Impact of Early Nutrition, Physical Activity and Sleep on the Fetal Programming of Disease in the Pregnancy: A Narrative Review
Abstract
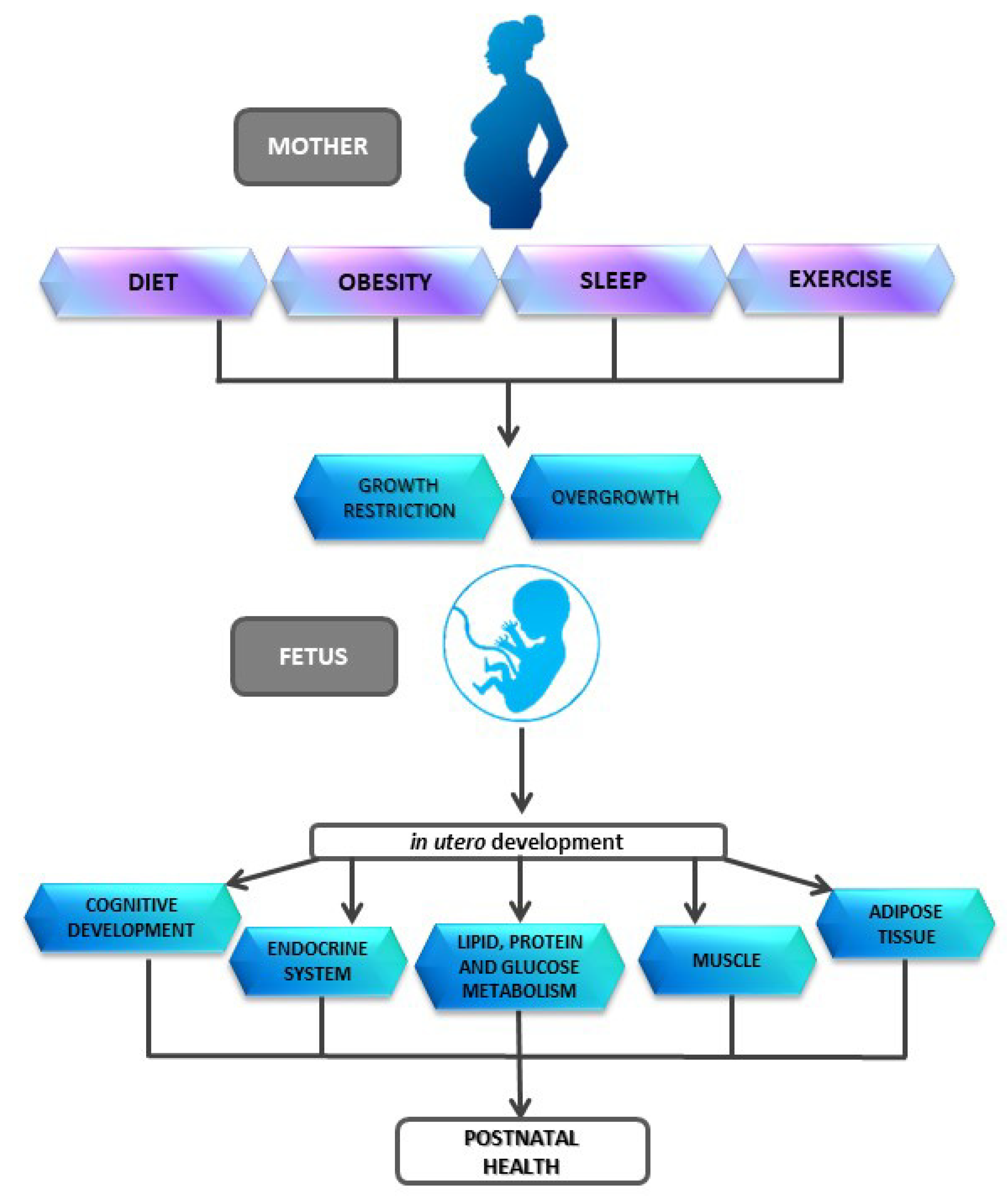
:1. Introduction
2. Materials and Methods
3. Maternal Diet and the Influence in Offspring Health
3.1. Influence of Maternal Diet on Cognitive Development and Behavior
3.2. Role of Malnutrition in Development and Lifelong Consequences
3.3. Metabolic Syndrome and Nutritional Programming
3.4. Excessive Calories Consumption in the Maternal Diet and Influence on the Development
4. Exercise and Metabolic Programming
5. Sleep and Metabolic Programming
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barker, D.J.; Gluckman, P.D.; Godfrey, K.M.; Harding, J.E.; Owens, J.A.; Robinson, J.S. Fetal nutrition and cardiovascular disease in adult life. Lancet 1993, 341, 938–941. [Google Scholar] [CrossRef]
- Thompson, J.A.; Regnault, T.R. In utero origins of adult insulin resistance and vascular dysfunction. Semin. Reprod. Med. 2011, 29, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Johnston, F.E. Mothers, babies, and disease in later life. By D. J. P. Barker. London: British Medical Journal Group, 1994. 34.95£ (cloth). Am. J. Hum. Biol. 1995, 7, 673–674. [Google Scholar] [CrossRef]
- Palmer, D.J.; Huang, R.C.; Craig, J.M.; Prescott, S.L. Nutritional influences on epigenetic programming: Asthma, allergy, and obesity. Immunol. Allergy Clin. N. Am. 2014, 34, 825–837. [Google Scholar] [CrossRef]
- Lee, H.S. Impact of Maternal Diet on the Epigenome during In Utero Life and the Developmental Programming of Diseases in Childhood and Adulthood. Nutrients 2015, 7, 9492–9507. [Google Scholar] [CrossRef] [Green Version]
- Simeoni, U.; Yzydorczyk, C.; Siddeek, B.; Benahmed, M. Epigenetics and neonatal nutrition. Early Hum. Dev. 2014, 90 (Suppl. 2), S23–S24. [Google Scholar] [CrossRef]
- Toca Mdel, C.; Tonietti, M.; Vecchiarelli, C. [Prenatal and postnatal nutrition: Long term impact on health]. Arch. Argent. Pediatr. 2015, 113, 248–253. [Google Scholar]
- Chango, A.; Pogribny, I.P. Considering maternal dietary modulators for epigenetic regulation and programming of the fetal epigenome. Nutrients 2015, 7, 2748–2770. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Xiao, X.; Zhang, Q.; Yu, M. DNA methylation: The pivotal interaction between early-life nutrition and glucose metabolism in later life. Br. J. Nutr. 2014, 112, 1850–1857. [Google Scholar] [CrossRef] [Green Version]
- Tarry-Adkins, J.L.; Ozanne, S.E. Mechanisms of early life programming: Current knowledge and future directions. Am. J. Clin. Nutr. 2011, 94, 1765s–1771s. [Google Scholar] [CrossRef] [Green Version]
- Lillycrop, K.A.; Burdge, G.C. Epigenetic mechanisms linking early nutrition to long term health. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Walker, S.O.; Hong, X.; Bartell, T.R.; Wang, X. Epigenetics and early life origins of chronic noncommunicable diseases. J. Adolesc. Health 2013, 52, S14–S21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hales, C.N.; Barker, D.J.P. The thrifty phenotype hypothesis: Type 2 diabetes. Br. Med. Bull. 2001, 60, 5–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fall, C.H.D. Fetal programming and the risk of noncommunicable disease. Indian J. Pediatrics 2013, 80 (Suppl. 1), S13–S20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vickers, M.H. Early life nutrition, epigenetics and programming of later life disease. Nutrients 2014, 6, 2165–2178. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.H. Fetal programming, epigenetics, and adult onset disease. Clin. Perinatol. 2014, 41, 815–831. [Google Scholar] [CrossRef]
- Wei, Y.; Schatten, H.; Sun, Q.Y. Environmental epigenetic inheritance through gametes and implications for human reproduction. Hum. Reprod. Update 2015, 21, 194–208. [Google Scholar] [CrossRef] [Green Version]
- Bellinger, L.; Sculley, D.V.; Langley-Evans, S.C. Exposure to undernutrition in fetal life determines fat distribution, locomotor activity and food intake in ageing rats. Int. J. Obes. 2006, 30, 729–738. [Google Scholar] [CrossRef] [Green Version]
- Collings, P.J.; Farrar, D.; Gibson, J.; West, J.; Barber, S.E.; Wright, J. Associations of Pregnancy Physical Activity with Maternal Cardiometabolic Health, Neonatal Delivery Outcomes and Body Composition in a Biethnic Cohort of 7305 Mother–Child Pairs: The Born in Bradford Study. Sports Med. 2020, 50, 615–628. [Google Scholar] [CrossRef] [Green Version]
