Milk Containing A2 β-Casein ONLY, as a Single Meal, Causes Fewer Symptoms of Lactose Intolerance than Milk Containing A1 and A2 β-Caseins in Subjects with Lactose Maldigestion and Intolerance: A Randomized, Double-Blind, Crossover Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subject Selection and Inclusion Criteria
2.2. Exclusion Criteria
2.3. Interventions
2.4. A1/A2 Analysis
2.5. Sugar, Protein, and Fat Analyses
2.6. Study Procedures
2.7. Study Endpoints
2.8. Study Ethics
2.9. Statistical Analyses
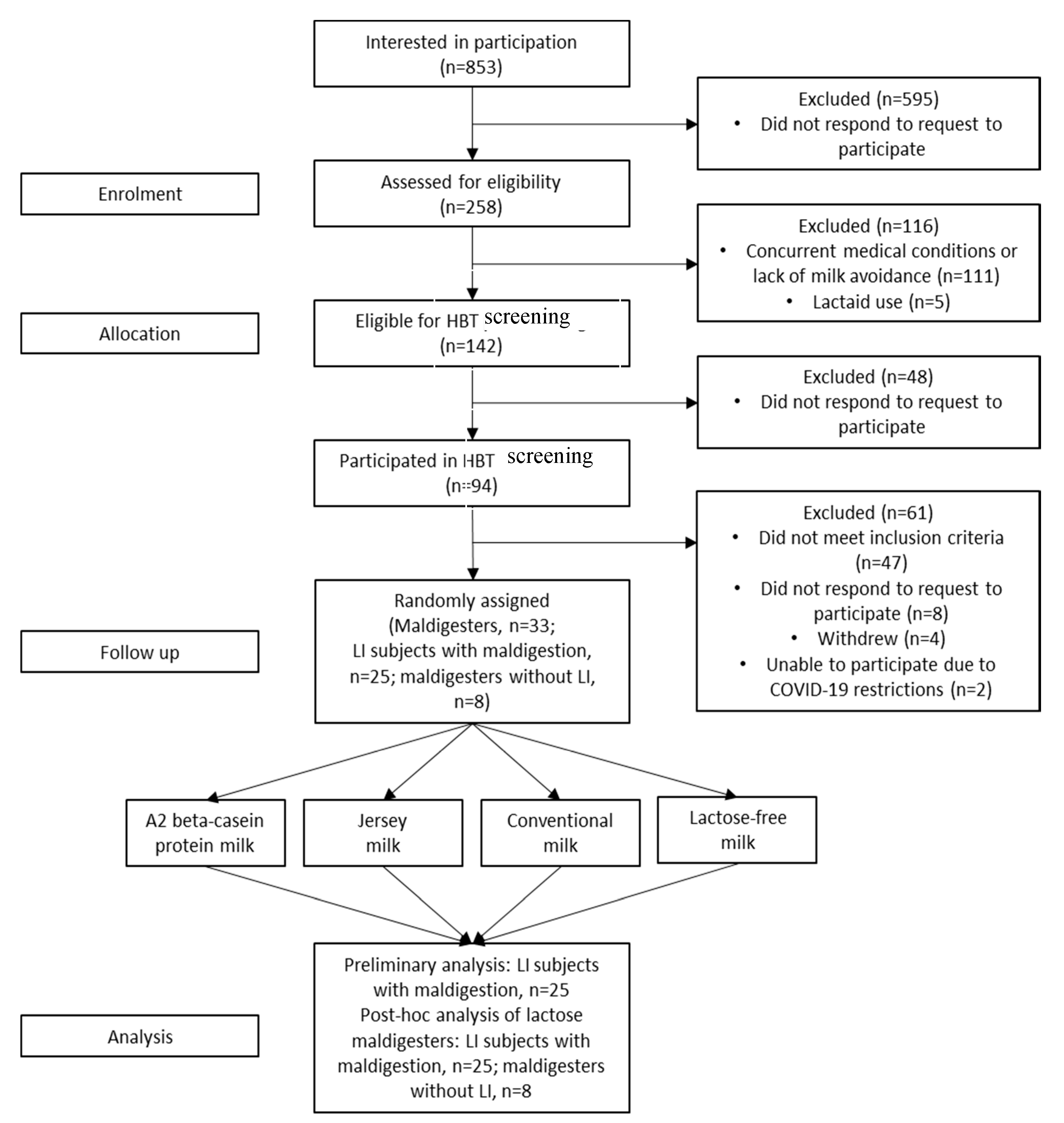
3. Results
3.1. Baseline and Demographic Characteristics
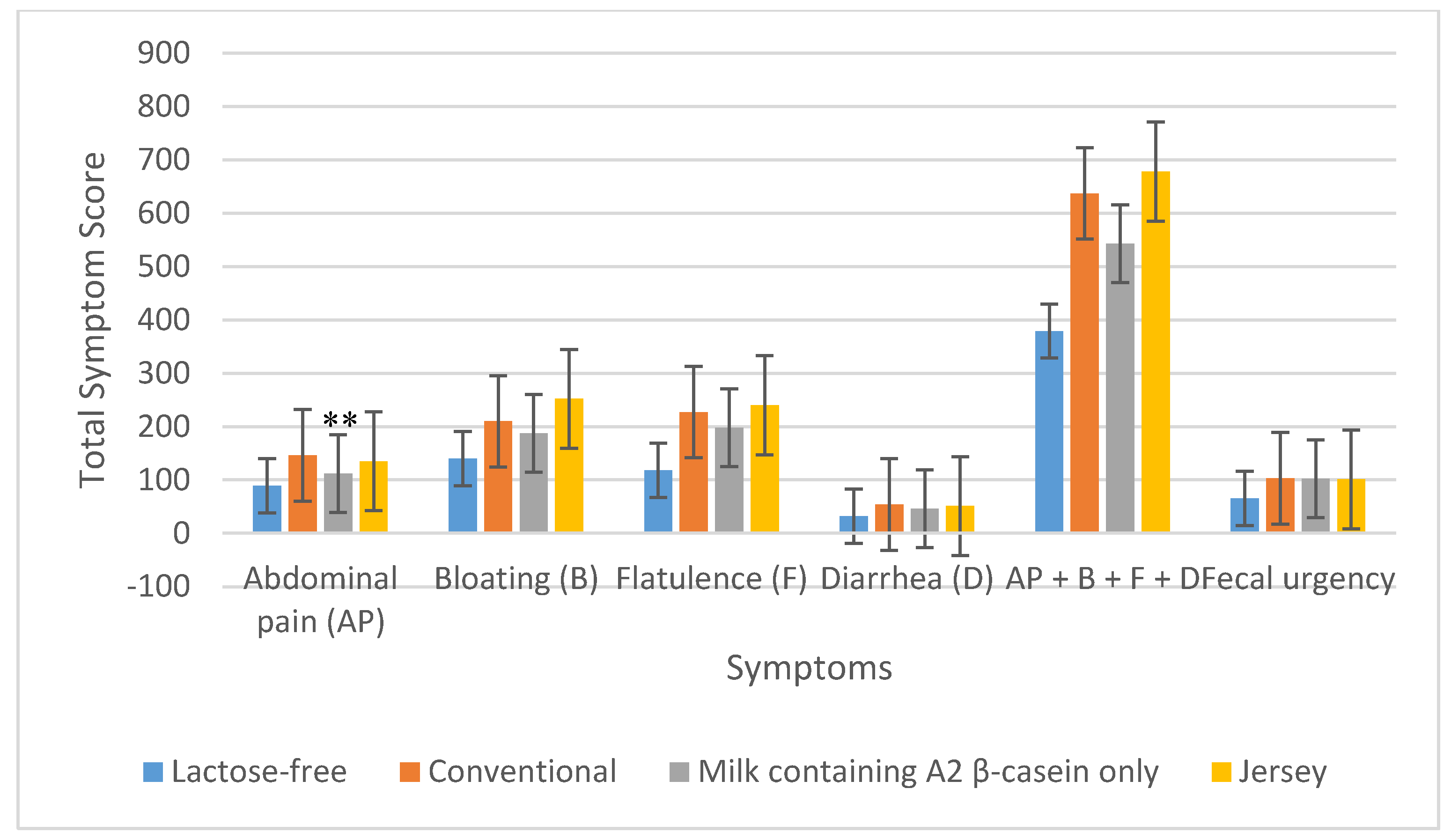
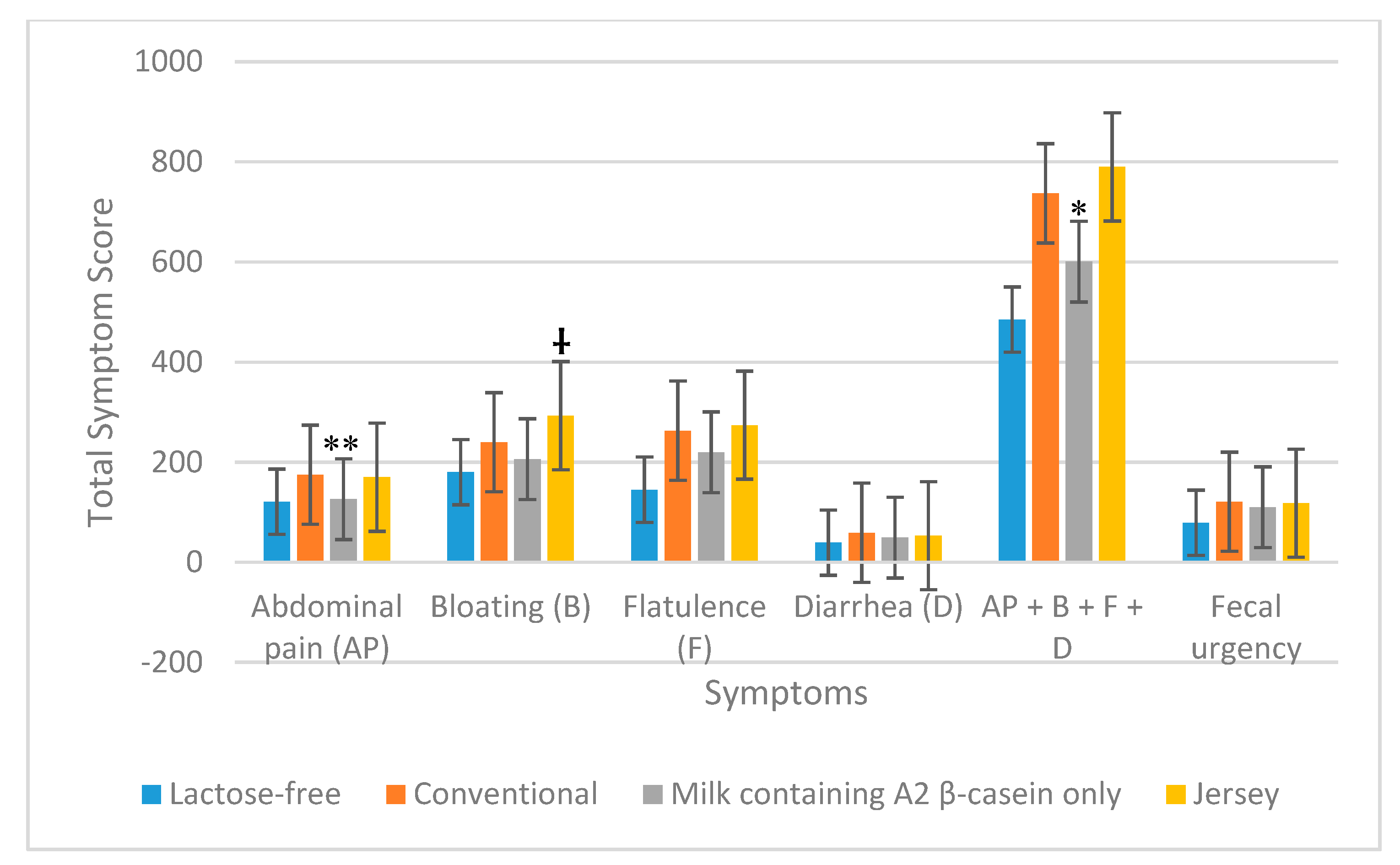
3.2. GI Symptoms
3.2.1. LI Subjects
3.2.2. All Maldigesters
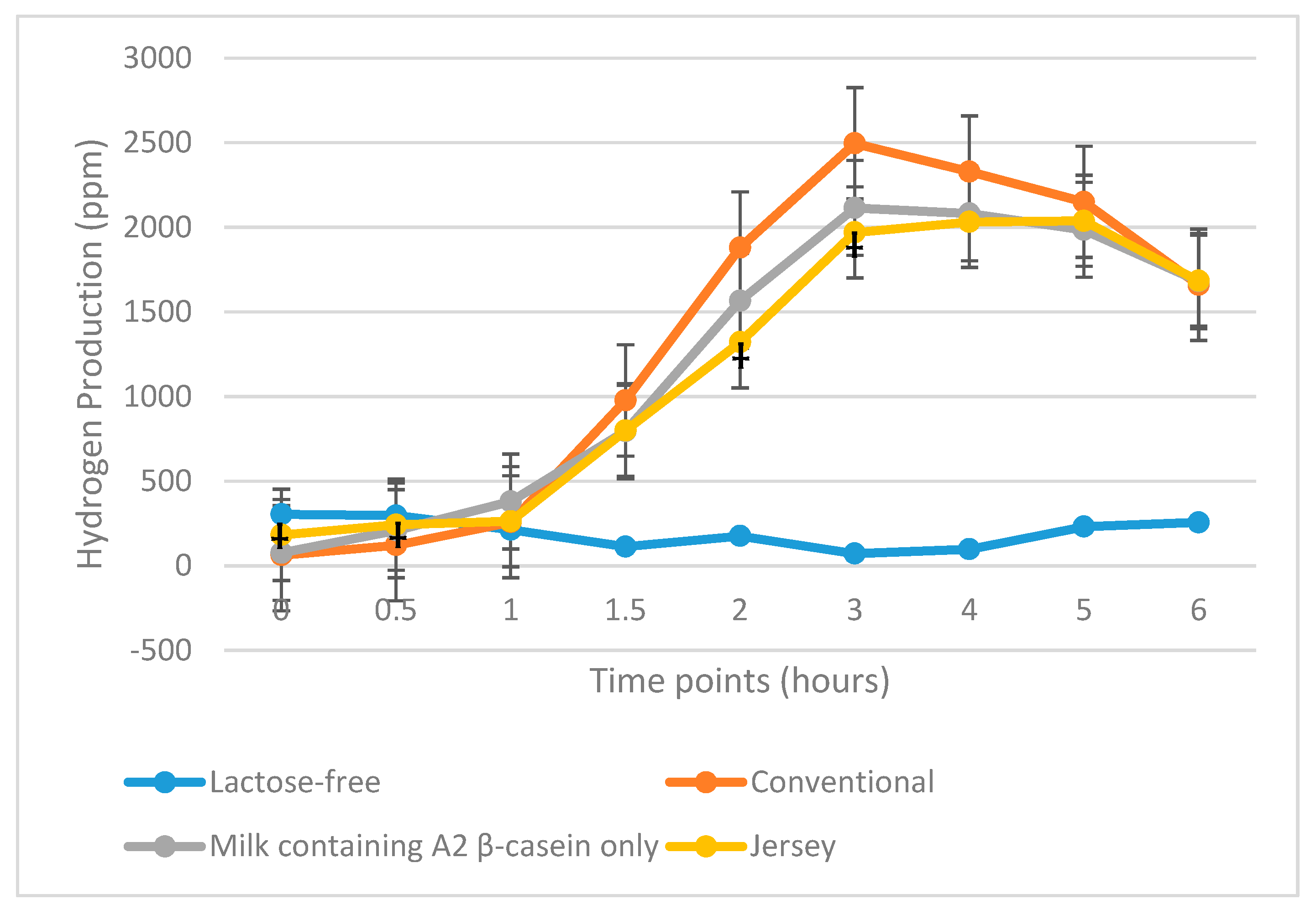
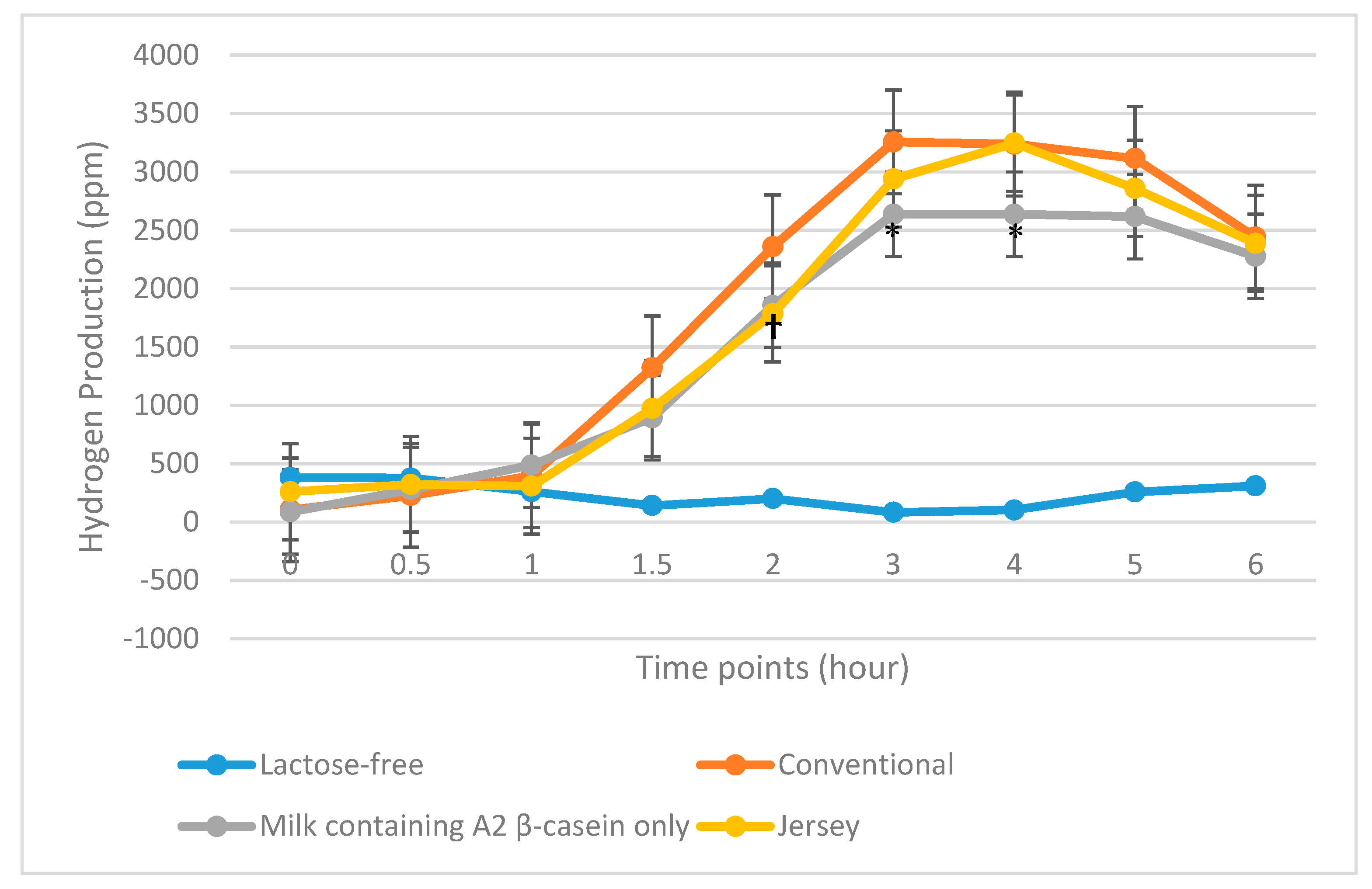
3.3. HBT Results
3.3.1. LI Subjects
3.3.2. All Maldigesters
