Dietary Fiber Intake Alters Gut Microbiota Composition but Does Not Improve Gut Wall Barrier Function in Women with Future Hypertensive Disorders of Pregnancy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Sample Collection
2.2. Fecal DNA Extraction
2.3. Bioinformatics Analysis
2.4. Zonulin Assay
2.5. Data Analysis
3. Results
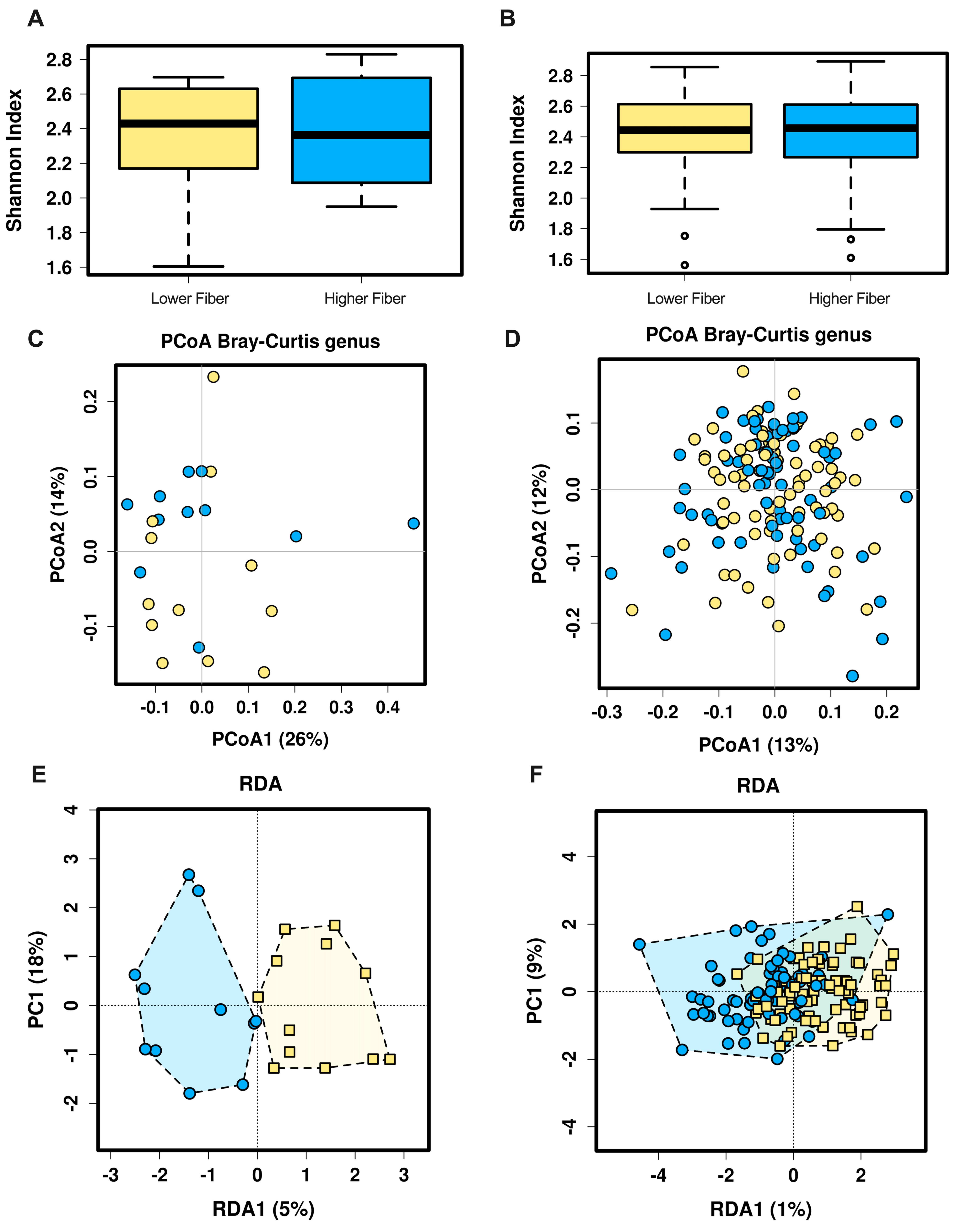
3.1. Overall Gut Microbiota Diversity
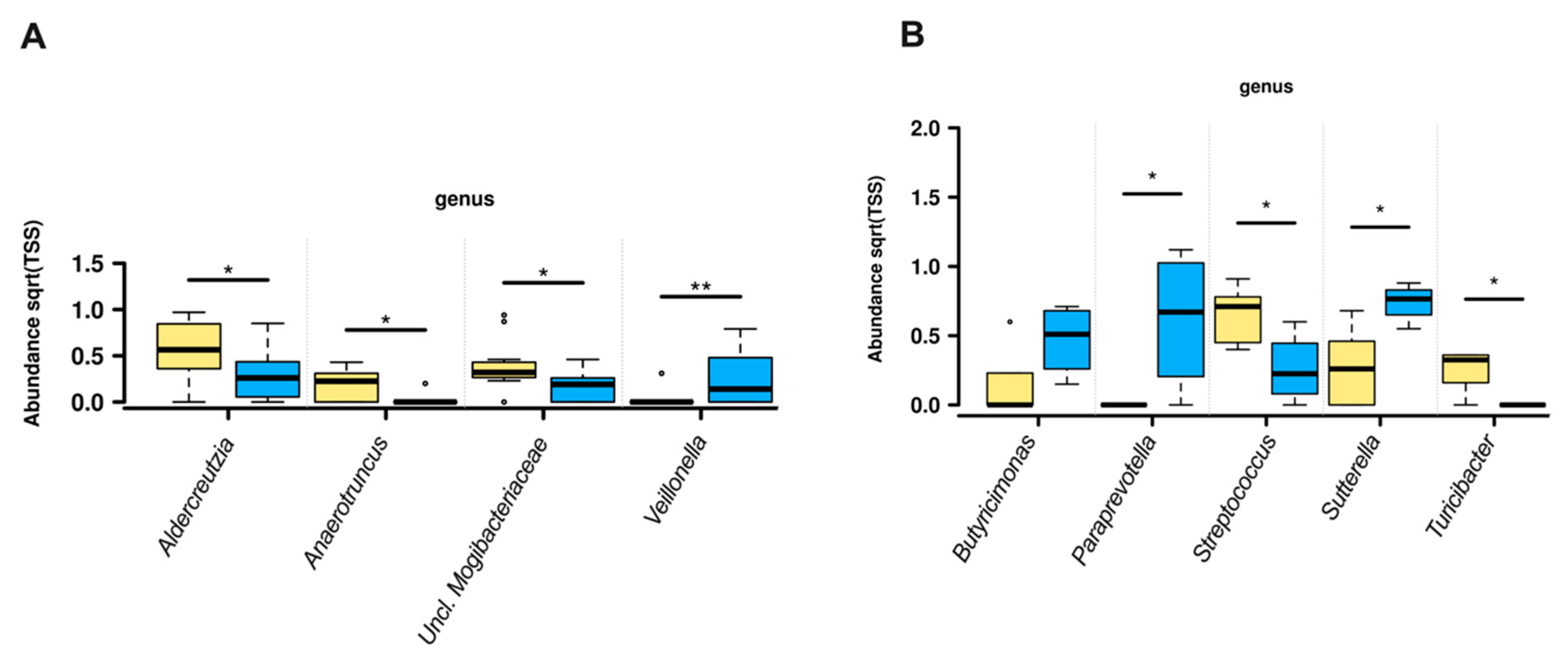
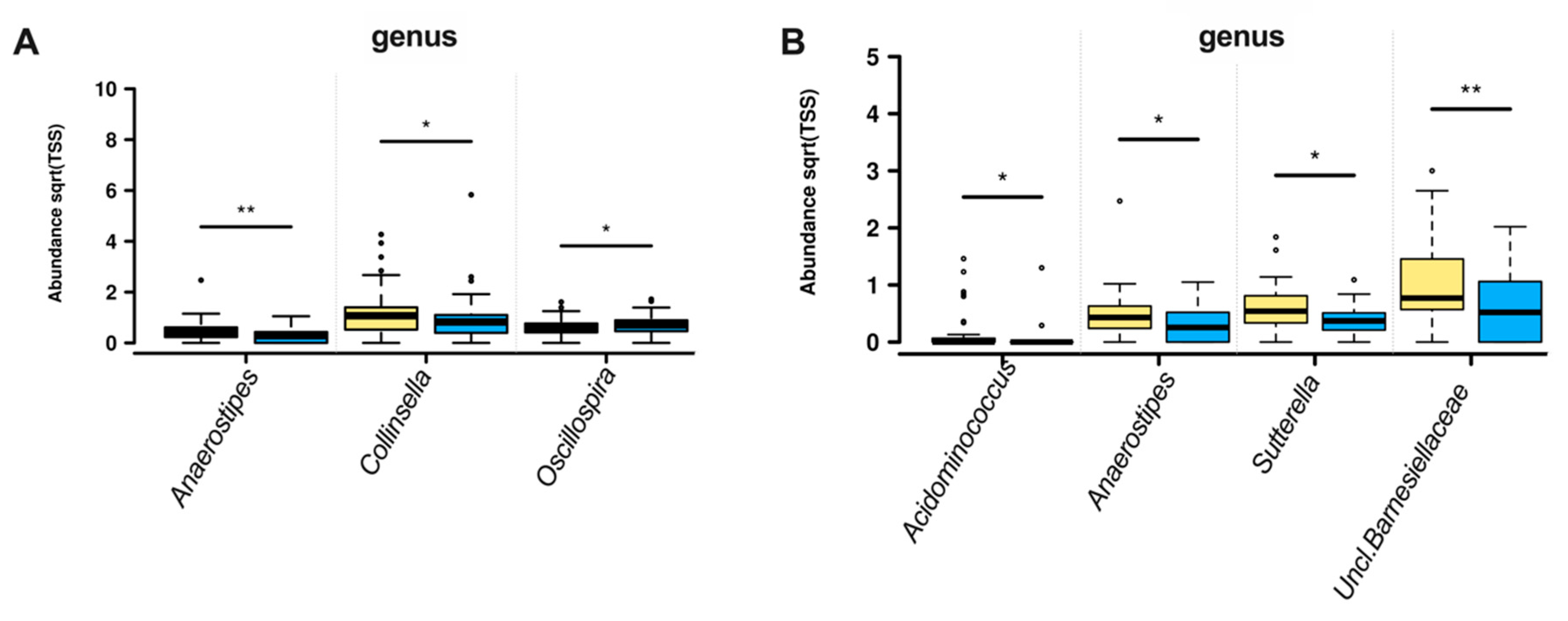
3.2. Gut Microbiota Composition
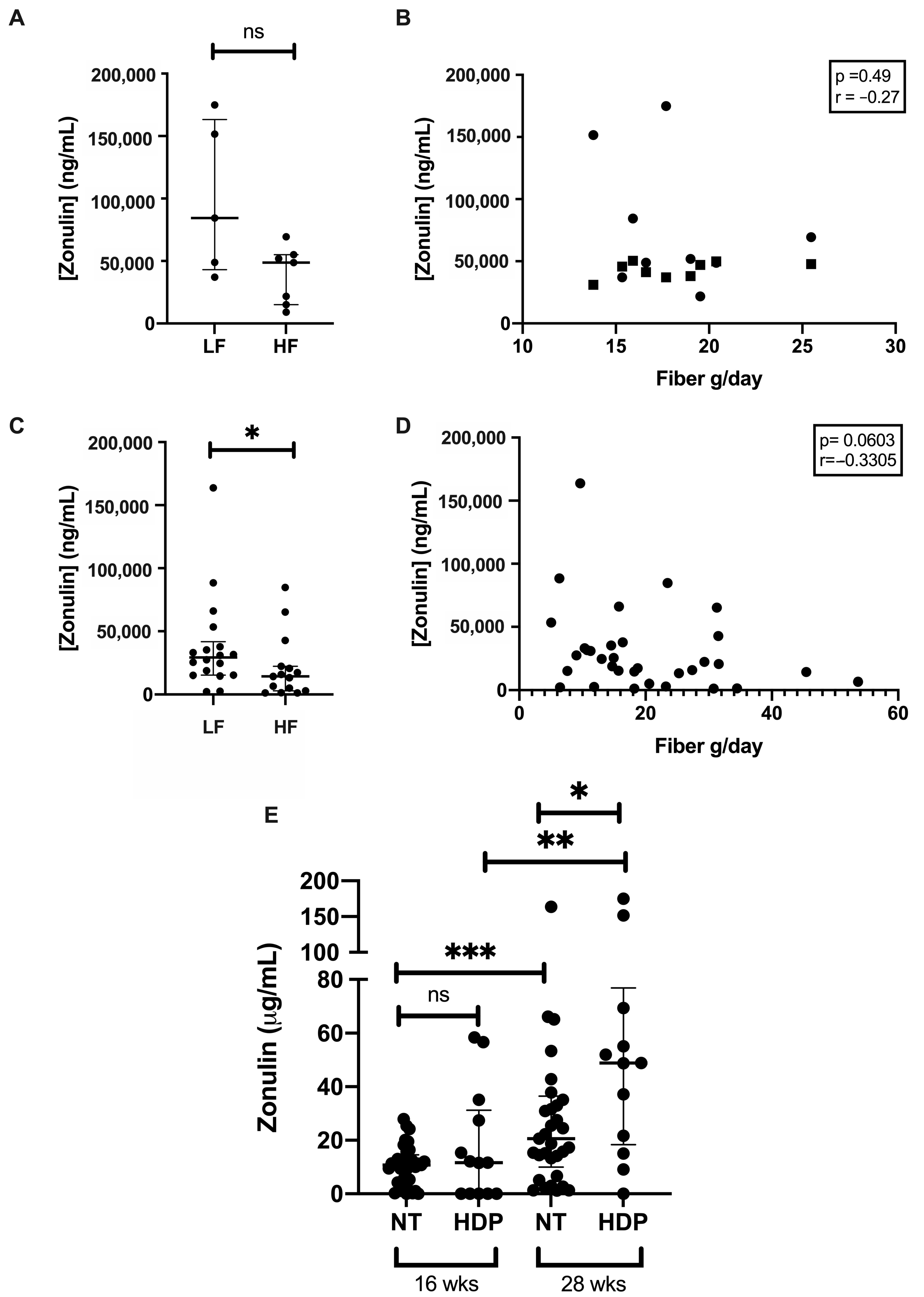
3.3. Gut Permeability
4. Discussion
4.1. Genera Diversity and Abundance with Fiber Intake
4.2. Gut Wall Barrier Function Across Gestation
4.3. Gut Wall Barrier Function and Fiber Intake
4.4. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Umesawa, M.; Kobashi, G. Epidemiology of hypertensive disorders in pregnancy: Prevalence, risk factors, predictors and prognosis. Hypertens. Res. 2017, 40, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Callaway, L.K.; Chang, A.M.; McIntyre, H.D.; Prins, J.B. The prevalence and impact of overweight and obesity in an Australian obstetric population. Med. J. Aust. 2006, 184, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, F.; Wang, Y.; Chen, J.; Tao, J.; Tian, G.; Wu, S.; Liu, W.; Cui, Q.; Geng, B.; et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017, 5, 14. [Google Scholar] [CrossRef]
- Bull, M.J.; Plummer, N.T. Part 1: The Human Gut Microbiome in Health and Disease. Integr. Med. Clin. J. 2014, 13, 17–22. [Google Scholar]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Kling Bäckhed, H.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host Remodeling of the Gut Microbiome and Metabolic Changes during Pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef]
