Association between Geriatric Nutritional Risk Index and Mortality in Older Trauma Patients in the Intensive Care Unit
Abstract
1. Background
2. Methods
2.1. Ethics Statement
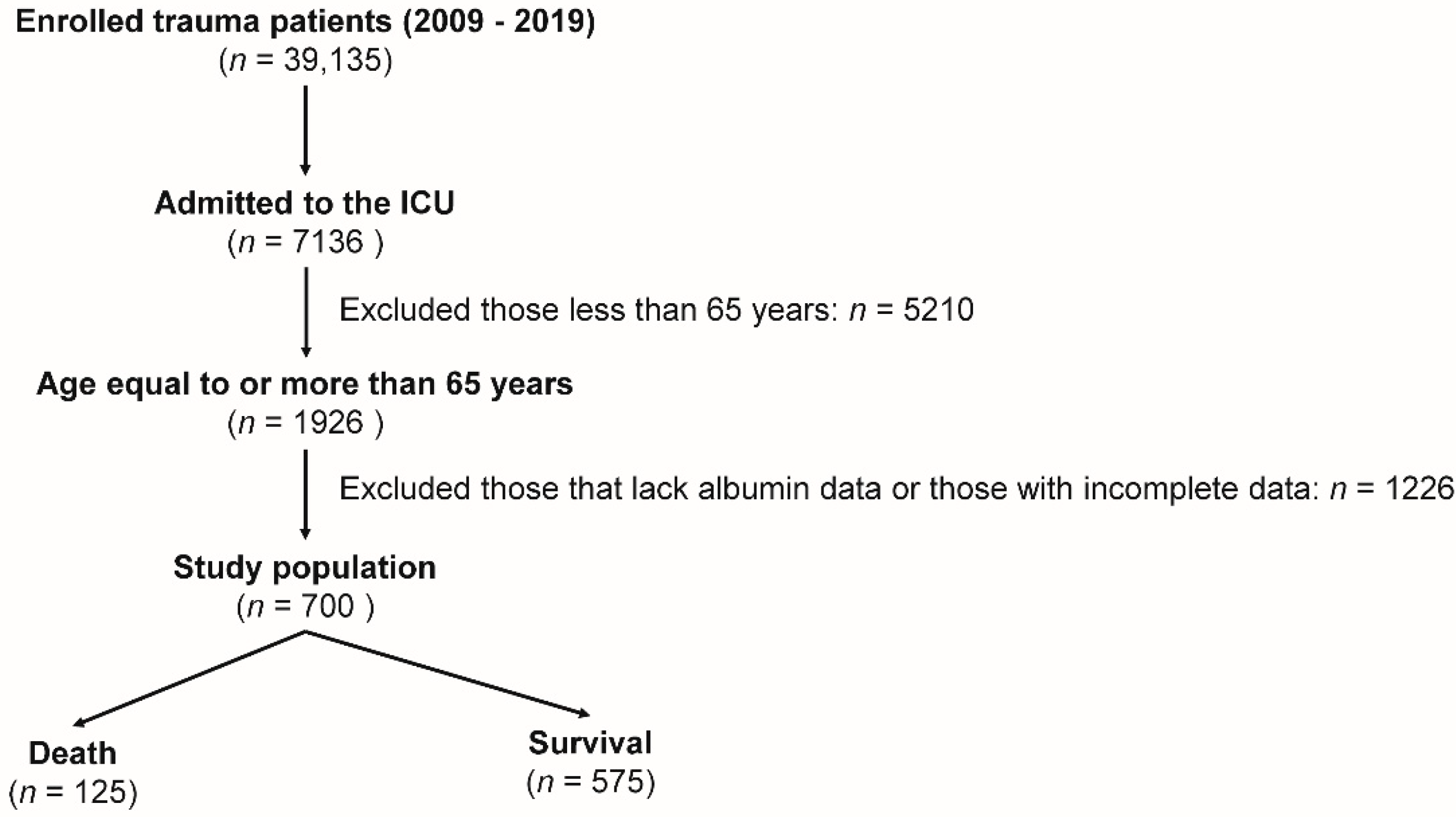
2.2. Study Population and Data Collection
2.3. Statistical Analyses
3. Results
3.1. Patient Demographics
3.2. Risk Factors for Mortality
3.3. Comparison of Patients with Low and High GNRI
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Amaral, T.F.; Matos, L.C.; Teixeira, M.A.; Tavares, M.M.; Alvares, L.; Antunes, A. Undernutrition and associated factors among hospitalized patients. Clin. Nutr. 2010, 29, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Cereda, E.; Pedrolli, C. The Geriatric Nutritional Risk Index. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gavazzi, G.; Krause, K.H. Ageing and infection. Lancet Infect. Dis. 2002, 2, 659–666. [Google Scholar] [CrossRef]
- Barker, L.A.; Gout, B.S.; Crowe, T.C. Hospital malnutrition: Prevalence, identification and impact on patients and the healthcare system. Int. J. Environ. Res. Public Health 2011, 8, 514–527. [Google Scholar] [CrossRef]
- Koekkoek, K.W.; van Zanten, A.R. Nutrition in the critically ill patient. Curr. Opin. Anaesthesiol. 2017, 30, 178–185. [Google Scholar] [CrossRef]
- White, J.V.; Guenter, P.; Jensen, G.; Malone, A.; Schofield, M. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: Characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J. Acad. Nutr. Diet. 2012, 112, 730–738. [Google Scholar] [CrossRef]
- Lew, C.C.H.; Yandell, R.; Fraser, R.J.L.; Chua, A.P.; Chong, M.F.F.; Miller, M. Association Between Malnutrition and Clinical Outcomes in the Intensive Care Unit: A Systematic Review [Formula: See text]. JPEN J. Parenter. Enter. Nutr. 2017, 41, 744–758. [Google Scholar] [CrossRef]
- Vargas, N.; Tibullo, L.; Landi, E.; Carifi, G.; Pirone, A.; Pippo, A.; Alviggi, I.; Tizzano, R.; Salsano, E.; Di Grezia, F.; et al. Caring for critically ill oldest old patients: A clinical review. Aging Clin. Exp. Res. 2017, 29, 833–845. [Google Scholar] [CrossRef]
- Rubinsky, M.D.; Clark, A.P. Early enteral nutrition in critically ill patients. Dimens. Crit. Care Nurs. DCCN 2012, 31, 267–274. [Google Scholar] [CrossRef]
- Durán Alert, P.; Milà Villarroel, R.; Formiga, F.; Virgili Casas, N.; Vilarasau Farré, C. Assessing risk screening methods of malnutrition in geriatric patients: Mini Nutritional Assessment (MNA) versus Geriatric Nutritional Risk Index (GNRI). Nutr. Hosp. 2012, 27, 590–598. [Google Scholar] [CrossRef]