- Niño Cruz, G.I.; Ramirez Varela, A.; da Silva, I.C.M.; Hallal, P.C.; Santos, I.S. Physical activity during pregnancy and offspring neurodevelopment: A systematic review. Paediatr. Perinat. Epidemiol. 2018, 32, 369–379. [Google Scholar] [CrossRef]
- Pires, G.N.; Benedetto, L.; Cortese, R.; Gozal, D.; Gulia, K.K.; Kumar, V.M.; Tufik, S.; Andersen, M.L. Effects of sleep modulation during pregnancy in the mother and offspring: Evidences from preclinical research. J. Sleep Res. 2020, n/a, e13135. [Google Scholar]
- Pot, G.K. Sleep and dietary habits in the urban environment: The role of chrono-nutrition. Proc. Nutr. Soc. 2018, 77, 189–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgieff, M.K. Nutrition and the developing brain: Nutrient priorities and measurement. Am. J. Clin. Nutr. 2007, 85, 614s–620s. [Google Scholar] [PubMed]
- Walker, C.D. Nutritional aspects modulating brain development and the responses to stress in early neonatal life. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 1249–1263. [Google Scholar] [CrossRef] [PubMed]
- Coo, H.; Fabrigar, L.; Davies, G.; Fitzpatrick, R.; Flavin, M. Are observed associations between a high maternal prepregnancy body mass index and offspring IQ likely to be causal? J. Epidemiol. Community Health 2019, 73, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Nivoit, P.; Morens, C.; Van Assche, F.A.; Jansen, E.; Poston, L.; Remacle, C.; Reusens, B. Established diet-induced obesity in female rats leads to offspring hyperphagia, adiposity and insulin resistance. Diabetologia 2009, 52, 1133–1142. [Google Scholar] [CrossRef] [Green Version]
- Howie, G.J.; Sloboda, D.M.; Kamal, T.; Vickers, M.H. Maternal nutritional history predicts obesity in adult offspring independent of postnatal diet. J. Physiol. 2009, 587, 905–915. [Google Scholar] [CrossRef]
- Ong, Z.Y.; Muhlhausler, B.S. Maternal “junk-food” feeding of rat dams alters food choices and development of the mesolimbic reward pathway in the offspring. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2011, 25, 2167–2179. [Google Scholar] [CrossRef] [Green Version]
- Breton, C.; Lukaszewski, M.A.; Risold, P.Y.; Enache, M.; Guillemot, J.; Rivière, G.; Delahaye, F.; Lesage, J.; Dutriez-Casteloot, I.; Laborie, C.; et al. Maternal prenatal undernutrition alters the response of POMC neurons to energy status variation in adult male rat offspring. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E462–E472. [Google Scholar] [CrossRef] [Green Version]
- Palou, M.; Priego, T.; Sánchez, J.; Palou, A.; Picó, C. Sexual dimorphism in the lasting effects of moderate caloric restriction during gestation on energy homeostasis in rats is related with fetal programming of insulin and leptin resistance. Nutr. Metab. 2010, 7, 69. [Google Scholar] [CrossRef] [Green Version]
- Manuel-Apolinar, L.; Rocha, L.; Damasio, L.; Tesoro-Cruz, E.; Zarate, A. Role of prenatal undernutrition in the expression of serotonin, dopamine and leptin receptors in adult mice: Implications of food intake. Mol. Med. Rep. 2014, 9, 407–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lussana, F.; Painter, R.C.; Ocke, M.C.; Buller, H.R.; Bossuyt, P.M.; Roseboom, T.J. Prenatal exposure to the Dutch famine is associated with a preference for fatty foods and a more atherogenic lipid profile. Am. J. Clin. Nutr. 2008, 88, 1648–1652. [Google Scholar] [CrossRef] [PubMed]
- Ayres, C.; Agranonik, M.; Portella, A.K.; Filion, F.; Johnston, C.C.; Silveira, P.P. Intrauterine growth restriction and the fetal programming of the hedonic response to sweet taste in newborn infants. Int. J. Pediatrics 2012, 2012, 657379. [Google Scholar] [CrossRef] [PubMed]
- Brion, M.-J.A.; Ness, A.R.; Rogers, I.; Emmett, P.; Cribb, V.; Davey Smith, G.; Lawlor, D.A. Maternal macronutrient and energy intakes in pregnancy and offspring intake at 10 y: Exploring parental comparisons and prenatal effects. Am. J. Clin. Nutr. 2010, 91, 748–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mennella, J.A.; Griffin, C.E.; Beauchamp, G.K. Flavor programming during infancy. Pediatrics 2004, 113, 840–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mennella, J.A.; Beauchamp, G.K. Flavor experiences during formula feeding are related to preferences during childhood. Early Hum. Dev. 2002, 68, 71–82. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, E.L.; Grayson, B.; Takahashi, D.; Robertson, N.; Maier, A.; Bethea, C.L.; Smith, M.S.; Coleman, K.; Grove, K.L. Chronic consumption of a high-fat diet during pregnancy causes perturbations in the serotonergic system and increased anxiety-like behavior in nonhuman primate offspring. J. Neurosci. 2010, 30, 3826–3830. [Google Scholar] [CrossRef] [Green Version]