- Luissint, A.C.; Parkos, C.A.; Nusrat, A. Inflammation and the Intestinal Barrier: Leukocyte-Epithelial Cell Interactions, Cell Junction Remodeling, and Mucosal Repair. Gastroenterology 2016, 151, 616–632. [Google Scholar] [CrossRef]
- Mor, G.; Cardenas, I. The immune system in pregnancy: A unique complexity. Am. J. Reprod. Immunol. 2010, 63, 425–433. [Google Scholar] [CrossRef]
- Lupp, C.; Robertson, M.L.; Wickham, M.E.; Sekirov, I.; Champion, O.L.; Gaynor, E.C.; Finlay, B.B. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe 2007, 2, 119–129. [Google Scholar] [CrossRef]
- Cheng, W.; Lu, J.; Li, B.; Lin, W.; Zhang, Z.; Wei, X.; Sun, C.; Chi, M.; Bi, W.; Yang, B.; et al. Effect of Functional Oligosaccharides and Ordinary Dietary Fiber on Intestinal Microbiota Diversity. Front. Microbiol. 2017, 8, 1750. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Rios-Covian, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de Los Reyes-Gavilan, C.G.; Salazar, N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Roytio, H.; Mokkala, K.; Vahlberg, T.; Laitinen, K. Dietary intake of fat and fibre according to reference values relates to higher gut microbiota richness in overweight pregnant women. Br. J. Nutr. 2017, 118, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; O’Riordan, M.X.D. Regulation of bacterial pathogenesis by intestinal short-chain Fatty acids. Adv. Appl. Microbiol. 2013, 85, 93–118. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Isolauri, E.; Laitinen, K.; Salminen, S. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am. J. Clin. Nutr. 2008, 88, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arango, L.F.; Barrett, H.L.; McIntyre, H.D.; Callaway, L.K.; Morrison, M.; Dekker Nitert, M.; Group, S.T. Connections Between the Gut Microbiome and Metabolic Hormones in Early Pregnancy in Overweight and Obese Women. Diabetes 2016, 65, 2214–2223. [Google Scholar] [CrossRef]
- Ferreira-Halder, C.V.; Faria, A.V.; Andrade, S.S. Action and function of Faecalibacterium prausnitzii in health and disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 643–648. [Google Scholar] [CrossRef]
- Peng, L.; Li, Z.-R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef]
- Lekva, T.; Norwitz, E.R.; Aukrust, P.; Ueland, T. Impact of Systemic Inflammation on the Progression of Gestational Diabetes Mellitus. Curr. Diabetes Rep. 2006, 16, 26. [Google Scholar] [CrossRef]
- Edwards, S.M.; Cunningham, S.A.; Dunlop, A.L.; Corwin, E.J. The Maternal Gut Microbiome during Pregnancy. MCN Am. J. Matern. Child Nurs. 2017, 42, 310–317. [Google Scholar] [CrossRef]
- Patti, A.M.; Pafili, K.; Papanas, N.; Rizzo, M. Metabolic disorders during pregnancy and postpartum cardiometabolic risk. Endocr. Connect. 2018, 7, E1–E4. [Google Scholar] [CrossRef] [PubMed]
- Nitert, M.D.; Barrett, H.L.; Foxcroft, K.; Tremellen, A.; Wilkinson, S.; Lingwood, B.; Tobin, J.M.; McSweeney, C.; O’Rourke, P.; McIntyre, H.D.; et al. SPRING: An RCT study of probiotics in the prevention of gestational diabetes mellitus in overweight and obese women. BMC Pregnancy Childbirth 2013, 13, 50. [Google Scholar] [CrossRef] [PubMed]
