.9 ± 4.5 | 2896.5 ± 159.2 | 6673.1 ± 180.1 |
| CLP-2 | 643.2 ± 24.8 | 583.2 ± 40.1 | 2115.7 ± 134.2 | 59.1 ± 17.1 | 6567.3 ± 409.6 | 9968.6 ± 541.3 |
| CLP-3 | 669.2 ± 53.3 | 740.0 ± 25.6 | 1914.2 ± 113.9 | 77.3 ± 1.7 | 6496.3 ± 330.1 | 9896.9 ± 463.8 |
| CLP-4 | 721.9 ± 29.9 | 1635.0 ± 25.4 | 1686.6 ± 54.9 | 79.2 ± 5.1 | 7345.2 ± 300.6 | 11466 ± 355.2 |
| CLP-5 | 969.5 ± 43.3 | 761.7 ± 36.9 | 2355.9 ± 190.3 | 98.0 ± 2.6 | 8554.3 ± 202.2 | 12739.4 ± 433.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, C.-C.; Tung, C.-W.; Chang, C.-W.; Tsai, C.-C.; Hsu, M.-C.; Wu, Y.-T. Potential Risk of Higenamine Misuse in Sports: Evaluation of Lotus Plumule Extract Products and a Human Study. Nutrients 2020, 12, 285. https://doi.org/10.3390/nu12020285
Yen C-C, Tung C-W, Chang C-W, Tsai C-C, Hsu M-C, Wu Y-T. Potential Risk of Higenamine Misuse in Sports: Evaluation of Lotus Plumule Extract Products and a Human Study. Nutrients. 2020; 12(2):285. https://doi.org/10.3390/nu12020285
Chicago/Turabian StyleYen, Ching-Chi, Chun-Wei Tung, Chih-Wei Chang, Chin-Chuan Tsai, Mei-Chich Hsu, and Yu-Tse Wu. 2020. "Potential Risk of Higenamine Misuse in Sports: Evaluation of Lotus Plumule Extract Products and a Human Study" Nutrients 12, no. 2: 285. https://doi.org/10.3390/nu12020285
APA StyleYen, C.-C., Tung, C.-W., Chang, C.-W., Tsai, C.-C., Hsu, M.-C., & Wu, Y.-T. (2020). Potential Risk of Higenamine Misuse in Sports: Evaluation of Lotus Plumule Extract Products and a Human Study. Nutrients, 12(2), 285. https://doi.org/10.3390/nu12020285