- Peleg-Raibstein, D.; Luca, E.; Wolfrum, C. Maternal high-fat diet in mice programs emotional behavior in adulthood. Behav. Brain Res. 2012, 233, 398–404. [Google Scholar] [CrossRef]
- Sasaki, A.; de Vega, W.C.; St-Cyr, S.; Pan, P.; McGowan, P.O. Perinatal high fat diet alters glucocorticoid signaling and anxiety behavior in adulthood. Neuroscience 2013, 240, 1–12. [Google Scholar] [CrossRef]
- Reyes-Castro, L.A.; Rodriguez, J.S.; Charco, R.; Bautista, C.J.; Larrea, F.; Nathanielsz, P.W.; Zambrano, E. Maternal protein restriction in the rat during pregnancy and/or lactation alters cognitive and anxiety behaviors of female offspring. Int. J. Dev. Neurosci. 2012, 30, 39–45. [Google Scholar] [CrossRef]
- Belluscio, L.M.; Berardino, B.G.; Ferroni, N.M.; Ceruti, J.M.; Cánepa, E.T. Early protein malnutrition negatively impacts physical growth and neurological reflexes and evokes anxiety and depressive-like behaviors. Physiol. Behav. 2014, 129, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Levay, E.A.; Paolini, A.G.; Govic, A.; Hazi, A.; Penman, J.; Kent, S. Anxiety-like behaviour in adult rats perinatally exposed to maternal calorie restriction. Behav. Brain Res. 2008, 191, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Akitake, Y.; Katsuragi, S.; Hosokawa, M.; Mishima, K.; Ikeda, T.; Miyazato, M.; Hosoda, H. Moderate maternal food restriction in mice impairs physical growth, behavior, and neurodevelopment of offspring. Nutr. Res. 2015, 35, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Bolton, J.L.; Bilbo, S.D. Developmental programming of brain and behavior by perinatal diet: Focus on inflammatory mechanisms. Dialogues Clin. Neurosci. 2014, 16, 307–320. [Google Scholar]
- Almeida, S.S.; Tonkiss, J.; Galler, J.R. Prenatal protein malnutrition affects exploratory behavior of female rats in the elevated plus-maze test. Physiol. Behav. 1996, 60, 675–680. [Google Scholar] [CrossRef]
- Hack, M.; Youngstrom, E.A.; Cartar, L.; Schluchter, M.; Taylor, H.G.; Flannery, D.; Klein, N.; Borawski, E. Behavioral outcomes and evidence of psychopathology among very low birth weight infants at age 20 years. Pediatrics 2004, 114, 932–940. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, A. Maternal pre-pregnancy obesity and risk for inattention and negative emotionality in children. J. Child. Psychol. Psychiatry 2010, 51, 134–143. [Google Scholar] [CrossRef]
- Plagemann, A.; Harder, T.; Brunn, M.; Harder, A.; Roepke, K.; Wittrock-Staar, M.; Ziska, T.; Schellong, K.; Rodekamp, E.; Melchior, K.; et al. Hypothalamic proopiomelanocortin promoter methylation becomes altered by early overfeeding: An epigenetic model of obesity and the metabolic syndrome. J. Physiol. 2009, 587, 4963–4976. [Google Scholar] [CrossRef]
- Grissom, N.M.; Reyes, T.M. Gestational overgrowth and undergrowth affect neurodevelopment: Similarities and differences from behavior to epigenetics. Int. J. Dev. Neurosci. Off. J. Int. Soc. Dev. Neurosci. 2013, 31, 406–414. [Google Scholar] [CrossRef]
- Portha, B.; Chavey, A.; Movassat, J. Early-life origins of type 2 diabetes: Fetal programming of the beta-cell mass. Exp. Diabetes Res. 2011, 2011, 105076. [Google Scholar] [CrossRef] [Green Version]
- Salam, R.A.; Das, J.K.; Bhutta, Z.A. Impact of intrauterine growth restriction on long-term health. Curr. Opin Clin. Nutr. Metab. Care 2014, 17, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Torreggiani, M.; Fois, A.; D’Alessandro, C.; Colucci, M.; Orozco Guillén, A.O.; Cupisti, A. Of Mice and Men: The Effect of Maternal Protein Restriction on Offspring’s Kidney Health. Are Studies on Rodents Applicable to Chronic Kidney Disease Patients? A Narrative Review. Nutrients 2020, 12, 1614. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, J.; Harambat, J.; Dubourg, L.; Guy, B.; Liutkus, A.; Canterino, I.; Kassaï, B.; Putet, G.; Cochat, P. Both extrauterine and intrauterine growth restriction impair renal function in children born very preterm. Kidney Int. 2009, 76, 445–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visentin, S.; Grisan, E.; Zanardo, V.; Bertin, M.; Veronese, E.; Cavallin, F.; Ambrosini, G.; Trevisanuto, D.; Cosmi, E. Developmental programming of cardiovascular risk in intrauterine growth-restricted twin fetuses according to aortic intima thickness. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2013, 32, 279–284. [Google Scholar] [CrossRef]