- Callaway, L.K.; McIntyre, H.D.; Barrett, H.L.; Foxcroft, K.; Tremellen, A.; Lingwood, B.E.; Tobin, J.M.; Wilkinson, S.; Kothari, A.; Morrison, M.; et al. Probiotics for the Prevention of Gestational Diabetes Mellitus in Overweight and Obese Women: Findings From the SPRING Double-blind Randomized Controlled Trial. Diabetes Care 2019, 42, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A.; Patterson, A.J.; Brown, W.J.; Ireland, P.; Giles, G. The Anti Cancer Council of Victoria FFQ: Relative validity of nutrient intakes compared with weighed food records in young to middle-aged women in a study of iron supplementation. Aust. N. Z. J. Public Health 2000, 24, 576–583. [Google Scholar] [CrossRef]
- Yu, Z.; Morrison, M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques 2004, 36, 808–812. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- McDonald, D.; Price, M.N.; Goodrich, J.; Nawrocki, E.P.; DeSantis, T.Z.; Probst, A.; Andersen, G.L.; Knight, R.; Hugenholtz, P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012, 6, 610–618. [Google Scholar] [CrossRef]
- Zakrzewski, M.; Proietti, C.; Ellis, J.J.; Hasan, S.; Brion, M.J.; Berger, B.; Krause, L. Calypso: A user-friendly web-server for mining and visualizing microbiome–environment interactions. Bioinformatics 2017, 33, 782–783. [Google Scholar] [CrossRef]
- Lowe, S.A.; Bowyer, L.; Lust, K.; McMahon, L.P.; Morton, M.; North, R.A.; Paech, M.; Said, J.M. SOMANZ guidelines for the management of hypertensive disorders of pregnancy 2014. Aust. N. Z. J. Obstet. Gynaecol. 2015, 55, e1–e29. [Google Scholar] [CrossRef]
- Konikoff, T.; Gophna, U. Oscillospira: A Central, Enigmatic Component of the Human Gut Microbiota. Trends Microbiol. 2016, 24, 523–524. [Google Scholar] [CrossRef] [PubMed]
- Schneider, E. ABC transporters catalyzing carbohydrate uptake. Res. Microbiol. 2001, 152, 303–310. [Google Scholar] [CrossRef]
