Psychobiotics: Mechanisms of Action, Evaluation Methods and Effectiveness in Applications with Food Products
Abstract
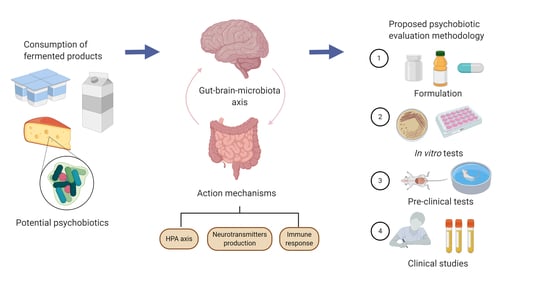
:1. Introduction
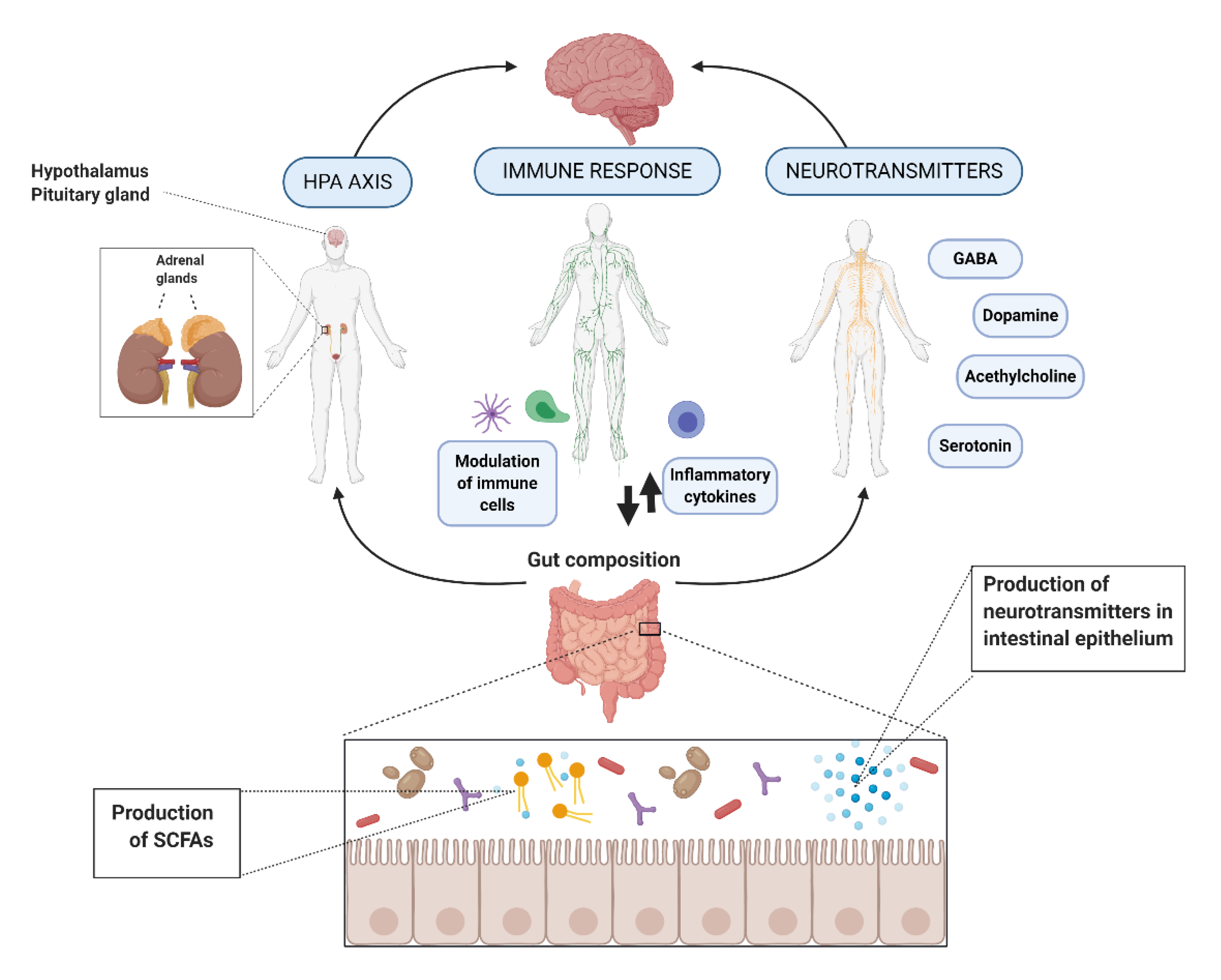
2. Action Mechanisms
2.1. Hypothalamic-Pituitary-Adrenal (HPA) Axis
2.2. Immune Response and Inflammation
2.3. Neurohormones and Neurotransmitters
2.3.1. Serotonin
2.3.2. Dopamine and Epinephrine
2.3.3. Gamma-Aminobutyric Acid (GABA) and Glutamate
2.3.4. Acetylcholine
2.4. Intermediate Substances and Metabolites
2.4.1. Vagus Nerve
2.4.2. Brain-Derived Neurotrophic Factor (BDNF)
2.4.3. Short-Chain Fatty Acids (SCFAs)
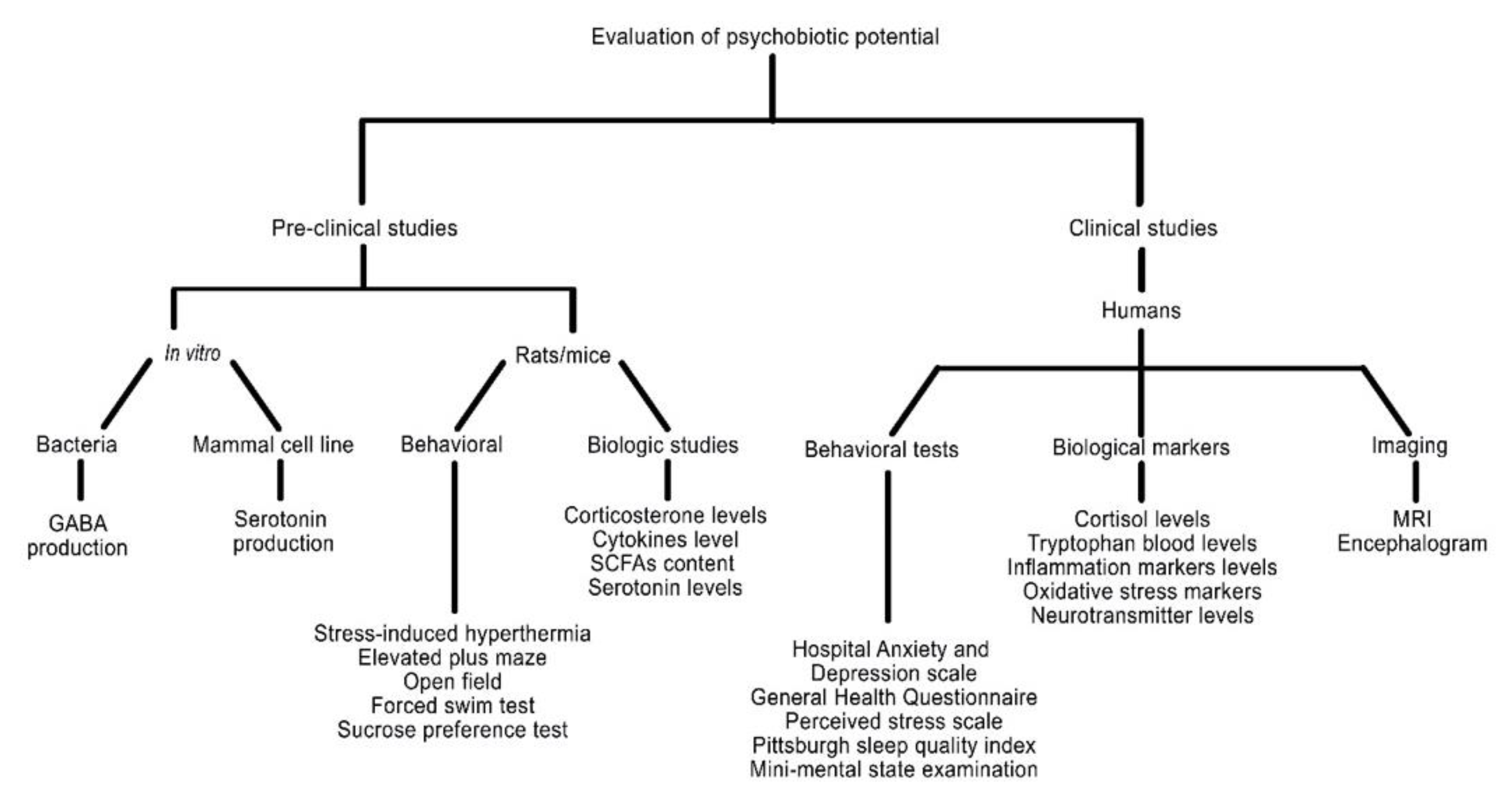
3. Evaluation Methods
4. Action Mechanisms Regarding the Psychobiotic Potential of Specific Strains and Formulations
4.1. Bacteria Strains with Psychobiotic Potential According to Its Possible Action Mechanism
4.2. Bacterial Formulations with Psychobiotic Potential According to Its Possible Action Mechanism