- Checkley, W.; West, K.P., Jr.; Wise, R.A.; Baldwin, M.R.; Wu, L.; LeClerq, S.C.; Christian, P.; Katz, J.; Tielsch, J.M.; Khatry, S.; et al. Maternal vitamin A supplementation and lung function in offspring. N. Engl. J. Med. 2010, 362, 1784–1794. [Google Scholar] [CrossRef] [Green Version]
- Kirkwood, B.R.; Hurt, L.; Amenga-Etego, S.; Tawiah, C.; Zandoh, C.; Danso, S.; Hurt, C.; Edmond, K.; Hill, Z.; Ten Asbroek, G.; et al. Effect of vitamin A supplementation in women of reproductive age on maternal survival in Ghana (ObaapaVitA): A cluster-randomised, placebo-controlled trial. Lancet 2010, 375, 1640–1649. [Google Scholar] [CrossRef]
- Edmond, K.; Hurt, L.; Fenty, J.; Amenga-Etego, S.; Zandoh, C.; Hurt, C.; Danso, S.; Tawiah, C.; Hill, Z.; ten Asbroek, A.H.A.; et al. Effect of vitamin A supplementation in women of reproductive age on cause-specific early and late infant mortality in rural Ghana: ObaapaVitA double-blind, cluster-randomised, placebo-controlled trial. BMJ Open 2012, 2, e000658. [Google Scholar] [CrossRef]
- Berry, R.J.; Li, Z.; Erickson, J.D.; Li, S.; Moore, C.A.; Wang, H.; Mulinare, J.; Zhao, P.; Wong, L.Y.; Gindler, J.; et al. Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect Prevention. N. Engl. J. Med. 1999, 341, 1485–1490. [Google Scholar] [CrossRef]
- Molloy, A.M.; Brody, L.C.; Mills, J.L.; Scott, J.M.; Kirke, P.N. The search for genetic polymorphisms in the homocysteine/folate pathway that contribute to the etiology of human neural tube defects. Birth Defects Res. Part. A Clin. Mol. Teratol. 2009, 85, 285–294. [Google Scholar] [CrossRef]
- Passerini, L.; Casey, G.J.; Biggs, B.A.; Cong, D.T.; Phu, L.B.; Phuc, T.Q.; Carone, M.; Montresor, A. Increased birth weight associated with regular pre-pregnancy deworming and weekly iron-folic acid supplementation for Vietnamese women. PLoS Negl. Trop Dis. 2012, 6, e1608. [Google Scholar] [CrossRef] [Green Version]
- Leung, B.M.; Wiens, K.P.; Kaplan, B.J. Does prenatal micronutrient supplementation improve children’s mental development? A systematic review. BMC Pregnancy Childbirth 2011, 11, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hales, C.N.; Barker, D.J.; Clark, P.M.; Cox, L.J.; Fall, C.; Osmond, C.; Winter, P.D. Fetal and infant growth and impaired glucose tolerance at age. BMJ 1991, 303, 1019–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barker, D.J.; Hales, C.N.; Fall, C.H.; Osmond, C.; Phipps, K.; Clark, P.M. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): Relation to reduced fetal growth. Diabetologia 1993, 36, 62–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Arah, O.A.; Liew, Z.; Cnattingius, S.; Olsen, J.; Sørensen, H.T.; Qin, G. Maternal diabetes during pregnancy and early onset of cardiovascular disease in offspring: Population based cohort study with 40 years of follow-up. BMJ 2019, 367, l6398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bieswal, F.; Ahn, M.T.; Reusens, B.; Holvoet, P.; Raes, M.; Rees, W.D.; Remacle, C. The importance of catch-up growth after early malnutrition for the programming of obesity in male rat. Obesity 2006, 14, 1330–1343. [Google Scholar] [CrossRef]
- Howie, G.J.; Sloboda, D.M.; Vickers, M.H. Maternal undernutrition during critical windows of development results in differential and sex-specific effects on postnatal adiposity and related metabolic profiles in adult rat offspring. Br. J. Nutr. 2012, 108, 298–307. [Google Scholar] [CrossRef] [Green Version]
- Neel, J.V. Diabetes mellitus: A “thrifty” genotype rendered detrimental by “progress”? Am. J. Hum. Genet. 1962, 14, 353–362. [Google Scholar]
- Hales, C.N.; Barker, D.J. Type 2 (non-insulin-dependent) diabetes mellitus: The thrifty phenotype hypothesis. Diabetologia 1992, 35, 595–601. [Google Scholar] [CrossRef]
- Burton, M.A.; Lillycrop, K.A. Nutritional modulation of the epigenome and its implication for future health. Proc. Nutr. Soc. 2019, 78, 305–312. [Google Scholar] [CrossRef]
- Breier, B.H.; Vickers, M.H.; Ikenasio, B.A.; Chan, K.Y.; Wong, W.P. Fetal programming of appetite and obesity. Mol. Cell Endocrinol. 2001, 185, 73–79. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A. The developmental origins of the metabolic syndrome. Trends Endocrinol. Metab. 2004, 15, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Phipps, K.; Barker, D.J.; Hales, C.N.; Fall, C.H.; Osmond, C.; Clark, P.M. Fetal growth and impaired glucose tolerance in men and women. Diabetologia 1993, 36, 225–228. [Google Scholar] [CrossRef] [Green Version]