- Pereira, M.A.; O’Reilly, E.; Augustsson, K.; Fraser, G.E.; Goldbourt, U.; Heitmann, B.L.; Hallmans, G.; Knekt, P.; Liu, S.; Pietinen, P.; et al. Dietary Fiber and Risk of Coronary Heart Disease: A Pooled Analysis of Cohort Studies. Arch. Intern. Med. 2004, 164, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Ao, H.; Peng, C. Gut Microbiota, Short-Chain Fatty Acids, and Herbal Medicines. Front. Pharm. 2018, 9, 1354. [Google Scholar] [CrossRef]
- Gilliland, S.E. Health and nutritional benefits from lactic acid bacteria. FEMS Microbiol. Rev. 1990, 7, 175–188. [Google Scholar] [CrossRef]
- Jefferson, A.; Adolphus, K. The Effects of Intact Cereal Grain Fibers, Including Wheat Bran on the Gut Microbiota Composition of Healthy Adults: A Systematic Review. Front. Nutr. 2019, 6, 33. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Sabater, M.; Ortega, F.; Ricart, W.; Fernández-Real, J.M. Circulating zonulin, a marker of intestinal permeability, is increased in association with obesity-associated insulin resistance. PLoS ONE 2012, 7, e37160. [Google Scholar] [CrossRef]
- Ajamian, M.; Steer, D.; Rosella, G.; Gibson, P.R. Serum zonulin as a marker of intestinal mucosal barrier function: May not be what it seems. PLoS ONE 2019, 14, e0210728. [Google Scholar] [CrossRef]
- Hu, G.X.; Chen, G.R.; Xu, H.; Ge, R.S.; Lin, J. Activation of the AMP activated protein kinase by short-chain fatty acids is the main mechanism underlying the beneficial effect of a high fiber diet on the metabolic syndrome. Med. Hypotheses 2010, 74, 123–126. [Google Scholar] [CrossRef]