3.4. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Inclusion and Exclusion Criteria for Phone Screening
- Ability/desire to provide informed consent
- Aged 18–65 years at screening
- Current or recent history of intolerance to and avoidance of dairy for at least 1 mo (by self-report and self-reported symptoms).
- Agreement to refrain from all other treatments and products used for dairy intolerance (e.g., Lactaid® dietary supplements; McNeil Nutritionals, LLC, Ft. Washington, PA, USA) during study involvement
- Willing to return for all study visits and complete all study related procedures, including fasting before and during the hydrogen breath tests (HBTs)
- Qualifying Lactose Challenge Symptom Score. Four symptom categories with severity measured from 0–5, as defined by one of the following:
- At least one score of “moderately severe” or “severe” on a single symptom during the 6 h HBT
- A score of “moderate” or greater for a single symptom at least two timepoints during the 6 h HBT
- At least one “moderate” score or greater for each of two symptoms during the 6 h HBT
- Able to understand and provide written informed consent in English.
- Allergic to milk
- Currently pregnant
- Currently lactating
- Cigarette smoking, or other use of tobacco or nicotine-containing products within 3 mo of screening
- Diagnosed with any of the following disorders known to be associated with abnormal gastrointestinal (GI) motility: gastroparesis, amyloidosis, neuromuscular diseases (including Parkinson’s disease), collagen vascular diseases, alcoholism, uremia, malnutrition, or untreated hypothyroidism
- History of surgery that alters normal GI tract function, including but not limited to: GI bypass surgery, bariatric surgery, gastric banding, vagotomy, fundoplication, pyloroplasty (N.B. history of uncomplicated abdominal surgeries such as removal of an appendix >12 months prior to screening will not be excluded)