- Fall, C.H.; Osmond, C.; Barker, D.J.; Clark, P.M.; Hales, C.N.; Stirling, Y.; Meade, T.W. Fetal and infant growth and cardiovascular risk factors in women. BMJ 1995, 310, 428–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stöger, R. The thrifty epigenotype: An acquired and heritable predisposition for obesity and diabetes? Bioessays 2008, 30, 156–166. [Google Scholar] [CrossRef]
- Desai, M.; Babu, J.; Ross, M.G. Programmed metabolic syndrome: Prenatal undernutrition and postweaning overnutrition. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R2306–R2314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bol, V.V.; Reusens, B.M.; Remacle, C.A. Postnatal catch-up growth after fetal protein restriction programs proliferation of rat preadipocytes. Obesity 2008, 16, 2760–2763. [Google Scholar] [CrossRef]
- Agote, M.; Goya, L.; Ramos, S.; Alvarez, C.; Gavete, M.L.; Pascual-Leone, A.M.; Escrivá, F. Glucose uptake and glucose transporter proteins in skeletal muscle from undernourished rats. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E1101–E1109. [Google Scholar] [CrossRef]
- Gavete, M.L.; Martín, M.A.; Alvarez, C.; Escrivá, F. Maternal food restriction enhances insulin-induced GLUT-4 translocation and insulin signaling pathway in skeletal muscle from suckling rats. Endocrinology 2005, 146, 3368–3378. [Google Scholar] [CrossRef] [Green Version]
- Escrivá, F.; Rodríguez, C.; Cacho, J.; Alvarez, C.; Portha, B.; Pascual-Leone, A.M. Glucose utilization and insulin action in adult rats submitted to prolonged food restriction. Am. J. Physiol. 1992, 263, E1–E7. [Google Scholar] [CrossRef]
- Bruss, M.D.; Khambatta, C.F.; Ruby, M.A.; Aggarwal, I.; Hellerstein, M.K. Calorie restriction increases fatty acid synthesis and whole body fat oxidation rates. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E108–E116. [Google Scholar] [CrossRef] [Green Version]
- Mackay, H.; Khazall, R.; Patterson, Z.R.; Wellman, M.; Abizaid, A. Rats perinatally exposed to food restriction and high-fat diet show differences in adipose tissue gene expression under chronic caloric restriction. Adipocyte 2013, 2, 237–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dulloo, A.G.; Jacquet, J.; Seydoux, J.; Montani, J.P. The thrifty ’catch-up fat’ phenotype: Its impact on insulin sensitivity during growth trajectories to obesity and metabolic syndrome. Int. J. Obes. 2006, 30 (Suppl. 4), S23–S35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dulloo, A.G. Thrifty energy metabolism in catch-up growth trajectories to insulin and leptin resistance. Best Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 155–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Magliano, D.J.; Zimmet, P.Z. The worldwide epidemiology of type 2 diabetes mellitus—Present and future perspectives. Nat. Rev. Endocrinol. 2011, 8, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Rughani, A.; Friedman, J.E.; Tryggestad, J.B. Type 2 Diabetes in Youth: The Role of Early Life Exposures. Curr. Diab. Rep. 2020, 20, 45. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Reynolds, R.M.; Prescott, S.L.; Nyirenda, M.; Jaddoe, V.W.V.; Eriksson, J.G.; Broekman, B.F.P. Influence of maternal obesity on the long-term health of offspring. Lancet. Diabetes Endocrinol. 2017, 5, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Woo Baidal, J.A.; Locks, L.M.; Cheng, E.R.; Blake-Lamb, T.L.; Perkins, M.E.; Taveras, E.M. Risk Factors for Childhood Obesity in the First 1,000 Days: A Systematic Review. Am. J. Prev. Med. 2016, 50, 761–779. [Google Scholar] [CrossRef]
- Bianco, M.E.; Josefson, J.L. Hyperglycemia During Pregnancy and Long-Term Offspring Outcomes. Curr. Diab. Rep. 2019, 19, 143. [Google Scholar] [CrossRef]
- Smith, J.; Cianflone, K.; Biron, S.; Hould, F.S.; Lebel, S.; Marceau, S.; Lescelleur, O.; Biertho, L.; Simard, S.; Kral, J.G.; et al. Effects of maternal surgical weight loss in mothers on intergenerational transmission of obesity. J. Clin. Endocrinol. Metab. 2009, 94, 4275–4283. [Google Scholar] [CrossRef] [Green Version]
- Beyerlein, A.; von Kries, R. Breastfeeding and body composition in children: Will there ever be conclusive empirical evidence for a protective effect against overweight? Am. J. Clin. Nutr. 2011, 94, 1772s–1775s. [Google Scholar] [CrossRef] [Green Version]
- Agostoni, C.; Baselli, L.; Mazzoni, M.B. Early nutrition patterns and diseases of adulthood: A plausible link? Eur. J. Intern. Med. 2013, 24, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Reifsnider, E.; Mendias, E. Early infant feeding influences and weight of children. In Childhood Obesity; IntechOpen: London, UK, 2012; pp. 15–52. [Google Scholar]