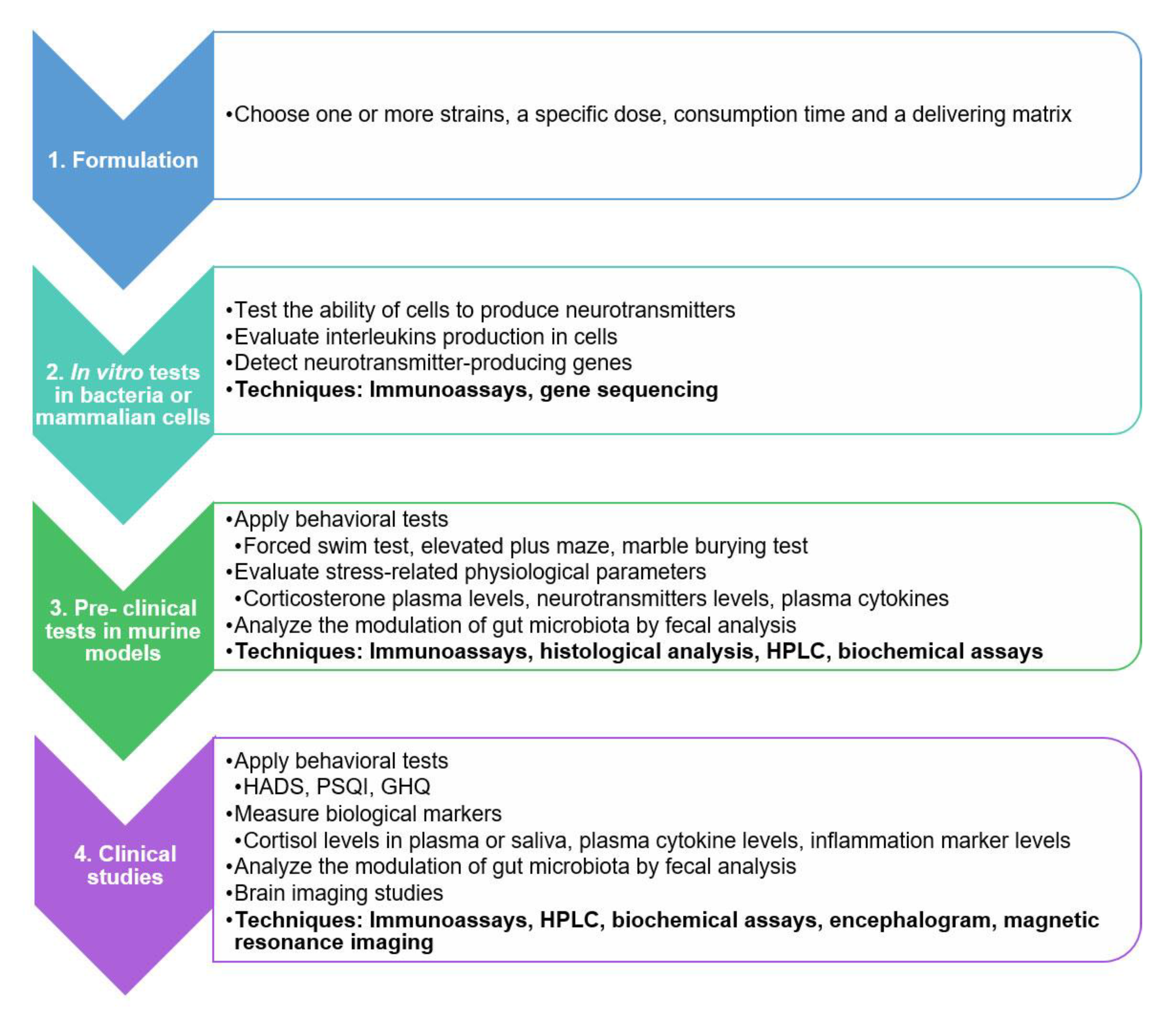
5. A Practical Guide for Evaluating the Psychobiotic Potential
6. Application of Potential Psychobiotic Strains on Fermented Foods and Beverages
6.1. Dairy Products
6.2. Soybean Products
6.3. Other Fermented Products
7. Future Tendencies and Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chisholm, D.; Sweeny, K.; Sheehan, P.; Rasmussen, B.; Smit, F.; Cuijpers, P.; Saxena, S. Scaling-up treatment of depression and anxiety: A global return on investment analysis. Lancet Psychiatry 2016, 3, 415–424. [Google Scholar] [CrossRef] [Green Version]
- Cohen, B.E.; Edmondson, D.; Kronish, I.M. State of the Art Review: Depression, Stress, Anxiety, and Cardiovascular Disease. Am. J. Hypertens. 2015, 28, 1295–1302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NIMH» Generalized Anxiety Disorder: When Worry Gets Out of Control. Available online: https://www.nimh.nih.gov/health/publications/generalized-anxiety-disorder-gad/index.shtml#pub3 (accessed on 19 October 2020).