- Past or present: organ transplant, chronic pancreatitis, pancreatic insufficiency, symptomatic biliary disease, celiac disease, chronic constipation, diverticulosis, inflammatory bowel disease, ulcerative colitis, Crohn’s disease, small intestine bacterial overgrowth syndrome, gastroparesis, gastro-esophageal reflux disease, irritable bowel syndrome, or any other medical condition with symptoms that could confound collection of adverse events
- Active ulcers, or history of severe ulcers
- Diabetes mellitus (type 1 and type 2)
- Congestive heart failure
- Human immunodeficiency virus, hepatitis B, or hepatitis C
- Body mass index > 35 kg/m2
- Recent bowel preparation for endoscopic or radiologic investigation within 4 weeks of screening (e.g., colonoscopy preparation)
- Use of concurrent therapy(ies) or other products (e.g., laxatives, stool softeners, Pepto Bismol®, Lactaid® dietary supplements) used for symptoms of dairy intolerance within 7 days of screening
- Chronic antacid and/or proton pump inhibitor use
- Recent use of systemic antibiotics, defined as use within 30 days prior to screening
- Recent high colonic enema, defined as use within 30 days prior to screening
- Any concurrent disease or symptoms that may interfere with assessment of the cardinal symptoms of dairy intolerance (i.e., gas, diarrhea, bloating, cramps, stomach pain)
- History of ethanol (alcohol) and/or drug abuse in the past 12 months
- Currently undergoing chemotherapy
- Use of any investigational drug or participation in any investigational study within 30 days prior to screening
- Prior enrollment in this study
- Any other conditions/issues noted by the study staff and/or Principal Investigator that would impact participation and/or protocol compliance.