- Koletzko, B.; von Kries, R.; Closa, R.; Escribano, J.; Scaglioni, S.; Giovannini, M.; Beyer, J.; Demmelmair, H.; Anton, B.; Gruszfeld, D.; et al. Can infant feeding choices modulate later obesity risk? Am. J. Clin. Nutr. 2009, 89, 1502s–1508s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fall, C.H.D. Evidence for the intra-uterine programming of adiposity in later life. Ann. Hum. Biol. 2011, 38, 410–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinberg, L.J.; Thompson, J.K. Obesity in Youth: Causes, Consequences, and Cures; American Psychological Association: Washington, DC, USA, 2009; p. 243. [Google Scholar] [CrossRef] [Green Version]
- Langley-Evans, S.C.; McMullen, S. Developmental origins of adult disease. Med. Princ. Pract. 2010, 19, 87–98. [Google Scholar] [CrossRef]
- Sullivan, E.L.; Grove, K.L. Metabolic imprinting in obesity. Forum Nutr. 2010, 63, 186–194. [Google Scholar]
- Şanlı, E.; Kabaran, S. Maternal Obesity, Maternal Overnutrition and Fetal Programming: Effects of Epigenetic Mechanisms on the Development of Metabolic Disorders. Curr. Genom. 2019, 20, 419–427. [Google Scholar] [CrossRef]
- Hanley, B.; Dijane, J.; Fewtrell, M.; Grynberg, A.; Hummel, S.; Junien, C.; Koletzko, B.; Lewis, S.; Renz, H.; Symonds, M.; et al. Metabolic imprinting, programming and epigenetics—A review of present priorities and future opportunities. Br. J. Nutr. 2010, 104 (Suppl. 1), S1–S25. [Google Scholar] [CrossRef] [Green Version]
- Marshall, N.E.; Guild, C.; Cheng, Y.W.; Caughey, A.B.; Halloran, D.R. Maternal superobesity and perinatal outcomes. Am. J. Obs. Gynecol. 2012, 206, 417.e411–e416. [Google Scholar] [CrossRef] [Green Version]
- Jarvie, E.; Hauguel-de-Mouzon, S.; Nelson, S.M.; Sattar, N.; Catalano, P.M.; Freeman, D.J. Lipotoxicity in obese pregnancy and its potential role in adverse pregnancy outcome and obesity in the offspring. Clin. Sci. 2010, 119, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Friis, C.M.; Paasche Roland, M.C.; Godang, K.; Ueland, T.; Tanbo, T.; Bollerslev, J.; Henriksen, T. Adiposity-related inflammation: Effects of pregnancy. Obesity 2013, 21, E124–E130. [Google Scholar] [CrossRef]
- Pacce, S.; Saure, C.; Mazza, C.S.; Garcia, S.; Tomzig, R.G.; Lopez, A.P.; Ribarola, L.; Krochick, G.A. Impact of maternal nutritional status before and during pregnancy on neonatal body composition: A cross-sectional study. Diabetes Metab. Syndr. 2016, 10, S7–S12. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Han, S.; Zhu, J.; Sun, X.; Ji, C.; Guo, X. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: A systematic review and meta-analysis. PLoS ONE 2013, 8, e61627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodwin, P.J.; Stambolic, V. Impact of the obesity epidemic on cancer. Annu. Rev. Med. 2015, 66, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Huffman, S.L. Nutrition in pregnancy and early childhood and associations with obesity in developing countries. Matern. Child. Nutr. 2013, 9 (Suppl. 1), 105–119. [Google Scholar] [CrossRef] [PubMed]
- Mohd-Shukri, N.A.; Duncan, A.; Denison, F.C.; Forbes, S.; Walker, B.R.; Norman, J.E.; Reynolds, R.M. Health Behaviours during Pregnancy in Women with Very Severe Obesity. Nutrients 2015, 7, 8431–8443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picó, C.; Palou, M.; Priego, T.; Sánchez, J.; Palou, A. Metabolic programming of obesity by energy restriction during the perinatal period: Different outcomes depending on gender and period, type and severity of restriction. Front. Physiol. 2012, 3, 436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konieczna, J.; García, A.P.; Sánchez, J.; Palou, M.; Palou, A.; Picó, C. Oral leptin treatment in suckling rats ameliorates detrimental effects in hypothalamic structure and function caused by maternal caloric restriction during gestation. PLoS ONE 2013, 8, e81906. [Google Scholar] [CrossRef]
- Delahaye, F.; Breton, C.; Risold, P.Y.; Enache, M.; Dutriez-Casteloot, I.; Laborie, C.; Lesage, J.; Vieau, D. Maternal perinatal undernutrition drastically reduces postnatal leptin surge and affects the development of arcuate nucleus proopiomelanocortin neurons in neonatal male rat pups. Endocrinology 2008, 149, 470–475. [Google Scholar] [CrossRef]