- Cereda, E.; Pusani, C.; Limonta, D.; Vanotti, A. The ability of the Geriatric Nutritional Risk Index to assess the nutritional status and predict the outcome of home-care resident elderly: A comparison with the Mini Nutritional Assessment. Br. J. Nutr. 2009, 102, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Poziomyck, A.K.; Weston, A.C.; Lameu, E.B.; Cassol, O.S.; Coelho, L.J.; Moreira, L.F. Preoperative nutritional assessment and prognosis in patients with foregut tumors. Nutr. Cancer 2012, 64, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Buzby, G.P.; Knox, L.S.; Crosby, L.O.; Eisenberg, J.M.; Haakenson, C.M.; McNeal, G.E.; Page, C.P.; Peterson, O.L.; Reinhardt, G.F.; Williford, W.O. Study protocol: A randomized clinical trial of total parenteral nutrition in malnourished surgical patients. Am. J. Clin. Nutr. 1988, 47, 366–381. [Google Scholar] [CrossRef]
- Labossiere, R.; Bernard, M.A. Nutritional considerations in institutionalized elders. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Ito, Y.; Ishii, H.; Aoyama, T.; Kamoi, D.; Kasuga, H.; Yasuda, K.; Maruyama, S.; Matsuo, S.; Murohara, T.; et al. Geriatric nutritional risk index accurately predicts cardiovascular mortality in incident hemodialysis patients. J. Cardiol. 2014, 64, 32–36. [Google Scholar] [CrossRef]
- Detsky, A.S.; McLaughlin, J.R.; Baker, J.P.; Johnston, N.; Whittaker, S.; Mendelson, R.A.; Jeejeebhoy, K.N. What is subjective global assessment of nutritional status? JPEN J. Parenter. Enter. Nutr. 1987, 11, 8–13. [Google Scholar] [CrossRef]
- Kondrup, J.; Allison, S.P.; Elia, M.; Vellas, B.; Plauth, M. ESPEN guidelines for nutrition screening 2002. Clin. Nutr. 2003, 22, 415–421. [Google Scholar] [CrossRef]
- Zhao, Y.; Xia, X.; Xie, D.; Liao, Y.; Wang, Y.; Chen, L.; Ge, N.; Yue, J. Geriatric Nutritional Risk Index can predict postoperative delirium and hospital length of stay in elderly patients undergoing non-cardiac surgery. Geriatr. Gerontol. Int. 2020, 20, 759–764. [Google Scholar] [CrossRef]
- Gu, W.; Zhang, G.; Sun, L.; Ma, Q.; Cheng, Y.; Zhang, H.; Shi, G.; Zhu, Y.; Ye, D. Nutritional screening is strongly associated with overall survival in patients treated with targeted agents for metastatic renal cell carcinoma. J. Cachexia Sarcopenia Muscle 2015, 6, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Lidoriki, I.; Schizas, D.; Frountzas, M.; Machairas, N.; Prodromidou, A.; Kapelouzou, A.; Karavokyros, I.; Pikoulis, E.; Kales, S.N.; Liakakos, T. GNRI as a prognostic factor for outcomes in cancer patients: A systematic review of the literature. Nutr. Cancer 2020, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.Y.; An, L.; Sun, D.W. Geriatric nutritional risk index predicts adverse outcomes in human malignancy: A meta-analysis. Dis. Markers 2019, 2019, 4796598. [Google Scholar] [CrossRef] [PubMed]
