The Origins of Salivary Vitamin A, Vitamin B12 and Vitamin D-Binding Proteins
Abstract
:1. Introduction
2. Materials and Method
2.1. Sample Collection
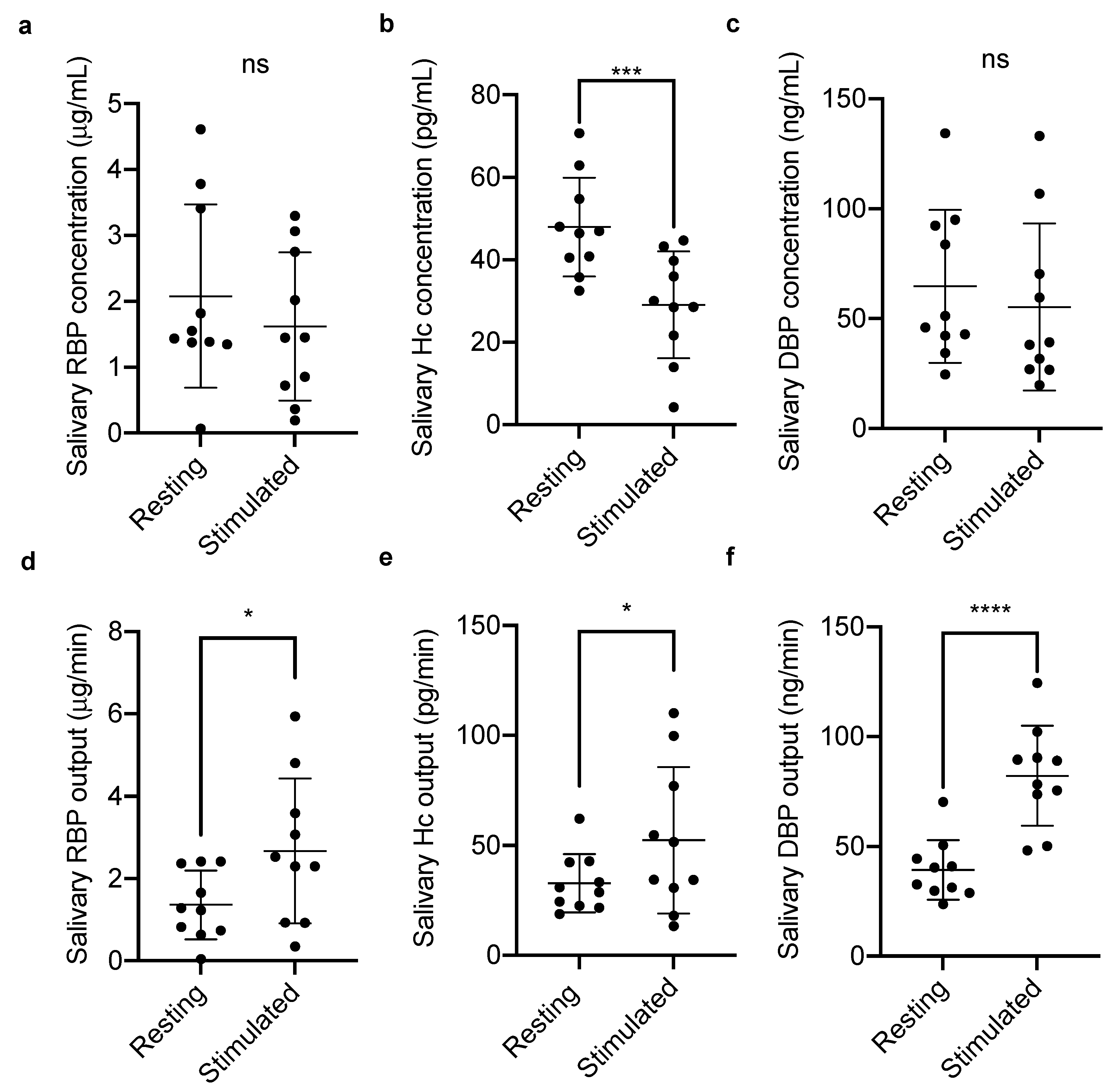
2.1.1. Salivary Vitamin-Binding Proteins at Rest and Stimulated Flow
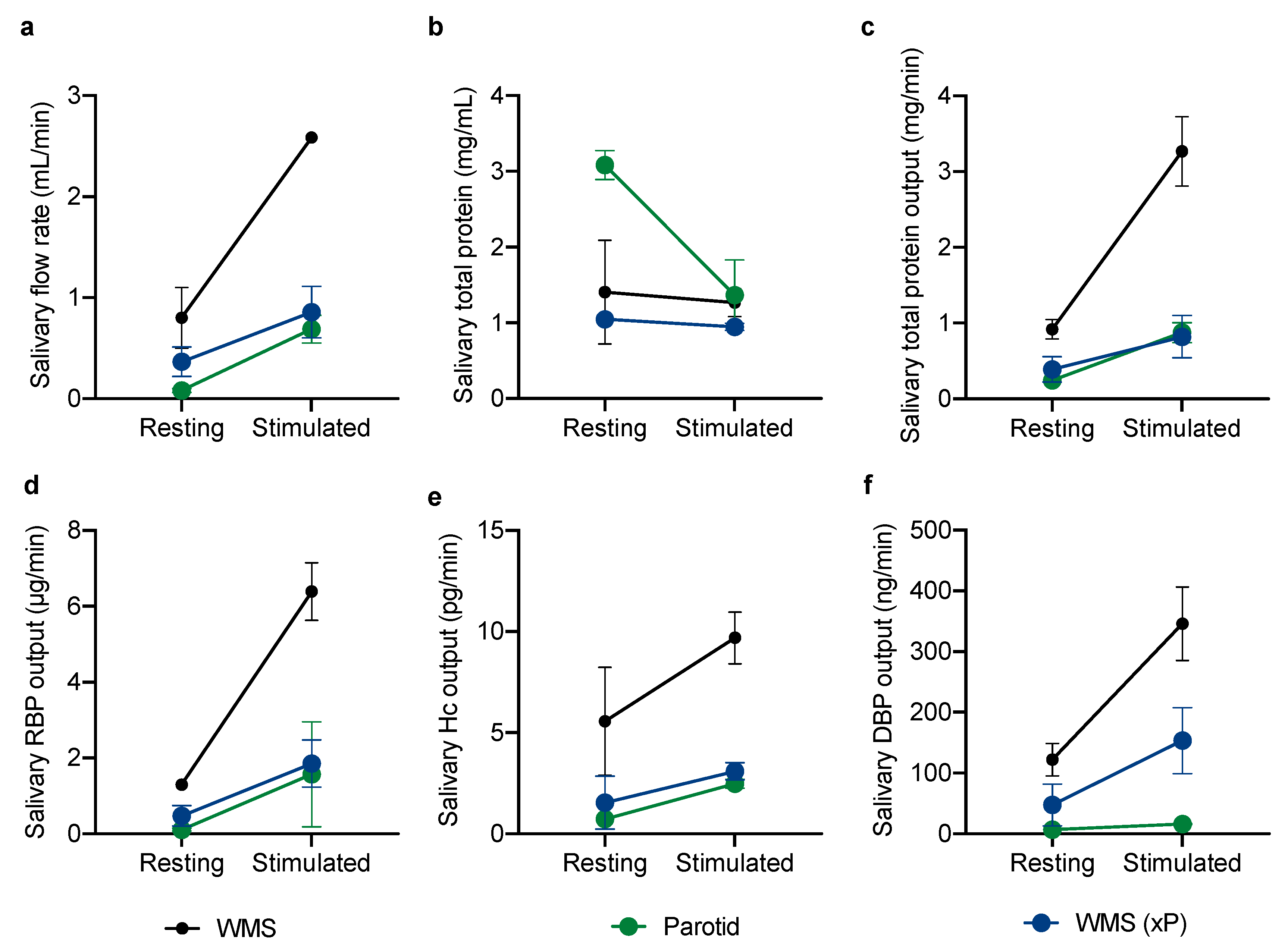
2.1.2. Glandular Contributions to the Vitamin-Binding Protein Output of Whole Mouth Saliva
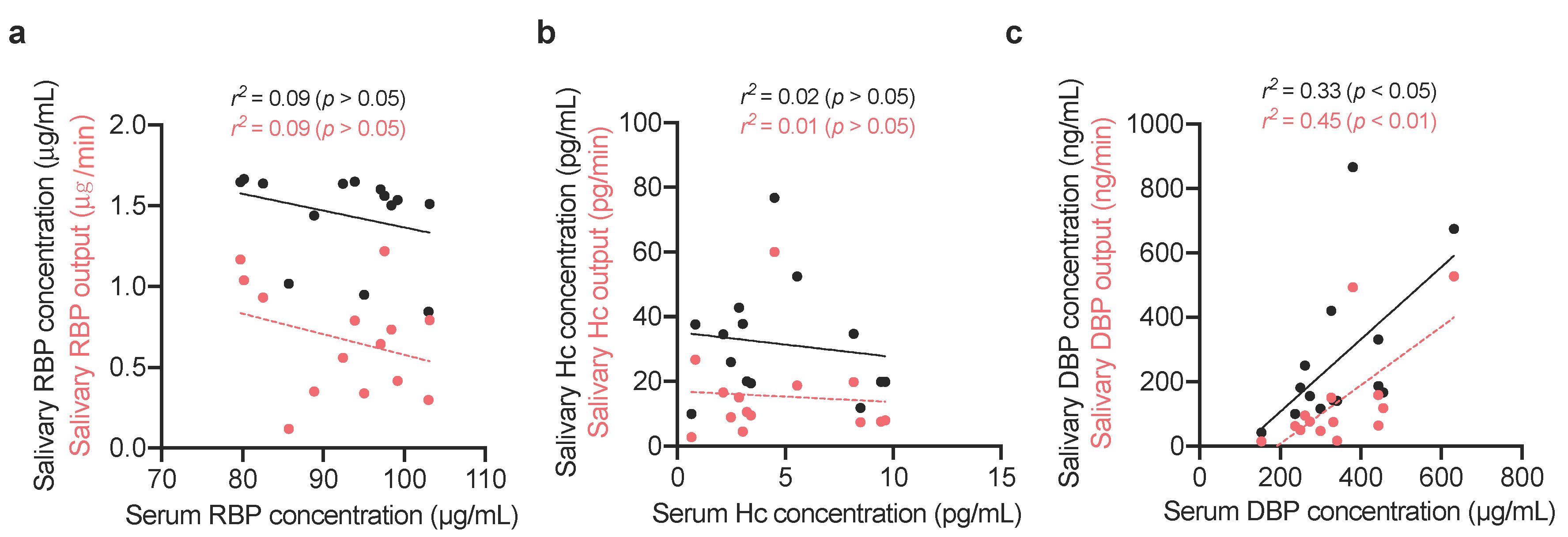
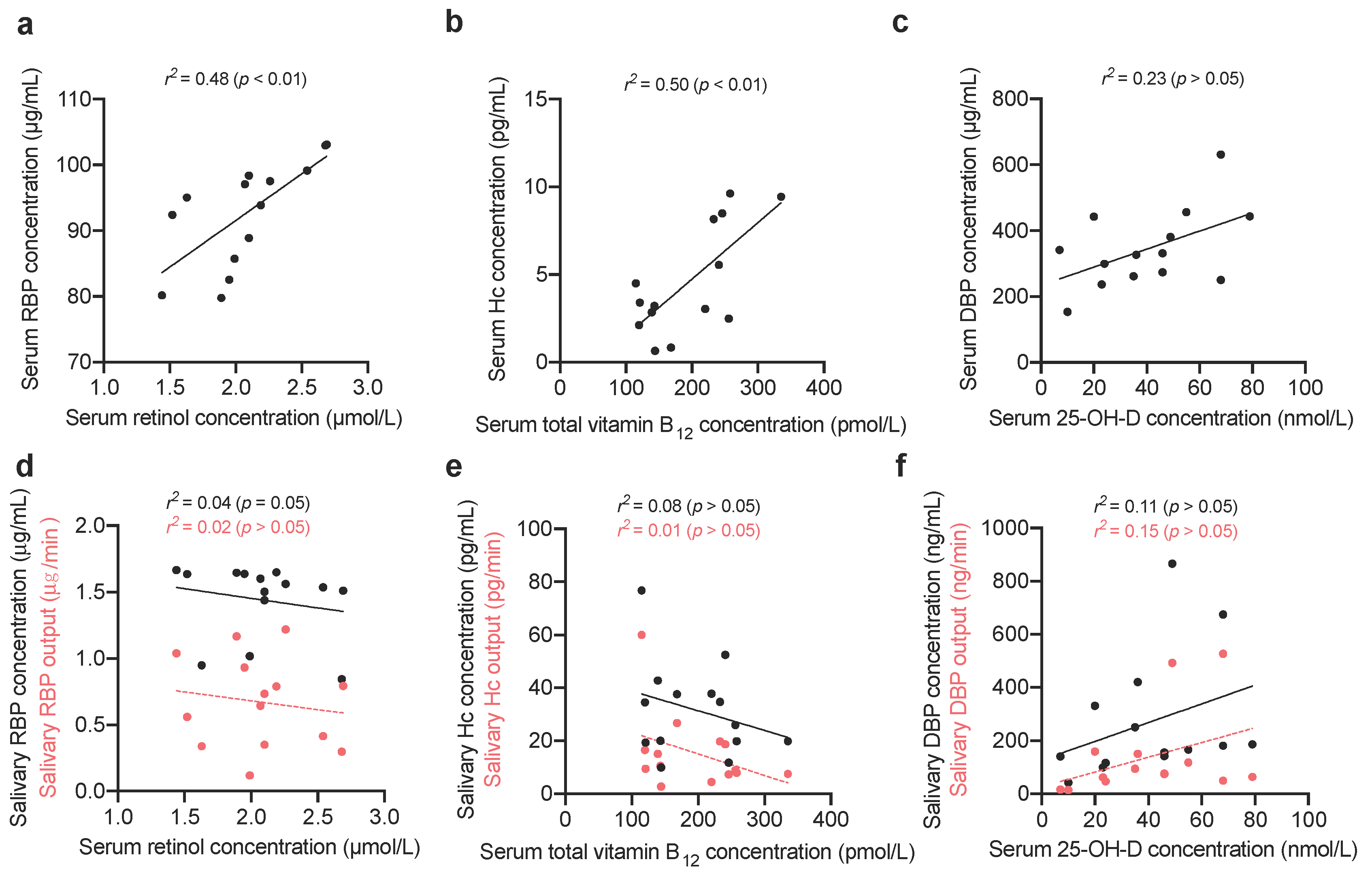
2.1.3. Comparing Vitamin-Binding Proteins Concentration with Systemic Vitamin Status
2.2. Biochemical Analysis
2.2.1. Quantification of Total Protein Content by Bicinchoninic Acid Assay
2.2.2. Quantification of Vitamin-Binding Proteins by Enzyme-Linked Immunosorbent Assay
2.2.3. Quantification of Retinol by High-Performance Liquid Chromatography
2.2.4. Quantification of Total Vitamin B12 & 25-OH-D by Chemiluminescent Microparticle Immunoassay
2.3. Statistical Analysis
2.4. Ethical Approval
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Loo, J.A.; Yan, W.; Ramachandran, P.; Wong, D.T. Comparative human salivary and plasma proteomes. J. Dent. Res. 2010, 89, 1016–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, M.; Yang, Y.; Guo, Z.; Shao, C.; Sun, H.; Zhang, Y.; Sun, Y.; Liu, Y.; Song, Y.; Zhang, L.; et al. A Comparative Proteomics Analysis of Five Body Fluids: Plasma, Urine, Cerebrospinal Fluid, Amniotic Fluid, and Saliva. Proteom. Clin. Appl. 2018, 12, e1800008. [Google Scholar] [CrossRef] [PubMed]
- Proctor, G.B. The physiology of salivary secretion. Periodontology 2000 2016, 70, 11–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, J.J.; Preshaw, P.M. Gingival crevicular fluid and saliva. Periodontology 2000 2016, 70, 7–10. [Google Scholar] [CrossRef]