- NIMH» Anxiety Disorders. Available online: https://www.nimh.nih.gov/health/topics/anxiety-disorders/index.shtml#part_145335 (accessed on 19 October 2020).
- NIMH» Depression. Available online: https://www.nimh.nih.gov/health/topics/depression/index.shtml#part_145399 (accessed on 19 October 2020).
- Wasilewski, A.; Zielińska, M.; Storr, M.; Fichna, J. Beneficial Effects of Probiotics, Prebiotics, Synbiotics, and Psychobiotics in Inflammatory Bowel Disease: Inflamm. Bowel Dis. 2015, 21, 1674–1682. [Google Scholar] [CrossRef] [PubMed]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar] [PubMed]
- Cryan, J.F.; O’Mahony, S.M. The microbiome-gut-brain axis: From bowel to behavior: From bowel to behavior. Neurogastroenterol. Motil. 2011, 23, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Cryan, J.F. Brain-Gut-Microbiota Axis and Mental Health. Psychosom. Med. 2017, 79, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Gao, X.-R.; Peng, L.; Ge, J.-F. Crosstalk between the microbiota-gut-brain axis and depression. Heliyon 2020, 6, e04097. [Google Scholar] [CrossRef]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26. [Google Scholar] [CrossRef]
- Foster, J.A.; McVey Neufeld, K.-A. Gut–brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef]
- Luang-In, V.; Katisart, T.; Konsue, A.; Nudmamud-Thanoi, S.; Narbad, A.; Saengha, W.; Wangkahart, E.; Pumriw, S.; Samappito, W.; Ma, N.L. Psychobiotic Effects of Multi-Strain Probiotics Originated from Thai Fermented Foods in a Rat Model. Food Sci. Anim. Resour. 2020. [Google Scholar] [CrossRef]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W.J. Psychobiotics and the Manipulation of Bacteria–Gut–Brain Signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lou De Santis, G.; Kavvadia, M.; Abd Almajeed Abbaas Alwardat, N.; Bigioni, G.; Zeppieri, C.; Cascapera, S.; De Lorenzo, A. Psychobiotics as integrative therapy for neuropsychiatric disorders with special emphasis on the microbiota-gut-brain axis. Biomed. Prev. Issues 2017, 2, 111. [Google Scholar] [CrossRef]
- Vaghef-Mehrabany, E.; Maleki, V.; Behrooz, M.; Ranjbar, F.; Ebrahimi-Mameghani, M. Can psychobiotics “mood” ify gut? An update systematic review of randomized controlled trials in healthy and clinical subjects, on anti-depressant effects of probiotics, prebiotics, and synbiotics. Clin. Nutr. 2020, 39, 1395–1410. [Google Scholar] [CrossRef] [PubMed]
- Farzi, A.; Fröhlich, E.E.; Holzer, P. Gut Microbiota and the Neuroendocrine System. Neurotherapeutics 2018, 15, 5–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ait-Belgnaoui, A.; Colom, A.; Braniste, V.; Ramalho, L.; Marrot, A.; Cartier, C.; Houdeau, E.; Theodorou, V.; Tompkins, T. Probiotic gut effect prevents the chronic psychological stress-induced brain activity abnormality in mice. Neurogastroenterol. Motil. 2014, 26, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Andersson, H.; Tullberg, C.; Ahrné, S.; Hamberg, K.; Lazou Ahrén, I.; Molin, G.; Sonesson, M.; Håkansson, Å. Oral Administration of Lactobacillus plantarum 299v Reduces Cortisol Levels in Human Saliva during Examination Induced Stress: A Randomized, Double-Blind Controlled Trial. Int. J. Microbiol. 2016, 2016, 8469018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schirmer, M.; Smeekens, S.P.; Vlamakis, H.; Jaeger, M.; Oosting, M.; Franzosa, E.A.; ter Horst, R.; Jansen, T.; Jacobs, L.; Bonder, M.J.; et al. Linking the Human Gut Microbiome to Inflammatory Cytokine Production Capacity. Cell 2016, 167, 1125–1136.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rea, K.; Dinan, T.G.; Cryan, J.F. The microbiome: A key regulator of stress and neuroinflammation. Neurobiol. Stress 2016, 4, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Obermeier, B.; Verma, A.; Ransohoff, R.M. The blood–brain barrier. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 133, pp. 39–59. ISBN 978-0-444-63432-0. [Google Scholar]
- Erny, D.; de Hrabě Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Leclercq, S.; Mian, F.M.; Stanisz, A.M.; Bindels, L.B.; Cambier, E.; Ben-Amram, H.; Koren, O.; Forsythe, P.; Bienenstock, J. Low-dose penicillin in early life induces long-term changes in murine gut microbiota, brain cytokines and behavior. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef]
- Wall, R.; Cryan, J.F.; Ross, R.P.; Fitzgerald, G.F.; Dinan, T.G.; Stanton, C. Bacterial neuroactive compounds produced by psychobiotics. In Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease; Lyte, M., Cryan, J.F., Eds.; Springer: New York, NY, USA, 2014; Volume 817, pp. 221–239. ISBN 978-1-4939-0896-7. [Google Scholar]
- Israelyan, N.; Margolis, K.G. Serotonin as a link between the gut-brain-microbiome axis in autism spectrum disorders. Pharmacol. Res. 2018, 132, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Denna, T.H.; Storkersen, J.N.; Gerriets, V.A. Beyond a neurotransmitter: The role of serotonin in inflammation and immunity. Pharmacol. Res. 2019, 140, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 2017, 112, 399–412. [Google Scholar] [CrossRef]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 2013, 18, 666–673. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, C.; Chakroborty, D.; Basu, S. Neurotransmitters as Regulators of Tumor Angiogenesis and Immunity: The Role of Catecholamines. J. Neuroimmune Pharmacol. 2013, 8, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, K. Role of Catecholamine Signaling in Brain and Nervous System Functions: New Insights from Mouse Molecular Genetic Study. J. Investig. Dermatol. Symp. Proc. 2001, 6, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Xing, B.; Li, Y.-C.; Gao, W.-J. Norepinephrine versus dopamine and their interaction in modulating synaptic function in the prefrontal cortex. Brain Res. 2016, 1641, 217–233. [Google Scholar] [CrossRef] [Green Version]