- Kushiyama, S.; Sakurai, K.; Kubo, N.; Tamamori, Y.; Nishii, T.; Tachimori, A.; Inoue, T.; Maeda, K. The preoperative geriatric nutritional risk index predicts postoperative complications in elderly patients with gastric cancer undergoing gastrectomy. In Vivo 2018, 32, 1667–1672. [Google Scholar] [CrossRef]
- Lee, K.; Ahn, J.M.; Kang, D.Y.; Ko, E.; Kwon, O.; Lee, P.H.; Lee, S.W.; Kim, D.H.; Kim, H.J.; Kim, J.B.; et al. Nutritional status and risk of all-cause mortality in patients undergoing transcatheter aortic valve replacement assessment using the geriatric nutritional risk index and the controlling nutritional status score. Clin. Res. Cardiol. 2020, 109, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Mii, S.; Guntani, A.; Kawakubo, E.; Shimazoe, H.; Ishida, M. Impact of the geriatric nutritional risk index on the long-term outcomes of patients undergoing open bypass for intermittent claudication. Circ. J. 2019, 83, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Shibata, K.; Yamamoto, M.; Kano, S.; Koyama, Y.; Shimura, T.; Kagase, A.; Yamada, S.; Kobayashi, T.; Tada, N.; Naganuma, T.; et al. Importance of Geriatric Nutritional Risk Index assessment in patients undergoing transcatheter aortic valve replacement. Am. Heart J. 2018, 202, 68–75. [Google Scholar] [CrossRef]
- Kubo, N.; Sakurai, K.; Tamura, T.; Toyokawa, T.; Tanaka, H.; Muguruma, K.; Yashiro, M.; Ohira, M. The impact of geriatric nutritional risk index on surgical outcomes after esophagectomy in patients with esophageal cancer. Esophagus 2019, 16, 147–154. [Google Scholar] [CrossRef]
- Yoo, J.W.; Ju, S.; Lee, S.J.; Cho, Y.J.; Lee, J.D.; Kim, H.C. Geriatric nutritional risk index is associated with 30-day mortality in patients with acute respiratory distress syndrome. Medicine 2020, 99, e20671. [Google Scholar] [CrossRef]
- Hsieh, C.H.; Hsu, S.Y.; Hsieh, H.Y.; Chen, Y.C. Differences between the sexes in motorcycle-related injuries and fatalities at a Taiwanese level I trauma center. Biomed. J. 2017, 40, 113–120. [Google Scholar] [CrossRef]