- Proctor, G.B.; Carpenter, G.H. Salivary secretion: Mechanism and neural regulation. Monogr. Oral Sci. 2014, 24, 14–29. [Google Scholar]
- Bader, M.; Dunkel, A.; Wenning, M.; Kohler, B.; Medard, G.; del Castillo, E.; Gholami, A.; Kuster, B.; Scherer, S.; Hofmann, T. Dynamic Proteome Alteration and Functional Modulation of Human Saliva Induced by Dietary Chemosensory Stimuli. J. Agric. Food Chem. 2018, 66, 5621–5634. [Google Scholar] [CrossRef]
- Dawes, C.; Pedersen, A.M.; Villa, A.; Ekstrom, J.; Proctor, G.B.; Vissink, A.; Aframian, D.; McGowan, R.; Aliko, A.; Narayana, N.; et al. The functions of human saliva: A review sponsored by the World Workshop on Oral Medicine VI. Arch. Oral Biol. 2015, 60, 863–874. [Google Scholar] [CrossRef]
- Chun, R.F. New perspectives on the vitamin D binding protein. Cell Biochem. Funct. 2012, 30, 445–456. [Google Scholar] [CrossRef]
- Kotnik, P.; Fischer-Posovszky, P.; Wabitsch, M. RBP4: A controversial adipokine. Eur. J. Endocrinol. 2011, 165, 703–711. [Google Scholar] [CrossRef] [Green Version]
- Christou, G.A.; Tselepis, A.D.; Kiortsis, D.N. The metabolic role of retinol binding protein 4: An update. Horm. Metab. Res. 2012, 44, 6–14. [Google Scholar] [CrossRef] [Green Version]
- White, P.; Cooke, N. The multifunctional properties and characteristics of vitamin D-binding protein. Trends Endocrinol. Metab. 2000, 11, 320–327. [Google Scholar] [CrossRef]