- Vogt, M.C.; Paeger, L.; Hess, S.; Steculorum, S.M.; Awazawa, M.; Hampel, B.; Neupert, S.; Nicholls, H.T.; Mauer, J.; Hausen, A.C.; et al. Neonatal insulin action impairs hypothalamic neurocircuit formation in response to maternal high-fat feeding. Cell 2014, 156, 495–509. [Google Scholar] [CrossRef] [Green Version]
- Ralevski, A.; Horvath, T.L. Developmental programming of hypothalamic neuroendocrine systems. Front. Neuroendocrinol. 2015, 39, 52–58. [Google Scholar] [CrossRef]
- Ramos-Lobo, A.M.; Teixeira, P.D.; Furigo, I.C.; Melo, H.M.; de M Lyra e Silva, N.; De Felice, F.G.; Donato, J., Jr. Long-term consequences of the absence of leptin signaling in early life. eLife 2019, 8, e40970. [Google Scholar] [CrossRef] [PubMed]
- Lillycrop, K.A.; Burdge, G.C. Epigenetic changes in early life and future risk of obesity. Int. J. Obes. 2011, 35, 72–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bumaschny, V.F.; Yamashita, M.; Casas-Cordero, R.; Otero-Corchón, V.; de Souza, F.S.; Rubinstein, M.; Low, M.J. Obesity-programmed mice are rescued by early genetic intervention. J. Clin. Investig. 2012, 122, 4203–4212. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, K.H.; Adams, J.M.; Jones, G.L.; Yamashita, M.; Schlapschy, M.; Skerra, A.; Rubinstein, M.; Low, M.J. Reprogramming the body weight set point by a reciprocal interaction of hypothalamic leptin sensitivity and Pomc gene expression reverts extreme obesity. Mol. Metab. 2016, 5, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Manzano, P.; Coll-Risco, I.; Van Poppel, M.N.M.; Segura-Jiménez, V.; Femia, P.; Romero-Gallardo, L.; Borges-Cosic, M.; Díaz-Castro, J.; Moreno-Fernández, J.; Ochoa-Herrera, J.J.; et al. Influence of a Concurrent Exercise Training Intervention during Pregnancy on Maternal and Arterial and Venous Cord Serum Cytokines: The GESTAFIT Project. J. Clin. Med. 2019, 8, 1862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsakiridis, I.; Bakaloudi, D.R.; Oikonomidou, A.C.; Dagklis, T.; Chourdakis, M. Exercise during pregnancy: A comparative review of guidelines. J. Perinat. Med. 2020, 48, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Mottola, M.F. Exercise prescription for overweight and obese women: Pregnancy and postpartum. Obs. Gynecol. Clin. N. Am. 2009, 36, 301–316. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, P.J.; Poudevigne, M.S.; Cress, M.E.; Motl, R.W.; Clapp, J.F., 3rd. Safety and efficacy of supervised strength training adopted in pregnancy. J. Phys. Act. Health 2011, 8, 309–320. [Google Scholar] [CrossRef] [Green Version]
- Petrov Fieril, K.; Fagevik Olsén, M.; Glantz, A.; Larsson, M. Experiences of exercise during pregnancy among women who perform regular resistance training: A qualitative study. Phys. Ther. 2014, 94, 1135–1143. [Google Scholar] [CrossRef] [Green Version]
- Roberts, J.M.; Lain, K.Y. Recent Insights into the pathogenesis of pre-eclampsia. Placenta 2002, 23, 359–372. [Google Scholar] [CrossRef]
- Bonzini, M.; Coggon, D.; Palmer, K.T. Risk of prematurity, low birthweight and pre-eclampsia in relation to working hours and physical activities: A systematic review. Occup. Environ. Med. 2007, 64, 228–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, B.B.; Amini, S.B.; Kappeler, K. Exercise in pregnancy: Effect on fitness and obstetric outcomes—A randomized trial. Med. Sci. Sports Exerc. 2012, 44, 2263–2269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soliman, A.; Lacasse, A.A.; Lanoix, D.; Sagrillo-Fagundes, L.; Boulard, V.; Vaillancourt, C. Placental melatonin system is present throughout pregnancy and regulates villous trophoblast differentiation. J. Pineal. Res. 2015, 59, 38–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamanna, S.; Geraci, S.A. Major sleep disorders among women: (women’s health series). South. Med. J. 2013, 106, 470–478. [Google Scholar] [CrossRef] [Green Version]
- Kovac, U.; Jasper, E.A.; Smith, C.J.; Baer, R.J.; Bedell, B.; Donovan, B.M.; Weathers, N.; Prosenc Zmrzljak, U.; Jelliffe-Pawlowski, L.L.; Rozman, D.; et al. The Association of Polymorphisms in Circadian Clock and Lipid Metabolism Genes With 2(nd) Trimester Lipid Levels and Preterm Birth. Front. Genet. 2019, 10, 540. [Google Scholar] [CrossRef] [Green Version]
- Parra, O.; Sánchez-Armengol, Á.; Capote, F.; Bonnin, M.; Arboix, A.; Campos-Rodríguez, F.; Pérez-Ronchel, J.; Durán-Cantolla, J.; Martínez-Null, C.; de la Peña, M.; et al. Efficacy of continuous positive airway pressure treatment on 5-year survival in patients with ischaemic stroke and obstructive sleep apnea: A randomized controlled trial. J. Sleep Res. 2015, 24, 47–53. [Google Scholar] [CrossRef]