- Hsieh, C.H.; Liu, H.T.; Hsu, S.Y.; Hsieh, H.Y.; Chen, Y.C. Motorcycle-related hospitalizations of the elderly. Biomed. J. 2017, 40, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.H.; Chen, Y.C.; Hsu, S.Y.; Hsieh, H.Y.; Chien, P.C. Defining polytrauma by abbreviated injury scale >/= 3 for a least two body regions is insufficient in terms of short-term outcome: A cross-sectional study at a level I trauma center. Biomed. J. 2018, 41, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Loftis, K.L.; Price, J.; Gillich, P.J. Evolution of the Abbreviated Injury Scale: 1990–2015. Traffic Inj. Prev. 2018, 19, S109–S113. [Google Scholar] [CrossRef] [PubMed]
- Committee on Medical Aspects of Automotive Safety. Rating the severity of tissue damage. I. The abbreviated scale. JAMA 1971, 215, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.Y.; Wu, S.C.; Rau, C.S.; Hsieh, T.M.; Liu, H.T.; Huang, C.Y.; Chou, S.E.; Su, W.T.; Hsieh, C.H. Impact of Adapting the Abbreviated Injury Scale (AIS)-2005 from AIS-1998 on injury severity scores and clinical outcome. Int. J. Environ. Res. Public Health 2019, 16, 5033. [Google Scholar] [CrossRef] [PubMed]
- Aharonson-Daniel, L.; Giveon, A.; Stein, M.; Israel Trauma, G.; Peleg, K. Different AIS triplets: Different mortality predictions in identical ISS and NISS. J. Trauma 2006, 61, 711–717. [Google Scholar] [CrossRef]
- Dong, X.R. Analysis of patients of multiple injuries with AIS-ISS and its clinical significance in the evaluation of the emergency managements. Zhonghua Wai Ke Za Zhi 1993, 31, 301–302. [Google Scholar]
- Cereda, E.; Vanotti, A. The new Geriatric Nutritional Risk Index is a good predictor of muscle dysfunction in institutionalized older patients. Clin. Nutr. 2007, 26, 78–83. [Google Scholar] [CrossRef]
- Han, J.Y.; Lee, K.H.; Kim, S.W.; Min, Y.J.; Cho, E.; Lee, Y.; Lee, S.H.; Kim, H.Y.; Lee, G.K.; Nam, B.H.; et al. A phase II study of poziotinib in patients with epidermal growth factor receptor (EGFR)-mutant lung adenocarcinoma who have acquired resistance to EGFR-tyrosine kinase inhibitors. Cancer Res. Treat. 2017, 49, 10–19. [Google Scholar] [CrossRef][Green Version]
- Artinyan, A.; Orcutt, S.T.; Anaya, D.A.; Richardson, P.; Chen, G.J.; Berger, D.H. Infectious postoperative complications decrease long-term survival in patients undergoing curative surgery for colorectal cancer: A study of 12,075 patients. Ann. Surg. 2015, 261, 497–505. [Google Scholar] [CrossRef]