- Blaner, W.S. Retinol-binding protein: The serum transport protein for vitamin A. Endocr. Rev. 1989, 10, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Graham, T.E.; Mody, N.; Preitner, F.; Peroni, O.D.; Zabolotny, J.M.; Kotani, K.; Quadro, L.; Kahn, B.B. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 2005, 436, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.R.; Goodman, D.S. The effects of diseases of the liver, thyroid, and kidneys on the transport of vitamin A in human plasma. J. Clin. Investig. 1971, 50, 2426–2436. [Google Scholar] [CrossRef] [PubMed]
- Fedosov, S.N.; Berglund, L.; Fedosova, N.U.; Nexo, E.; Petersen, T.E. Comparative analysis of cobalamin binding kinetics and ligand protection for intrinsic factor, transcobalamin, and haptocorrin. J. Biol. Chem. 2002, 277, 9989–9996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- England, J.M.; Down, M.C.; Wise, I.J.; Linnell, J.C. The transport of endogenous vitamin B12 in normal human serum. Clin. Sci. Mol. Med. 1976, 51, 47–52. [Google Scholar] [CrossRef]
- Fedosov, S.N. Physiological and molecular aspects of cobalamin transport. Subcell. Biochem. 2012, 56, 347–367. [Google Scholar]
- Morkbak, A.L.; Poulsen, S.S.; Nexo, E. Haptocorrin in humans. Clin. Chem. Lab. Med. 2007, 45, 1751–1759. [Google Scholar] [CrossRef]
- Wickramasinghe, S.N.; Fida, S. Correlations between holo-transcobalamin II, holo-haptocorrin, and total B12 in serum samples from healthy subjects and patients. J. Clin. Pathol. 1993, 46, 537–539. [Google Scholar] [CrossRef]
- Nexo, E.; Hvas, A.M.; Bleie, O.; Refsum, H.; Fedosov, S.N.; Vollset, S.E.; Schneede, J.; Nordrehaug, J.E.; Ueland, P.M.; Nygard, O.K. Holo-transcobalamin is an early marker of changes in cobalamin homeostasis. A randomized placebo-controlled study. Clin. Chem. 2002, 48, 1768–1771. [Google Scholar] [CrossRef]