- Asano, Y.; Hiramoto, T.; Nishino, R.; Aiba, Y.; Kimura, T.; Yoshihara, K.; Koga, Y.; Sudo, N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G1288–G1295. [Google Scholar] [CrossRef] [Green Version]
- Sudo, N. Biogenic Amines: Signals Between Commensal Microbiota and Gut Physiology. Front. Endocrinol. 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samardzic, J.; Jadzic, D.; Hencic, B.; Jancic, J.; Strac, D.S. Introductory chapter: GABA/Glutamate balance: A key for normal brain functioning. In GABA and Glutamate—New Developments in Neurotransmission Research; Samardzic, J., Ed.; InTech: London, UK, 2018; ISBN 978-953-51-3821-1. [Google Scholar]
- Siragusa, S.; De Angelis, M.; Di Cagno, R.; Rizzello, C.G.; Coda, R.; Gobbetti, M. Synthesis of γ-Aminobutyric Acid by Lactic Acid Bacteria Isolated from a Variety of Italian Cheeses. Appl. Environ. Microbiol. 2007, 73, 7283–7290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valenzuela, J.A.; Flórez, A.B.; Vázquez, L.; Vasek, O.M.; Mayo, B. Production of γ-aminobutyric acid (GABA) by lactic acid bacteria strains isolated from traditional, starter-free dairy products made of raw milk. Benef. Microbes 2019, 10, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Yunes, R.A.; Poluektova, E.U.; Dyachkova, M.S.; Klimina, K.M.; Kovtun, A.S.; Averina, O.V.; Orlova, V.S.; Danilenko, V.N. GABA production and structure of gadB/gadC genes in Lactobacillus and Bifidobacterium strains from human microbiota. Anaerobe 2016, 42, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Strandwitz, P.; Kim, K.H.; Terekhova, D.; Liu, J.K.; Sharma, A.; Levering, J.; McDonald, D.; Dietrich, D.; Ramadhar, T.R.; Lekbua, A.; et al. GABA-modulating bacteria of the human gut microbiota. Nat. Microbiol. 2019, 4, 396–403. [Google Scholar] [CrossRef]
- Picciotto, M.R.; Higley, M.J.; Mineur, Y.S. Acetylcholine as a Neuromodulator: Cholinergic Signaling Shapes Nervous System Function and Behavior. Neuron 2012, 76, 116–129. [Google Scholar] [CrossRef] [Green Version]
- Roshchina, V.V. Evolutionary considerations of neurotransmitters in microbial, plant, and animal cells. In Microbial Endocrinology; Lyte, M., Freestone, P.P.E., Eds.; Springer: New York, NY, USA, 2010; pp. 17–52. ISBN 978-1-4419-5575-3. [Google Scholar]
- Forsythe, P.; Bienenstock, J.; Kunze, W.A. Vagal pathways for microbiome-brain-gut axis communication. In Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease; Lyte, M., Cryan, J.F., Eds.; Springer: New York, NY, USA, 2014; Volume 817, pp. 115–133. ISBN 978-1-4939-0896-7. [Google Scholar]
- Fülling, C.; Dinan, T.G.; Cryan, J.F. Gut Microbe to Brain Signaling: What Happens in Vagus. Neuron 2019, 101, 998–1002. [Google Scholar] [CrossRef] [Green Version]
- Bonaz, B.; Bazin, T.; Pellissier, S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front. Neurosci. 2018, 12. [Google Scholar] [CrossRef] [Green Version]
- Maqsood, R.; Stone, T.W. The Gut-Brain Axis, BDNF, NMDA and CNS Disorders. Neurochem. Res. 2016, 41, 2819–2835. [Google Scholar] [CrossRef]
- Bistoletti, M.; Caputi, V.; Baranzini, N.; Marchesi, N.; Filpa, V.; Marsilio, I.; Cerantola, S.; Terova, G.; Baj, A.; Grimaldi, A.; et al. Antibiotic treatment-induced dysbiosis differently affects BDNF and TrkB expression in the brain and in the gut of juvenile mice. PLoS ONE 2019, 14, e0212856. [Google Scholar] [CrossRef] [Green Version]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majeed, M.; Nagabhushanam, K.; Arumugam, S.; Majeed, S.; Ali, F. Bacillus coagulans MTCC 5856 for the management of major depression with irritable bowel syndrome: A randomised, double-blind, placebo controlled, multi-centre, pilot clinical study. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, A.P.; Hutch, W.; Borre, Y.E.; Kennedy, P.J.; Temko, A.; Boylan, G.; Murphy, E.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Bifidobacterium longum 1714 as a translational psychobiotic: Modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl. Psychiatry 2016, 6, e939. [Google Scholar] [CrossRef] [Green Version]
- Pinto-Sanchez, M.I.; Hall, G.B.; Ghajar, K.; Nardelli, A.; Bolino, C.; Lau, J.T.; Martin, F.-P.; Cominetti, O.; Welsh, C.; Rieder, A.; et al. Probiotic Bifidobacterium longum NCC3001 Reduces Depression Scores and Alters Brain Activity: A Pilot Study in Patients with Irritable Bowel Syndrome. Gastroenterology 2017, 153, 448–459.e8. [Google Scholar] [CrossRef]
- Miyaoka, T.; Kanayama, M.; Wake, R.; Hashioka, S.; Hayashida, M.; Nagahama, M.; Okazaki, S.; Yamashita, S.; Miura, S.; Miki, H.; et al. Clostridium butyricum MIYAIRI 588 as Adjunctive Therapy for Treatment-Resistant Major Depressive Disorder: A Prospective Open-Label Trial. Clin. Neuropharmacol. 2018, 41, 151–155. [Google Scholar] [CrossRef]
- Adikari, A.M.G.C.P.; Adikari, A.M.G.C.; Appukutty, M.; Kuan, G. Effects of Daily Probiotic Supplementation on Football Player’s Stress and Anxiety; Atlantis Press: Pairs, France, 2019. [Google Scholar]
- Kato-Kataoka, A.; Nishida, K.; Takada, M.; Suda, K.; Kawai, M.; Shimizu, K.; Kushiro, A.; Hoshi, R.; Watanabe, O.; Igarashi, T.; et al. Fermented milk containing Lactobacillus casei strain Shirota prevents the onset of physical symptoms in medical students under academic examination stress. Benef. Microbes 2016, 7, 153–156. [Google Scholar] [CrossRef]
- Nishida, K.; Sawada, D.; Kawai, T.; Kuwano, Y.; Fujiwara, S.; Rokutan, K. Para-psychobiotic Lactobacillus gasseri CP 2305 ameliorates stress-related symptoms and sleep quality. J. Appl. Microbiol. 2017, 123, 1561–1570. [Google Scholar] [CrossRef] [Green Version]
- Takada, M.; Nishida, K.; Kataoka-Kato, A.; Gondo, Y.; Ishikawa, H.; Suda, K.; Kawai, M.; Hoshi, R.; Watanabe, O.; Igarashi, T.; et al. Probiotic Lactobacillus casei strain Shirota relieves stress-associated symptoms by modulating the gut–brain interaction in human and animal models. Neurogastroenterol. Motil. 2016, 28, 1027–1036. [Google Scholar] [CrossRef] [Green Version]