References
- Phelan, M.; Aherne, A.; Fitzgerald, R.J.; O’Brien, N.M. Casein-derived bioactive peptides: Biological effects, industrial uses, safety aspects and regulatory status. Int. Dairy J. 2009, 19, 643–654. [Google Scholar] [CrossRef]
- Formaggioni, P.; Summer, A.; Malacarne, M.; Mariani, P. Milk protein polymorphism: Detection and diffusion of the genetic variants in Bos genus. Ann. Fac. Med. Vet. Univ. Parma 1999, 19, 127–165. [Google Scholar] [CrossRef] [Green Version]
- Ng-Kwai-Hang, K.F.; Grosclaude, F. Genetic polymorphism of milk proteins. In Advanced Dairy Chemistry—1 Proteins; Fox, P.F., McSweeney, P.L.H., Eds.; Springer: Boston, MA, USA, 2003; pp. 739–816. [Google Scholar] [CrossRef]
- Bradley, D.G.; MacHugh, D.E.; Cunningham, P.; Loftus, R.T. Mitochondrial diversity and the origins of African and European cattle. Proc. Natl. Acad. Sci. USA 1996, 93, 5131–5135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacHugh, D.E.; Shriver, M.D.; Loftus, R.T.; Cunningham, P.; Bradley, D.G. Microsatellite DNA variation and the evolution, domestication and phylogeography of taurine and zebu cattle (Bos taurus and Bos indicus). Genetics 1997, 146, 1071–1086. [Google Scholar] [PubMed]
- De Noni, I. Release of β-casomorphins 5 and 7 during simulated gastrointestinal digestion of bovine β-casein variants and milk-based infant formulas. Food Chem. 2008, 110, 897–903. [Google Scholar] [CrossRef]
- De Noni, I.; Cattaneo, S. Occurrence of β-casomorphins 5 and 7 in commercial dairy products and in their digests following in vitro simulated gastrointestinal digestion. Food Chem. 2010, 119, 560–566. [Google Scholar] [CrossRef]
- Jinsmaa, Y.; Yoshikawa, M. Enzymatic release of neocasomorphin and beta-casomorphin from bovine beta-casein. Peptides 1999, 20, 957–962. [Google Scholar] [CrossRef]
- Ul Haq, M.R.; Kapila, R.; Kapila, S. Release of beta-casomorphin-7/5 during simulated gastrointestinal digestion of milk beta-casein variants from Indian crossbred cattle (Karan Fries). Food Chem. 2015, 168, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Boutrou, R.; Gaudichon, C.; Dupont, D.; Jardin, J.; Airinei, G.; Marsset-Baglieri, A.; Benamouzig, R.; Tome, D.; Leonil, J. Sequential release of milk protein-derived bioactive peptides in the jejunum in healthy humans. Am. J. Clin. Nutr. 2013, 97, 1314–1323. [Google Scholar] [CrossRef] [Green Version]
- Ul Haq, M.R.; Kapila, R.; Shandilya, U.K.; Kapila, S. Impact of milk derived β-casomorphins on physiological functions and trends in research: A review. Int. J. Food Prop. 2014, 17, 1726–1741. [Google Scholar] [CrossRef] [Green Version]
- Brooke-Taylor, S.; Dwyer, K.; Woodford, K.; Kost, N. Systematic review of the gastrointestinal effects of A1 compared with A2 beta-casein. Adv. Nutr. 2017, 8, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Deth, R.; Clarke, A.; Ni, J.; Trivedi, M. Clinical evaluation of glutathione concentrations after consumption of milk containing different subtypes of beta-casein: Results from a randomized, cross-over clinical trial. Nutr. J. 2016, 15, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pizzorno, J. Glutathione! Integr. Med. (Encinitas) 2014, 13, 8–12. [Google Scholar] [PubMed]
- He, M.; Sun, J.; Jiang, Z.Q.; Yang, Y.X. Effects of cow’s milk beta-casein variants on symptoms of milk intolerance in Chinese adults: A multicentre, randomised controlled study. Nutr. J. 2017, 16, 72. [Google Scholar] [CrossRef] [Green Version]
- Ho, S.; Woodford, K.; Kukuljan, S.; Pal, S. Comparative effects of A1 versus A2 beta-casein on gastrointestinal measures: A blinded randomised cross-over pilot study. Eur. J. Clin. Nutr. 2014, 68, 994–1000. [Google Scholar] [CrossRef]