- Harrington, D.J. Laboratory assessment of vitamin B12 status. J. Clin. Pathol. 2017, 70, 168–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonnesen, P.; Thim, L.; Nexo, E. Epidermal Growth-Factor and Haptocorrin in Nasal Secretion. Scand. J. Clin. Lab. Investig. 1990, 50, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Nexo, E. Cobalamin Binding-Proteins in Human Seminal Plasma. Scand. J. Clin. Lab. Investig. 1992, 52, 647–652. [Google Scholar] [CrossRef]
- Hansen, M.; Brynskov, J.; Christensen, P.A.; Krintel, J.J.; Gimsing, P. Cobalamin Binding-Proteins (Haptocorrin and Transcobalamin) in Human Cerebrospinal-Fluid. Scand. J. Haematol. 1985, 34, 209–212. [Google Scholar] [CrossRef]
- Greibe, E.; Lildballe, D.L.; Streym, S.; Vestergaard, P.; Rejnmark, L.; Mosekilde, L.; Nexo, E. Cobalamin and haptocorrin in human milk and cobalamin-related variables in mother and child: A 9-mo longitudinal study. Am. J. Clin. Nutr. 2013, 98, 389–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, M.J.; Rasmussen, M.R.; Andersen, C.B.; Nexo, E.; Moestrup, S.K. Vitamin B12 transport from food to the body’s cells—A sophisticated, multistep pathway. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 345–354. [Google Scholar] [CrossRef]
- Bikle, D.D.; Schwartz, J. Vitamin D Binding Protein, Total and Free Vitamin D Levels in Different Physiological and Pathophysiological Conditions. Front. Endocrinol. 2019, 10, 317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chun, R.F.; Shieh, A.; Gottlieb, C.; Yacoubian, V.; Wang, J.; Hewison, M.; Adams, J.S. Vitamin D Binding Protein and the Biological Activity of Vitamin D. Front. Endocrinol. 2019, 10, 718. [Google Scholar] [CrossRef]