- Savignac, H.M.; Kiely, B.; Dinan, T.G.; Cryan, J.F. Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neurogastroenterol. Motil. 2014, 26, 1615–1627. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; O’Riordan, K.J.; Lee, Y.; Wang, G.; Zhao, J.; Zhang, H.; Cryan, J.F.; Chen, W. Towards a psychobiotic therapy for depression: Bifidobacterium breve CCFM1025 reverses chronic stress-induced depressive symptoms and gut microbial abnormalities in mice. Neurobiol. Stress 2020, 12, 100216. [Google Scholar] [CrossRef] [PubMed]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Kiely, B.; Cryan, J.F.; Dinan, T.G. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 2010, 170, 1179–1188. [Google Scholar] [CrossRef]
- Hao, Z.; Wang, W.; Guo, R.; Liu, H. Faecalibacterium prausnitzii (ATCC 27766) has preventive and therapeutic effects on chronic unpredictable mild stress-induced depression-like and anxiety-like behavior in rats. Psychoneuroendocrinology 2019, 104, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wang, T.; Liang, S.; Hu, X.; Li, W.; Jin, F. Ingestion of Lactobacillus strain reduces anxiety and improves cognitive function in the hyperammonemia rat. Sci. China Life Sci. 2014, 57, 327–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morshedi, M.; Valenlia, K.B.; Hosseinifard, E.S.; Shahabi, P.; Abbasi, M.M.; Ghorbani, M.; Barzegari, A.; Sadigh-Eteghad, S.; Saghafi-Asl, M. Beneficial psychological effects of novel psychobiotics in diabetic rats: The interaction among the gut, blood and amygdala. J. Nutr. Biochem. 2018, 57, 145–152. [Google Scholar] [CrossRef]
- Liu, Y.-W.; Liu, W.-H.; Wu, C.-C.; Juan, Y.-C.; Wu, Y.-C.; Tsai, H.-P.; Wang, S.; Tsai, Y.-C. Psychotropic effects of Lactobacillus plantarum PS128 in early life-stressed and naïve adult mice. Brain Res. 2016, 1631, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [Green Version]
- Kantak, P.A.; Bobrow, D.N.; Nyby, J.G. Obsessive–compulsive-like behaviors in house mice are attenuated by a probiotic (Lactobacillus rhamnosus GG). Behav. Pharmacol. 2014, 25, 71–79. [Google Scholar] [CrossRef]
- Kazemi, A.; Noorbala, A.A.; Azam, K.; Eskandari, M.H.; Djafarian, K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clin. Nutr. 2019, 38, 522–528. [Google Scholar] [CrossRef]
- Steenbergen, L.; Sellaro, R.; van Hemert, S.; Bosch, J.A.; Colzato, L.S. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain. Behav. Immun. 2015, 48, 258–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akkasheh, G.; Kashani-Poor, Z.; Tajabadi-Ebrahimi, M.; Jafari, P.; Akbari, H.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z.; Esmaillzadeh, A. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. Nutrition 2016, 32, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Kouchaki, E.; Tamtaji, O.R.; Salami, M.; Bahmani, F.; Daneshvar Kakhaki, R.; Akbari, E.; Tajabadi-Ebrahimi, M.; Jafari, P.; Asemi, Z. Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2017, 36, 1245–1249. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.-F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M.; et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011, 105, 755–764. [Google Scholar] [CrossRef] [Green Version]
- Yunes, R.A.; Poluektova, E.U.; Vasileva, E.V.; Odorskaya, M.V.; Marsova, M.V.; Kovalev, G.I.; Danilenko, V.N. A Multi-strain Potential Probiotic Formulation of GABA-Producing Lactobacillus plantarum 90sk and Bifidobacterium adolescentis 150 with Antidepressant Effects. Probiotics Antimicrob. Proteins 2020, 12, 973–979. [Google Scholar] [CrossRef]
- Tian, P.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacterium with the role of 5-hydroxytryptophan synthesis regulation alleviates the symptom of depression and related microbiota dysbiosis. J. Nutr. Biochem. 2019, 66, 43–51. [Google Scholar] [CrossRef]
- Selhub, E.M.; Logan, A.C.; Bested, A.C. Fermented foods, microbiota, and mental health: Ancient practice meets nutritional psychiatry. J. Physiol. Anthropol. 2014, 33. [Google Scholar] [CrossRef] [Green Version]
- Hilimire, M.R.; DeVylder, J.E.; Forestell, C.A. Fermented foods, neuroticism, and social anxiety: An interaction model. Psychiatry Res. 2015, 228, 203–208. [Google Scholar] [CrossRef]
- Chung, Y.-C.; Jin, H.-M.; Cui, Y.; Kim, D.S.; Jung, J.M.; Park, J.-I.; Jung, E.-S.; Choi, E.-K.; Chae, S.-W. Fermented milk of Lactobacillus helveticus IDCC3801 improves cognitive functioning during cognitive fatigue tests in healthy older adults. J. Funct. Foods 2014, 10, 465–474. [Google Scholar] [CrossRef]
- Ohsawa, K.; Nakamura, F.; Uchida, N.; Mizuno, S.; Yokogoshi, H. Lactobacillus helveticus -fermented milk containing lactononadecapeptide (NIPPLTQTPVVVPPFLQPE) improves cognitive function in healthy middle-aged adults: A randomised, double-blind, placebo-controlled trial. Int. J. Food Sci. Nutr. 2018, 69, 369–376. [Google Scholar] [CrossRef]
- Ko, C.Y.; Lin, H.-T.V.; Tsai, G.J. Gamma-aminobutyric acid production in black soybean milk by Lactobacillus brevis FPA 3709 and the antidepressant effect of the fermented product on a forced swimming rat model. Process Biochem. 2013, 48, 559–568. [Google Scholar] [CrossRef]
- Musa, N.H.; Mani, V.; Lim, S.M.; Vidyadaran, S.; Abdul Majeed, A.B.; Ramasamy, K. Lactobacilli-fermented cow’s milk attenuated lipopolysaccharide-induced neuroinflammation and memory impairment in vitro and in vivo. J. Dairy Res. 2017, 84, 488–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benton, D.; Williams, C.; Brown, A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur. J. Clin. Nutr. 2007, 61, 355–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tillisch, K.; Labus, J.; Kilpatrick, L.; Jiang, Z.; Stains, J.; Ebrat, B.; Guyonnet, D.; Legrain–Raspaud, S.; Trotin, B.; Naliboff, B.; et al. Consumption of Fermented Milk Product with Probiotic Modulates Brain Activity. Gastroenterology 2013, 144, 1394–1401.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbari, E.; Asemi, Z.; Daneshvar Kakhaki, R.; Bahmani, F.; Kouchaki, E.; Tamtaji, O.R.; Hamidi, G.A.; Salami, M. Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Alzheimer’s Disease: A Randomized, Double-Blind and Controlled Trial. Front. Aging Neurosci. 2016, 8. [Google Scholar] [CrossRef] [Green Version]