- Nathan, H.; Yin, H.; Wong, S.L. Postoperative complications and long-term survival after complex cancer resection. Ann. Surg. Oncol. 2017, 24, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Choi, H.S.; Ko, Y.G.; Yun, D.H. Performance of the Geriatric Nutritional Risk Index in predicting 28-day hospital mortality in older adult patients with sepsis. Clin. Nutr. 2013, 32, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Fruchtenicht, A.V.; Poziomyck, A.K.; Kabke, G.B.; Loss, S.H.; Antoniazzi, J.L.; Steemburgo, T.; Moreira, L.F. Nutritional risk assessment in critically ill cancer patients: Systematic review. Rev. Bras. Ter. Intensiva 2015, 27, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.; et al. ESPEN guideline: Clinical nutrition in surgery. Clin. Nutr. 2017, 36, 623–650. [Google Scholar] [CrossRef]
- Caillet, P.; Liuu, E.; Raynaud Simon, A.; Bonnefoy, M.; Guerin, O.; Berrut, G.; Lesourd, B.; Jeandel, C.; Ferry, M.; Rolland, Y.; et al. Association between cachexia, chemotherapy and outcomes in older cancer patients: A systematic review. Clin. Nutr. 2017, 36, 1473–1482. [Google Scholar] [CrossRef]
| Variables | Death n = 125 | Survival n = 575 | p-Value |
|---|---|---|---|
| Gender | 0.696 | ||
| Male, n (%) | 79 (63.2) | 374 (65.0) | |
| Female, n (%) | 46 (36.8) | 201 (35.0) | |
| Age (years) | 76.4 ± 7.5 | 75.7 ± 7.0 | 0.386 |
| BMI | 23.6 ± 4.5 | 23.4 ± 4.0 | 0.604 |
| Albumin (g/dl) | 3.0 ± 0.8 | 3.3 ± 0.6 | <0.001 |
| GNRI | 89.8 ± 12.9 | 94.2 ± 12.0 | <0.001 |
| Co-morbidities | |||
| DM, n (%) | 39 (31.2) | 165 (28.7) | 0.577 |
| HTN, n (%) | 59 (47.2) | 333 (57.9) | 0.029 |
| CAD, n (%) | 22 (17.6) | 83 (14.4) | 0.369 |
| CHF, n (%) | 4 (3.2) | 12 (2.1) | 0.450 |
| CVA, n (%) | 12 (9.6) | 58 (10.1) | 0.869 |
| ESRD, n (%) | 13 (10.4) | 24 (4.2) | 0.005 |
| COPD, n (%) | 4 (3.2) | 19 (3.3) | 0.953 |
| ISS, median (IQR) | 25 (16–29) | 16 (13–25) | <0.001 |
| 1–15, n (%) | 12 (9.6) | 158 (27.5) | <0.001 |
| 16–24, n (%) | 44 (35.2) | 267 (46.4) | 0.022 |
| ≥25, n (%) | 69 (55.2) | 150 (26.1) | <0.001 |
| LOS in hospital (days) | 19.7 ± 20.9 | 24.8 ± 18.4 | 0.006 |
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Gender | 0.9 (0.62–1.38) | 0.696 | 0.9 (0.57–1.33) | 0.511 |
| Age | 1.0 (0.99–1.04) | 0.385 | 1.0 (0.99–1.05) | 0.129 |
| GNRI | 0.97 (0.95–0.99) | <0.001 | 0.97 (0.95–0.99) | 0.001 |
| HTN | 0.7 (0.44–0.96) | 0.029 | 0.7 (0.47–1.09) | 0.120 |
| ESRD | 2.7 (1.32–5.39) | 0.006 | 3.6 (1.70–7.67) | 0.001 |
| ISS | 1.1 (1.05–1.10) | <0.001 | 1.1 (1.05–1.10) | <0.001 |
| GNRI: Variables | <82 n = 128 | 82 to <92 n = 191 | 92 to ≤98 n = 136 | >98 n = 245 | p-Value |
|---|---|---|---|---|---|