- Capra, S. Nutrient Reference Values for Australia and New Zealand: Including Recommended Dietary Intakes; Commonwealth of Australia: Canberra, Australia, 2006. [Google Scholar]
| Fiber Condition | Hypertensive Disorders of Pregnancy | Normotensive | ||||
|---|---|---|---|---|---|---|
| Lower Fiber | Higher Fiber | p-Value | Lower Fiber | Higher Fiber | p-Value | |
| N | 10 | 12 | NA | 79 | 73 | NA |
| Maternal age (years) | 33 (1.34) | 38 (1.43) | 0.05 | 32 (0.55) | 33 (0.53) | 0.67 |
| Maternal BMI (kg/m2) | 34.9 (2.36) | 34.2 (1.28) | 0.77 | 34.9 (0.62) | 33.9 (0.67) | 0.31 |
| Excess weight gain (n) (Y/N/No Data) | 2/8/0 | 6/6/0 | 0.20 | 24/44/11 | 31/31/11 | 0.12 |
| Ethnicity | NA | 0.06 | ||||
| Caucasian n (%) | 90.0 | 91.7 | 86.1 | 90.4 | ||
| Indian n (%) | 0 | 0 | 1.3 | 0 | ||
| Asian n (%) | 0 | 8.3 | 7.6 | 0 | ||
| Other n (%) | 10.0 | 0 | 5.1 | 9.6 | ||
| SBP^ (mmHg) | 114 (2.10) | 115 (4.39) | 0.87 | 111 (1.02) | 109 (0.98) | 0.08 |
| DBP^ (mmHg) | 70 (2.34) | 70 (2.93) | 0.90 | 66 (0.86) | 65 (0.87) | 0.50 |
| Fasting glucose^ (mmol/L) | 4.3 (0.12) | 4.5 (0.07) | 0.24 | 4.3 (0.05) | 4.3 (0.05) | 0.81 |
| C-Peptide^ (nmol/L) | 1.1 (0.16) | 0.9 (0.08) | 0.39 | 0.9 (0.04) | 0.8 (0.04) | 0.03 |
| Insulin^ (mU/L) | 11.2 (1.22) | 10.0 (1.78) | 0.60 | 10.8 (0.68) | 8.7 (0.54) | 0.02 |
| Total cholesterol^ (mmol/L) | 6.9 (0.27) | 6.2 (0.43) | 0.21 | 6.6 (0.14) | 6.7 (0.12) | 0.34 |
| Circulating Triglycerides^ (mmol/L) | 2.6 (0.25) | 1.9 (0.23) | 0.08 | 2.1 (0.10) | 2.0 (0.08) | 0.48 |
| Daily Energy Intake^ (kJ) | 5351 (430) | 6880 (278) | 0.0088 | 5307 (128) | 8022 (221) | <0.0001 |
| Fiber intake^ (g/day) | 12.8 (0.87) | 22.8 (1.18) | <0.0001 | 13.6 (0.3) | 25.4 (0.8) | <0.0001 |
| Energy-corrected fiber intake^ (mg/kJ) | 2.0 (0.11) | 2.7 (0.20) | 0.005 | 2.6 (0.1) | 3.0 (0.1) | <0.0001 |