- Reid, S.N.S.; Ryu, J.; Kim, Y.; Jeon, B.H. The Effects of Fermented Laminaria japonica on Short-Term Working Memory and Physical Fitness in the Elderly. Evid. Based Complement. Alternat. Med. 2018, 2018, 8109621. [Google Scholar] [CrossRef] [Green Version]
- Hwang, Y.-H.; Park, S.; Paik, J.-W.; Chae, S.-W.; Kim, D.-H.; Jeong, D.-G.; Ha, E.; Kim, M.; Hong, G.; Park, S.-H.; et al. Efficacy and Safety of Lactobacillus Plantarum C29-Fermented Soybean (DW2009) in Individuals with Mild Cognitive Impairment: A 12-Week, Multi-Center, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2019, 11, 305. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.M.; Yu, K.W.; Kang, D.H.; Suh, H.J. Anti-stress and anti-fatigue effect of fermented rice bran. Phytother. Res. 2002, 16, 700–702. [Google Scholar] [CrossRef]
- Ton, A.M.M.; Campagnaro, B.P.; Alves, G.A.; Aires, R.; Côco, L.Z.; Arpini, C.M.; Guerra e Oliveira, T.; Campos-Toimil, M.; Meyrelles, S.S.; Pereira, T.M.C.; et al. Oxidative Stress and Dementia in Alzheimer’s Patients: Effects of Synbiotic Supplementation. Oxid. Med. Cell. Longev. 2020, 2020, 2638703. [Google Scholar] [CrossRef]
- Van de Wouw, M.; Walsh, A.M.; Crispie, F.; van Leuven, L.; Lyte, J.M.; Boehme, M.; Clarke, G.; Dinan, T.G.; Cotter, P.D.; Cryan, J.F. Distinct actions of the fermented beverage kefir on host behaviour, immunity and microbiome gut-brain modules in the mouse. Microbiome 2020, 8, 67. [Google Scholar] [CrossRef]
- Butler, M.I.; Bastiaanssen, T.F.S.; Long-Smith, C.; Berding, K.; Morkl, S.; Cusack, A.-M.; Strain, C.; Busca, K.; Porteous-Allen, P.; Claesson, M.J.; et al. Recipe for a Healthy Gut: Intake of Unpasteurised Milk Is Associated with Increased Lactobacillus Abundance in the Human Gut Microbiome. Nutrients 2020, 12, 1468. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.A.; Jazayeri, S.; Khosravi-Darani, K.; Solati, Z.; Mohammadpour, N.; Asemi, Z.; Adab, Z.; Djalali, M.; Tehrani-Doost, M.; Hosseini, M.; et al. The effects of probiotics on mental health and hypothalamic–pituitary–adrenal axis: A randomized, double-blind, placebo-controlled trial in petrochemical workers. Nutr. Neurosci. 2016, 19, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Nishihira, J.; Kagami-Katsuyama, H.; Tanaka, A.; Nishimura, M.; Kobayashi, T.; Kawasaki, Y. Elevation of natural killer cell activity and alleviation of mental stress by the consumption of yogurt containing Lactobacillus gasseri SBT2055 and Bifidobacterium longum SBT2928 in a double-blind, placebo-controlled clinical trial. J. Funct. Foods 2014, 11, 261–268. [Google Scholar] [CrossRef]
- Savaiano, D.A.; Hutkins, R.W. Yogurt, cultured fermented milk, and health: A systematic review. Nutr. Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, F.F.; Farias, D. de P. Psychobiotics: An emerging alternative to ensure mental health amid the COVID-19 outbreak? Trends Food Sci. Technol. 2020, 103, 386–387. [Google Scholar] [CrossRef]
| Bacteria | Model | Dose | Findings | Possible Mechanism | Reference |
|---|---|---|---|---|---|
| Single-strain probiotic | |||||
| Bacillus coagulans MTCC 5856 | Clinical trial | 2 billion spores | Robust efficacy for the treatment of patients experiencing IBS symptoms with major depressive disorder. | Production of SCFAsand antimicrobial and anti-inflammatory substances. | [52] |
| Bifidobacterium longum 1714 | Clinical trial | 1 × 109 CFU/day | Reduced stress and improved memory. | Brain-Derived Neurotrophic Factor (BDNF) synthesis through vagal activity. | [53] |
| Bifidobacterium longum NCC3001 | Clinical trial | 1 × 1010 CFU/g | Reduction in depression scores on Hospital Anxiety and Depression Scale and reduced responses to negative emotional stimuli in multiple brain areas. | Release of neuroactive compounds through vagal signaling as well as BDNF regulation. | [54] |
| Clostridium butyricum MIYAIRI 588 | Clinical trial | 60 mg/day | In combination with antidepressants, is effective in the treatment of treatment-resistant major depressive disorder. | Regulation of proinflammatory agents. | [55] |
| Lactobacillus casei Shirota | Clinical trial | 1 × 109 over 8 weeks | Decrease in the cognitive state anxiety scores, somatic state, and perceived stress scale. | [56] | |
| Lactobacillus casei Shirota | Clinical trial | 100 mL of a fermented beverage containing more than 1 × 109 CFU/mL/day | Salivary cortisol and plasma L-tryptophan levels were increased in the placebo group, while the experimental group had higher fecal serotonin levels. Lower rate of subjects experiencing common abdominal and cold symptoms, and total number of days experiencing these physical symptoms. | Hypothalamic-pituitary-adrenal (HPA) axis modulation and promotion of serotonin synthesis. | [57] |
| Lactobacillus gasseri CP2305 | Clinical trial | 1 × 1010 CFU | Stress-associated behaviors were improved, as well as the sleeping quality. The parabiotic administration also prevented increases in basal salivary cortisol release and expression of stress-responsive microRNAs. | Regulation of inflammation mechanisms. | [58] |
| Lactobacillus casei Shirota | Clinical trial and in vivo murine model | Milk fermented with 1 × 109 CFU/mL | Increases in salivary cortisol levels and incidence rate of physical symptoms were significantly suppressed. In rats, water avoidance stress-induced increases in plasma corticosterone were suppressed, and the number of corticotrophin releasing factor-expressing cells in the paraventricular nucleus was reduced. | HPA axis regulation. | [59] |