- Jianqin, S.; Leiming, X.; Lu, X.; Yelland, G.W.; Ni, J.; Clarke, A.J. Effects of milk containing only A2 beta casein versus milk containing both A1 and A2 beta casein proteins on gastrointestinal physiology, symptoms of discomfort, and cognitive behavior of people with self-reported intolerance to traditional cows’ milk. Nutr. J. 2015, 15, 35. [Google Scholar] [CrossRef] [Green Version]
- Sheng, X.; Li, Z.; Ni, J.; Yelland, G. Effects of conventional milk versus milk containing only A2 beta-casein on digestion in Chinese children: A randomized study. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 375–382. [Google Scholar] [CrossRef]
- Suarez, F.L.; Savaiano, D.A.; Levitt, M.D. A comparison of symptoms after the consumption of milk or lactose-hydrolyzed milk by people with self-reported severe lactose intolerance. N. Engl. J. Med. 1995, 333, 1–4. [Google Scholar] [CrossRef]
- Milan, A.M.; Shrestha, A.; Karlström, H.J.; Martinsson, J.A.; Nilsson, N.J.; Perry, J.K.; Day, L.; Barnett, M.P.G.; Cameron-Smith, D. Comparison of the impact of bovine milk β-casein variants on digestive comfort in females self-reporting dairy intolerance: A randomized controlled trial. Am. J. Clin. Nutr. 2020, 111, 149–160. [Google Scholar] [CrossRef]
- Misselwitz, B.; Butter, M.; Verbeke, K.; Fox, M.R. Update on lactose malabsorption and intolerance: Pathogenesis, diagnosis and clinical management. Gut 2019, 68, 2080–2091. [Google Scholar] [CrossRef] [Green Version]
- Di Costanzo, M.; Berni Canani, R. Lactose Intolerance: Common misunderstandings. Ann. Nutr. Metab. 2018, 73 (Suppl. 4), 30–37. [Google Scholar] [CrossRef]
- Hertzler, S.R.; Savaiano, D.A. Colonic adaptation to daily lactose feeding in lactose maldigesters reduces lactose intolerance. Am. J. Clin. Nutr. 1996, 64, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Savaiano, D.A.; Ritter, A.J.; Klaenhammer, T.R.; James, G.M.; Longcore, A.T.; Chandler, J.R.; Walker, W.A.; Foyt, H.L. Improving lactose digestion and symptoms of lactose intolerance with a novel galacto-oligosaccharide (RP-G28): A randomized, double-blind clinical trial. Nutr. J. 2013, 12, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezaie, A.; Buresi, M.; Lembo, A.; Lin, H.; McCallum, R.; Rao, S.; Schmulson, M.; Valdovinos, M.; Zakko, S.; Pimentel, M. Hydrogen and methane-based breath testing in gastrointestinal disorders: The North American consensus. Am. J. Gastroenterol. 2017, 112, 775–784. [Google Scholar] [CrossRef] [Green Version]
- Wilt, T.J.; Shaukat, A.; Shamliyan, T.; Taylor, B.C.; MacDonald, R.; Tacklind, J.; Rutks, I.; Schwarzenberg, S.J.; Kane, R.L.; Levitt, M. Lactose intolerance and health. Evid. Rep. Technol. Assess (Full Rep.) 2010, 192, 410. [Google Scholar]
- AOAC International. Official Methods of Analysis of AOAC International, 18th ed.; Methods 989.05, 932.05, 986.25, 945.48B, 968.06 and 992.15; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Mason, B.S.; Slover, H.T. A Gas chromatographic method for the determination of sugars in foods. J. Agric. Food Chem. 1971, 19, 551–554. [Google Scholar] [CrossRef]
- Brobst, K.M. Gas-Liquid Chromatography of Trimethylsilyl Derivatives, Methods in Carbohydrate Chemistry; Academic Press: New York, NY, USA, 1972; Volume 6, pp. 3–8. [Google Scholar] [CrossRef]
- Barnett, M.P.; McNabb, W.C.; Roy, N.C.; Woodford, K.B.; Clarke, A.J. Dietary A1 beta-casein affects gastrointestinal transit time, dipeptidyl peptidase-4 activity, and inflammatory status relative to A2 beta-casein in Wistar rats. Int. J. Food Sci. Nutr. 2014, 65, 720–727. [Google Scholar] [CrossRef]