- Haddad, J.G.; Hillman, L.; Rojanasathit, S. Human serum binding capacity and affinity for 25-hydroxyergocalciferol and 25-hydroxycholecalciferol. J. Clin. Endocrinol. Metab. 1976, 43, 86–91. [Google Scholar] [CrossRef]
- Haddad, J.G., Jr.; Walgate, J. Radioimmunoassay of the binding protein for vitamin D and its metabolites in human serum: Concentrations in normal subjects and patients with disorders of mineral homeostasis. J. Clin. Investig. 1976, 58, 1217–1222. [Google Scholar] [CrossRef]
- Verboven, C.; Bogaerts, I.; Waelkens, E.; Rabijns, A.; van Baelen, H.; Bouillon, R.; de Ranter, C. Actin-DBP: The perfect structural fit? Acta Crystallogr. D Biol. Crystallogr. 2003, 59 Pt 2, 263–273. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Meng, H.X.; Xu, L.; Zhang, L.; Shi, D.; Feng, X.H.; Lu, R.F.; Chen, Z.B. Vitamin D-Binding Protein Levels in Plasma and Gingival Crevicular Fluid of Patients with Generalized Aggressive Periodontitis. Int. J. Endocrinol. 2014, 2014, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cashman, K.D.; van den Heuvel, E.G.; Schoemaker, R.J.; Preveraud, D.P.; Macdonald, H.M.; Arcot, J. 25-Hydroxyvitamin D as a Biomarker of Vitamin D Status and Its Modeling to Inform Strategies for Prevention of Vitamin D Deficiency within the Population. Adv. Nutr. 2017, 8, 947–957. [Google Scholar] [CrossRef] [Green Version]
- Dastani, Z.; Berger, C.; Langsetmo, L.; Fu, L.; Wong, B.Y.; Malik, S.; Goltzman, D.; Cole, D.E.; Richards, J.B. In healthy adults, biological activity of vitamin D, as assessed by serum PTH, is largely independent of DBP concentrations. J. Bone Miner. Res. 2014, 29, 494–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbott Diagnostics. Architect B12, REF 7K61, G6-0569/R10; Abbott Diagnostics: Chicago, IL, USA, 2015. [Google Scholar]