| Bifidobacterium breve 1205 | In vivo murine model | 1 × 109 CFU/mL | Reduced anxiety in the marble-burying test and induced lower anxiety in the elevated plus maze. | Immune system regulation and gut hormones secretion. | [60] |
| Bifidobacterium breve CCFM1025 | In vivo murine model | 0.1 mL/10 g body weight daily at 1 × 109 CFU/mL | Reduced depression and anxiety behaviors. The hyperactive HPA response and inflammation were also alleviated. Expression of the brain-derived neurotrophic factor was increased, while chronic stress was restored. | Capacity of SCFAs to improve serotonin levels, and regulation of the HPA axis and BDNF synthesis. | [61] |
| Bifidobacterium infantis 35624 | In vivo murine model | 1 × 1010 live bacterial cells/100 mL drinking/day | Normalization of the immune response, reversal of behavioral deficits, and restoration of basal noradrenaline concentrations in the brain. | Anti-inflammatory properties. | [62] |
| Bifidobacterium longum 1714 | In vivo murine model | 1 × 109 CFU/mL | Reduced anxiety in the marble-burying test; decreased stress-induced hyperthermia, lower anxiety in the elevated plus maze, and antidepressant-like behavior in the tail suspension test. | Immune system regulation and gut hormones secretion. | [60] |
| Faecalibacterium prausnitzii ATCC 27766 | In vivo murine model | 1 × 109 CFU by oral gavage | Preventive and therapeutic effects on depression and anxiety behavior, higher levels of SCFAs in the cecum, and higher levels of cytokines interleukin-10 in the plasma. Corticosterone, C-reaction protein, and Interleukin-6 levels were normalized. | SCFAs synthesis, immune system stimulation, and HPA axis regulation. | [63] |
| Lactobacillus helveticus NS8 | In vivo murine model | 1 × 109 CFU/mL in drinking water | The anxiolytic effect in hyperammonia-treated rats was associated with a reduction in hippocampal serotonin 5-HTP levels. | Downregulation of inflammation and serotonin metabolism. | [64] |
| Lactobacillus plantarum ATCC 8014 | In vivo murine model | Bacterial suspensions at 1 × 107 CFU/mL | Favorable effects on oxidative markers of the blood and amygdala, as well as on concentrations of amygdala serotonin and brain-derived neurotrophic factor (BDNF). Beneficial effects were observed on the elevated plus maze and forced swimming tests. | HPA axis downregulation due to oxidative stress reduction. | [65] |
| Lactobacillus plantarum PS128 | In vivo murine model | 1 × 109 CFU/mouse/day by gavage | Anxiety behavior in naïve adult mice was reduced, whereas the depressive behaviors were reduced in early life stressed mice. Early life stressed mice-induced elevation of serum corticosterone decreased, inflammatory cytokine levels reduced, and anti-inflammatory cytokine levels increased. Dopamine levels in rose in both groups, whereas serotonin level was increased in the naïve adult mice. | HPA axis regulation, modulation of the immune system, and synthesis of neuroactive compounds. | [66] |
| Lactobacillus rhamnosus JB-1 | In vivo murine model | 1 × 109 CFU | Reduction of the content of corticosterone and restricted behaviors associated with depression and anxiety. Neurochemical and behavioral effects were absent in mice after vagotomy. | GABA synthesis and regulation of the HPA axis. | [67] |
| Lactobacillus rhamnosus GG | In vivo murine model | 1 × 109 CFU | Attenuated OCD-like behavior induction: Increased perseverative open-field locomotion, stereotypic turning, and marble burying. | Brain serotogenic system. | [68] |
| Formulations (multi-strain probiotics | |||||
| Lactobacillus helveticus R0052 and Bifidofacterium longum R0175 | Clinical trial | 1 × 109 CFU/5 g | Significant decrease in Beck Depression Inventory (BDI) score. The kynurenine/tryptophan ratio decreased significantly in the probiotic group, while the tryptophan/isoleucine ratio increased. | Modulation of neurotransmitters synthesis and metabolism. | [69] |
| Bifidobacterium bifidum W23, Bifidobacterium lactis W52, Lactobacillus acidophilus W37, Lactobacillus brevis W63, Lactobacillus casei W56, Lactobacillus salivarius W24, and Lactococcus lactis (W19 and W58) | Clinical trial | 2.5 × 109 CFU/g | Reduced overall cognitive reactivity to sad mood. | [70] | |
| Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium bifidum | Clinical trial | 2 × 109 CFU/g of each strain | Significant improvement in depression scores on the Beck Depression Inventory, and reduced serum insulin and increased plasma total glutathione levels. | Decreased oxidative stress. | [71] |
| Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum and Lactobacillus fermentum | Clinical trial | 1 capsule containing 2 × 109 CFU/g daily for 12 weeks | Improved expanded disability status scale, Beck Depression Inventory, general health questionnaire, and depression anxiety and stress scale. Changes in C-reactive protein, plasma nitric oxide metabolites, and malondialdehyde. | [72] | |
| Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 | Clinical trial and in vivo murine model | 3 × 109 CFU/stick daily for 30 days | Reduction of anxiety-like behavior in rats, alleviation of psychological distress in volunteers, and a reduction in urinary free cortisol levels. | HPA axis regulation. | [73] |
| Bifidobacterium longum subsp. infantis E41 and Bifidobacterium breve M2CF22M7 | In vivo murine model | 1 × 109 CFU | Reduction in depressive behaviors of mice as well as increased the level of 5-hydroxytryptamine and brain-derived neurotrophic factor concentration in the brain. | Downregulation of HPA axis, and changes in levels of 5-HTP through SCFAs synthesis. | [64] |
| Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 | In vivo murine model | 1 × 109 CFU in 0.9% NaCl | Attenuated HPA axis and autonomic nervous system activities in response to water avoidance stress, and reduced cFos protein expression in different brain areas. Probiotic pretreatment prevented hippocampal neurogenesis and expression changes in hypothalamic genes involved in synaptic plasticity. | Changes in brain plasticity due to BDNF production, and HPA axis regulation. | [18]) |
| Lactobacillus plantarum 90sk and Bifidobacterium adolescentis 150 | In vivo murine model | 1 dose (0.5 mL) of the mixture of 1 × 108 CFU L. plantarum 90sk and 1 × 107 CFU B. adolescentis 150 | Reduced depressive-like behavior in the forced swimming test; the effect was similar to that of fluoxetine. | GABA production. | [74] |
| Food Product | Strain(s) | Model | Doses | Psychobiotic Effects | Reference |
|---|---|---|---|---|---|
| Black soybean milk | Lactobacillus helveticus IDCC3801 | Clinical trial | 500, 1000, or 2000 mg of tablets of skim milk powder fermented for 12 weeks | Improvements in cognitive function in healthy old adults | [78] |
| Lactobacillus helveticus | Clinical trial | 190 g of fermented milk once a day for 8 weeks | Improved cognitive function in healthy middle-aged adults | [79] | |
| Lactobacillus brevis FPA 3709 | In vivo murine model | 35 mg/kg or 70 mg/kg body weight by oral gavage for 28 days | Antidepressant effect without side effects in rat models | [80] | |
| Lactobacillus fermentum LAB9 or Lactobacillus casei LABPC | In vivo murine model | Daily administration by oral gavage for 28 days | There was a restoration of cholinergic neurotransmission and attenuation of neuroinflammation in mice | [81] | |
| Fermented milk | Lactobacillus gasseri CP2305 | Clinical trial | 190 g of fermented milk once a day for 5 weeks | Improved sleep quality and alleviated stress-associated symptoms in healthy students | [58] |
| Lactobacillus casei Shirota | Clinical trial | 100 mL of fermented milk once a day for 8 weeks | Increased fecal serotonin levels and reduced physical symptoms in healthy subjects when exposed to stressful situations | [57] | |
| Clinical trial | 65 mL of probiotic-containing milk drink for 3 weeks | Improved the mood of adults whose mood was initially poor/depressive | [82] | ||
| Bifidobacterium animalis subsp lactis I-2494, Streptococcus thermophilus I-1630, Lactobacillus bulgaricus I-1632 and I-1519, and Lactococcus lactis subsp. lactis I-1631 | Clinical trial | 125 g of fermented milk twice daily for 4 weeks | Affected the activity of brain regions that control central processing of emotion and sensation in healthy women | [83] | |
| Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum, and Lactobacillus fermentum | Clinical trial | 200 mL/day for 12 weeks | Positively affected cognitive function and some metabolic statuses in Alzheimer’s disease patients (60–95 years old) | [84] | |
| Lactobacillus casei Shirota YIT 9029 | Clinical trial and In vivo murine model | 100 mL of fermented milk once a day for 8 weeks | Physical symptoms of stressed subjects were reduced | [59] | |
| Fermented Laminaria japonica | Lactobacillus brevis BJ20 | Clinical trial | 1.5 g/day of fermented Laminaria japonica for 6 weeks | Provided a protective mechanism against cognitive impairment associated with dementia in elderly | [85] |
| Fermented soybean | Lactobacillus plantarum C29 | Clinical trial | 800 mg/day for 12 weeks | Improved cognitive function in individuals with Mild Cognitive Impairment | [86] |
| Fermented rice bran | Saccharomyces cerevisae IFO 2346 | In vivo murine model | 1 g/kg/day of a hot water extract of fermented rice bran | Demonstrated anti-stress and anti-fatigue effects in rats and mice | [87] |
| Kefir | Acetobacter aceti, Acetobacter sp., Lactobacillus delbrueckii, Lactobacillus fermentum, Lactobacillus fructivorans, Enterococcus faecium, Leuconostoc spp., Lactobacillus kefiranofaciens, Candida famata, and Candida krusei | Clinical trial | 2 mL/Kg/daily for 90 days | Improvement in memory, visual-spatial/abstraction abilities, and executive/language functions | [88] |
| Lactobacillus reuteri | In vivo murine model | Daily administration 1 h by oral gavage for 3 weeks | Increased capacity of GABA production in the gut microbiota of mouse | [89] | |
| Unpasteurized milk and dairy products | Lactobacilli | Clinical trial | Free consumption before and after 12 weeks | Decreased stress and anxiety scores in adults | [90] |
| Yogur | Lactobacillus acidophilus LA5 and Bifidobacterium lactis BB12 | Clinical trial | 100 g once a day for 6 weeks | Improvement of adults in depression anxiety and stress scale scores | [91] |
| Lactobacillus gasseri SBT2055 and Bifidobacterium longum SBT2928 | Clinical trial | 100 g once a day for 12 weeks | Levels of the stress-induced hormone adrenocorticotrophic hormone significantly decreased in adults | [92] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Toro-Barbosa, M.; Hurtado-Romero, A.; Garcia-Amezquita, L.E.; García-Cayuela, T. Psychobiotics: Mechanisms of Action, Evaluation Methods and Effectiveness in Applications with Food Products. Nutrients 2020, 12, 3896. https://doi.org/10.3390/nu12123896
Del Toro-Barbosa M, Hurtado-Romero A, Garcia-Amezquita LE, García-Cayuela T. Psychobiotics: Mechanisms of Action, Evaluation Methods and Effectiveness in Applications with Food Products. Nutrients. 2020; 12(12):3896. https://doi.org/10.3390/nu12123896
Chicago/Turabian StyleDel Toro-Barbosa, Mariano, Alejandra Hurtado-Romero, Luis Eduardo Garcia-Amezquita, and Tomás García-Cayuela. 2020. "Psychobiotics: Mechanisms of Action, Evaluation Methods and Effectiveness in Applications with Food Products" Nutrients 12, no. 12: 3896. https://doi.org/10.3390/nu12123896
APA StyleDel Toro-Barbosa, M., Hurtado-Romero, A., Garcia-Amezquita, L. E., & García-Cayuela, T. (2020). Psychobiotics: Mechanisms of Action, Evaluation Methods and Effectiveness in Applications with Food Products. Nutrients, 12(12), 3896. https://doi.org/10.3390/nu12123896