| Carbohydrates (g/day) | 122.7 (11.76) | 171.1 (6.31) | 0.0003 | 131.9 (3.4) | 215.1 (6.7) | <0.0001 |
| Starch (g/day) | 62.8 (7.54) | 86.3 (4.58) | 0.018 | 66.6 (2.1) | 116.6 (4.6) | <0.0001 |
| Protein (g/day) | 71.1 (6.74) | 80.0 (5.14) | 0.31 | 62.4 (2.0) | 99.4 (3.4) | <0.0001 |
| Total Fats (g/day) | 56.6 (5.72) | 71.6 (4.59) | 0.05 | 55.2 (1.6) | 88.1 (3.2) | <0.0001 |
| Saturated Fats (g/day) | 24.2 (2.47) | 30.8 (2.75) | 0.08 | 24.6 (0.8) | 38.4 (1.5) | <0.0001 |
| Fetal Sex (F/M) | 4/5/1 | 7/5/0 | 0.71 | 37/26 | 28/31 | 0.28 |
| Birth weight (g) | 3528 (91.37) | 3511 (129.7) | 0.91 | 3605 (82) | 3572 (64) | 0.75 |
| HDP No GDM | Normotensive | p-Value | |
|---|---|---|---|
| N | 22 | 152 | NA |
| Maternal age (years) | 36 (1.1) | 35 (0.4) | 0.62 |
| Maternal BMI (kg/m2) | 34.4 (1.3) | 34.4 (0.5) | 0.99 |
| Ethnicity | 0.94 | ||
| Caucasian n (%) | 20 (91) | 134 (88) | |
| Indian n (%) | 0 (0) | 1 (<1) | |
| Asian n (%) | 1 (4.5) | 6 (4) | |
| Other n (%) | 1 (4.5) | 11 (7) | |
| SBP (mmHg) | 114 (2.5) | 110 (0.7) | 0.04 |
| DBP (mmHg) | 70 (1.9) | 66 (0.6) | 0.01 |
| Fasting glucose (mmol/L) | 4.4 (0.1) | 4.3 (0.0) | 0.24 |
| C-Peptide (nmol/L) | 1.0 (0.1) | 0.9 (0.0) | 0.13 |
| Insulin (mU/L) | 11.2 (1.2) | 9.8 (0.4) | 0.26 |
| Total cholesterol (mmol/L) | 6.5 (0.3) | 6.6 (0.1) | 0.65 |
| Circulating Triglycerides (mmol/L) | 2.2 (0.2) | 2.1 (0.1) | 0.49 |
| Daily Energy Intake (kJ) | 6185 (293) | 6512 (166) | 0.46 |
| Fiber intake (g/day) | 18.3 (1.3) | 19.3 (0.6) | 0.58 |
| Energy-corrected fiber intake (mg/kJ) | 3.0 (0.2) | 2.8 (0.1) | 0.29 |
| Carbohydrates (g/day) | 149.1 (8.1) | 171.9 (5.0) | 0.09 |