- Trivedi, M.S.; Shah, J.S.; Al-Mughairy, S.; Hodgson, N.W.; Simms, B.; Trooskens, G.A.; Van Criekinge, W.; Deth, R.C. Food-derived opioid peptides inhibit cysteine uptake with redox and epigenetic consequences. J. Nutr. Biochem. 2014, 25, 1011–1018. [Google Scholar] [CrossRef] [Green Version]
- Yadav, S.; Yadav, N.D.S.; Gheware, A.; Kulshreshtha, A.; Sharma, P.; Singh, V.P. Oral feeding of cow milk containing A1 variant of β casein induces pulmonary inflammation in male Balb/c mice. Sci. Rep. 2020, 10, 8053. [Google Scholar] [CrossRef]
| Age, mean (range); years | 25 (19–50) |
| Bodyweight, mean (range); kg | 71 |
| Height, mean (range); cm | 170 |
| BMI, mean (range); kg/m2 | 24 |
| Male/female, n/n | 15/18 |
| Lactose intolerant maldigesters (meeting QLCSS; hydrogen > 20 ppm), n | 25 |
| Lactose tolerant maldigesters (hydrogen ≤ 20 ppm), n | 8 |
| Race, n | |
| Asian | 14 |
| African American | 4 |
| Caucasian | 14 |
| American Indian | 1 |
| Ethnicity, n | |
| Hispanic | 5 |
| Non-Hispanic | 26 |
| Unknown | 2 |
| Nutrient | Milk Containing A2 β-Casein Only | Jersey Milk | Conventional Milk | Lactose-Free Milk |
|---|---|---|---|---|
| Protein (g/serving) | 3.14 | 3.95 | 3.30 | 3.21 |
| Fat (g/serving) | 2.10 | 2.00 | 1.90 | 2.00 |
| Lactose (g/serving) | 4.70 | 4.40 | 4.60 | 0.13 |
| Carbohydrate (g/serving) | 4.70 | 4.40 | 4.60 | N/A |
| Calories (kcal/serving) | 0.0541 | 0.0500 | 0.0500 | 0.0500 |
| A1 β-casein protein (%) | 0.00 | 25.00 | 75.00 | 60.00 |
| A2 β-casein protein (%) | 100.00 | 75.00 | 25.00 | 40.00 |
| Criteria | Pairs | LI Subjects (n = 25) | Lactose Maldigesters (n = 33; 25 LI Subjects + 8 Maldigesters) | ||
|---|---|---|---|---|---|
| Total | p-Values | Total | p-Values | ||
| Total hydrogen produced per subject (ppm) | Conventional milk Milk containing A2 β-casein only | 11,935 10,892 | 0.31 | 16,460 13,771 | 0.04 |
| Conventional milk Jersey milk | 11,935 10,533 | 0.09 | 16,460 15,079 | 0.44 | |
| Total symptom scores a | Conventional milk Milk containing A2 β-casein only | 637 543 | 0.13 | 737 601 | 0.04 |
| Conventional milk Jersey milk | 637 678 | 0.55 | 737 790 | 0.17 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramakrishnan, M.; Eaton, T.K.; Sermet, O.M.; Savaiano, D.A. Milk Containing A2 β-Casein ONLY, as a Single Meal, Causes Fewer Symptoms of Lactose Intolerance than Milk Containing A1 and A2 β-Caseins in Subjects with Lactose Maldigestion and Intolerance: A Randomized, Double-Blind, Crossover Trial. Nutrients 2020, 12, 3855. https://doi.org/10.3390/nu12123855
Ramakrishnan M, Eaton TK, Sermet OM, Savaiano DA. Milk Containing A2 β-Casein ONLY, as a Single Meal, Causes Fewer Symptoms of Lactose Intolerance than Milk Containing A1 and A2 β-Caseins in Subjects with Lactose Maldigestion and Intolerance: A Randomized, Double-Blind, Crossover Trial. Nutrients. 2020; 12(12):3855. https://doi.org/10.3390/nu12123855
Chicago/Turabian StyleRamakrishnan, Monica, Tracy K. Eaton, Omer M. Sermet, and Dennis A. Savaiano. 2020. "Milk Containing A2 β-Casein ONLY, as a Single Meal, Causes Fewer Symptoms of Lactose Intolerance than Milk Containing A1 and A2 β-Caseins in Subjects with Lactose Maldigestion and Intolerance: A Randomized, Double-Blind, Crossover Trial" Nutrients 12, no. 12: 3855. https://doi.org/10.3390/nu12123855
APA StyleRamakrishnan, M., Eaton, T. K., Sermet, O. M., & Savaiano, D. A. (2020). Milk Containing A2 β-Casein ONLY, as a Single Meal, Causes Fewer Symptoms of Lactose Intolerance than Milk Containing A1 and A2 β-Caseins in Subjects with Lactose Maldigestion and Intolerance: A Randomized, Double-Blind, Crossover Trial. Nutrients, 12(12), 3855. https://doi.org/10.3390/nu12123855