| Gender | 0.862 | ||||
| Male, n (%) | 86(67.2) | 124(64.9) | 89(65.4) | 154(62.9) | |
| Female, n (%) | 42(32.8) | 67(35.1) | 47(34.6) | 91(37.1) | |
| Age (years) | 78.2 ± 7.5 * | 75.9 ± 7.5 | 76.1 ± 6.3 | 74.4 ± 6.7 | <0.001 |
| BMI | 19.9 ± 2.7 * | 21.7 ± 2.9 * | 23.6 ± 2.9 * | 26.5 ± 3.7 | <0.001 |
| Albumin (g/dl) | 2.5 ± 0.6 * | 3.0 ± 0.5 * | 3.3 ± 0.5 * | 3.8 ± 0.5 | <0.001 |
| Co-morbidities | |||||
| DM, n (%) | 31(24.2) | 51(26.7) | 39(28.7) | 83(33.9) | 0.193 |
| HTN, n (%) | 57(44.5) * | 99(51.8) * | 79(58.1) | 157(64.1) | 0.002 |
| CAD, n (%) | 14(10.9) | 30(15.7) | 25(18.4) | 36(14.7) | 0.396 |
| CHF, n (%) | 3(2.3) | 5(2.6) | 5(3.7) | 3(1.2) | 0.474 |
| CVA, n (%) | 9(7.0) | 19(9.9) | 18(13.2) | 24(9.8) | 0.416 |
| ESRD, n (%) | 4(31.1) | 12(6.3) | 8(5.9) | 13(5.3) | 0.644 |
| COPD, n (%) | 6(4.7) | 6(3.1) | 5(3.7) | 6(2.4) | 0.703 |
| ISS, median (IQR) | 17(16–25) | 17(16–25) | 17(16–25) | 16(13–25) | 0.345 |
| 1–15, n (%) | 31(24.2) | 39(20.4) | 28(20.6) | 72(29.4) | 0.110 |
| 16–24, n (%) | 53(41.4) | 92(48.2) | 64(47.1) | 102(41.6) | 0.438 |
| ≥ 25, n (%) | 44(34.4) | 60(31.4) | 44(32.4) | 71(29.0) | 0.742 |
| Mortality, n (%) | 34(26.6) * | 37(19.4) | 22(16.2) | 32(13.1) | 0.012 |
| LOS in hospital (days) | 26.5 ± 21.6 * | 25.6 ± 18.7 | 24.4 ± 20.2 | 20.9 ± 16.7 | 0.020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.-T.; Wu, S.-C.; Tsai, C.-H.; Li, C.; Chou, S.-E.; Su, W.-T.; Hsu, S.-Y.; Hsieh, C.-H. Association between Geriatric Nutritional Risk Index and Mortality in Older Trauma Patients in the Intensive Care Unit. Nutrients 2020, 12, 3861. https://doi.org/10.3390/nu12123861
Liu H-T, Wu S-C, Tsai C-H, Li C, Chou S-E, Su W-T, Hsu S-Y, Hsieh C-H. Association between Geriatric Nutritional Risk Index and Mortality in Older Trauma Patients in the Intensive Care Unit. Nutrients. 2020; 12(12):3861. https://doi.org/10.3390/nu12123861
Chicago/Turabian StyleLiu, Hang-Tsung, Shao-Chun Wu, Ching-Hua Tsai, Chi Li, Sheng-En Chou, Wei-Ti Su, Shiun-Yuan Hsu, and Ching-Hua Hsieh. 2020. "Association between Geriatric Nutritional Risk Index and Mortality in Older Trauma Patients in the Intensive Care Unit" Nutrients 12, no. 12: 3861. https://doi.org/10.3390/nu12123861
APA StyleLiu, H.-T., Wu, S.-C., Tsai, C.-H., Li, C., Chou, S.-E., Su, W.-T., Hsu, S.-Y., & Hsieh, C.-H. (2020). Association between Geriatric Nutritional Risk Index and Mortality in Older Trauma Patients in the Intensive Care Unit. Nutrients, 12(12), 3861. https://doi.org/10.3390/nu12123861