| Starch (g/day) | 72.3 (4.9) | 90.59 (3.2) | 0.03 |
| Protein (g/day) | 75.9 (4.2) | 80.2 (2.5) | 0.52 |
| Total Fats (g/day) | 64.9 (3.9) | 71.67 (2.2) | 0.27 |
| Saturated Fats (g/day) | 27.8 (2.0) | 31.2 (1.0) | 0.21 |
| Fetal Sex (F/M) | 11/10 | 65/57 | >0.99 |
| Birth weight (g) | 3518 (82) | 3589 (52) | 0.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomsett, K.I.; Barrett, H.L.; Dekker, E.E.; Callaway, L.K.; McIntyre, D.H.; Dekker Nitert, M. Dietary Fiber Intake Alters Gut Microbiota Composition but Does Not Improve Gut Wall Barrier Function in Women with Future Hypertensive Disorders of Pregnancy. Nutrients 2020, 12, 3862. https://doi.org/10.3390/nu12123862
Tomsett KI, Barrett HL, Dekker EE, Callaway LK, McIntyre DH, Dekker Nitert M. Dietary Fiber Intake Alters Gut Microbiota Composition but Does Not Improve Gut Wall Barrier Function in Women with Future Hypertensive Disorders of Pregnancy. Nutrients. 2020; 12(12):3862. https://doi.org/10.3390/nu12123862
Chicago/Turabian StyleTomsett, Kate I., Helen L. Barrett, Evelyn E. Dekker, Leonie K. Callaway, David H. McIntyre, and Marloes Dekker Nitert. 2020. "Dietary Fiber Intake Alters Gut Microbiota Composition but Does Not Improve Gut Wall Barrier Function in Women with Future Hypertensive Disorders of Pregnancy" Nutrients 12, no. 12: 3862. https://doi.org/10.3390/nu12123862
APA StyleTomsett, K. I., Barrett, H. L., Dekker, E. E., Callaway, L. K., McIntyre, D. H., & Dekker Nitert, M. (2020). Dietary Fiber Intake Alters Gut Microbiota Composition but Does Not Improve Gut Wall Barrier Function in Women with Future Hypertensive Disorders of Pregnancy. Nutrients, 12(12), 3862. https://doi.org/10.3390/nu12123862