- Pien, G.W.; Pack, A.I.; Jackson, N.; Maislin, G.; Macones, G.A.; Schwab, R.J. Risk factors for sleep-disordered breathing in pregnancy. Thorax 2014, 69, 371–377. [Google Scholar] [CrossRef] [Green Version]
- Warland, J.; Dorrian, J.; Morrison, J.L.; O’Brien, L.M. Maternal sleep during pregnancy and poor fetal outcomes: A scoping review of the literature with meta-analysis. Sleep Med. Rev. 2018, 41, 197–219. [Google Scholar] [CrossRef]
- Jiang, F. Sleep and Early Brain Development. Ann. Nutr. Metab. 2019, 75 (Suppl. 1), 44–54. [Google Scholar] [CrossRef]
- Nakamura, Y.; Tamura, H.; Kashida, S.; Takayama, H.; Yamagata, Y.; Karube, A.; Sugino, N.; Kato, H. Changes of serum melatonin level and its relationship to feto-placental unit during pregnancy. J. Pineal. Res. 2001, 30, 29–33. [Google Scholar] [CrossRef]
- Okatani, Y.; Okamoto, K.; Hayashi, K.; Wakatsuki, A.; Tamura, S.; Sagara, Y. Maternal-fetal transfer of melatonin in pregnant women near term. J. Pineal. Res. 1998, 25, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Nehme, P.A.; Amaral, F.G.; Middleton, B.; Lowden, A.; Marqueze, E.; França-Junior, I.; Antunes, J.L.F.; Cipolla-Neto, J.; Skene, D.J.; Moreno, C.R.C. Melatonin profiles during the third trimester of pregnancy and health status in the offspring among day and night workers: A case series. Neurobiol. Sleep Circadian Rhythm. 2019, 6, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, C.M.; Hu, X.Q.; Zhao, Y. Relationship between melatonin receptor 1B and insulin receptor substrate 1 polymorphisms with gestational diabetes mellitus: A systematic review and meta-analysis. Sci. Rep. 2014, 4, 6113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendez, N.; Halabi, D.; Spichiger, C.; Salazar, E.R.; Vergara, K.; Alonso-Vasquez, P.; Carmona, P.; Sarmiento, J.M.; Richter, H.G.; Seron-Ferre, M.; et al. Gestational Chronodisruption Impairs Circadian Physiology in Rat Male Offspring, Increasing the Risk of Chronic Disease. Endocrinology 2016, 157, 4654–4668. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Tamura, H.; Cruz, M.H.; Fuentes-Broto, L. Clinical relevance of melatonin in ovarian and placental physiology: A review. Gynecol. Endocrinol. 2014, 30, 83–89. [Google Scholar] [CrossRef]
- Zlotos, D.P.; Jockers, R.; Cecon, E.; Rivara, S.; Witt-Enderby, P.A. MT1 and MT2 melatonin receptors: Ligands, models, oligomers, and therapeutic potential. J. Med. Chem. 2014, 57, 3161–3185. [Google Scholar] [CrossRef]
- Markwald, R.R.; Melanson, E.L.; Smith, M.R.; Higgins, J.; Perreault, L.; Eckel, R.H.; Wright, K.P., Jr. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc. Natl. Acad. Sci. USA 2013, 110, 5695–5700. [Google Scholar] [CrossRef] [Green Version]
- Terrón, M.P.; Delgado-Adámez, J.; Pariente, J.A.; Barriga, C.; Paredes, S.D.; Rodríguez, A.B. Melatonin reduces body weight gain and increases nocturnal activity in male Wistar rats. Physiol. Behav. 2013, 118, 8–13. [Google Scholar] [CrossRef]
- Onaolapo, A.Y.; Onaolapo, O.J. Circadian dysrhythmia-linked diabetes mellitus: Examining melatonin’s roles in prophylaxis and management. World J. Diabetes 2018, 9, 99–114. [Google Scholar] [CrossRef]
- Hsu, C.-N.; Huang, L.-T.; Tain, Y.-L. Perinatal Use of Melatonin for Offspring Health: Focus on Cardiovascular and Neurological Diseases. Int. J. Mol. Sci. 2019, 20, 5681. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Fernandez, J.; Ochoa, J.J.; Lopez-Frias, M.; Diaz-Castro, J. Impact of Early Nutrition, Physical Activity and Sleep on the Fetal Programming of Disease in the Pregnancy: A Narrative Review. Nutrients 2020, 12, 3900. https://doi.org/10.3390/nu12123900
Moreno-Fernandez J, Ochoa JJ, Lopez-Frias M, Diaz-Castro J. Impact of Early Nutrition, Physical Activity and Sleep on the Fetal Programming of Disease in the Pregnancy: A Narrative Review. Nutrients. 2020; 12(12):3900. https://doi.org/10.3390/nu12123900
Chicago/Turabian StyleMoreno-Fernandez, Jorge, Julio J. Ochoa, Magdalena Lopez-Frias, and Javier Diaz-Castro. 2020. "Impact of Early Nutrition, Physical Activity and Sleep on the Fetal Programming of Disease in the Pregnancy: A Narrative Review" Nutrients 12, no. 12: 3900. https://doi.org/10.3390/nu12123900
APA StyleMoreno-Fernandez, J., Ochoa, J. J., Lopez-Frias, M., & Diaz-Castro, J. (2020). Impact of Early Nutrition, Physical Activity and Sleep on the Fetal Programming of Disease in the Pregnancy: A Narrative Review. Nutrients, 12(12), 3900. https://doi.org/10.3390/nu12123900