- Baeten, J.M.; Richardson, B.A.; Bankson, D.D.; Wener, M.H.; Kreiss, J.K.; Lavreys, L.; Mandaliya, K.; Bwayo, J.J.; McClelland, R.S. Use of serum retinol-binding protein for prediction of vitamin A deficiency: Effects of HIV-1 infection, protein malnutrition, and the acute phase response. Am. J. Clin. Nutr. 2004, 79, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, S.J.; Mondul, A.M.; Kopp, W.; Rager, H.; Virtamo, J.; Albanes, D. Circulating 25-hydroxyvitamin D, vitamin D-binding protein and risk of prostate cancer. Int. J. Cancer 2013, 132, 2940–2947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proctor, G.B.; Carpenter, G.H. Regulation of salivary gland function by autonomic nerves. Auton. Neurosci. 2007, 133, 3–18. [Google Scholar] [CrossRef]
- Proctor, G.B.; Carpenter, G.H. Chewing stimulates secretion of human salivary secretory immunoglobulin A. J. Dent. Res. 2001, 80, 909–913. [Google Scholar] [CrossRef]
- Carpenter, G.H. The secretion, components, and properties of saliva. Annu. Rev. Food Sci. Technol. 2013, 4, 267–276. [Google Scholar] [CrossRef]
- Christensen, M.E.; Hansen, H.S.; Poulsen, S.S.; Bretlau, P.; Nexo, E. Immunohistochemical and quantitative changes in salivary EGF, amylase and haptocorrin following radiotherapy for oral cancer. Acta Otolaryngol. 1996, 116, 137–143. [Google Scholar] [CrossRef]
- Denny, P.; Hagen, F.K.; Hardt, M.; Liao, L.; Yan, W.; Arellanno, M.; Bassilian, S.; Bedi, G.S.; Boontheung, P.; Cociorva, D.; et al. The proteomes of human parotid and submandibular/sublingual gland salivas collected as the ductal secretions. J. Proteome Res. 2008, 7, 1994–2006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schipper, R.G.; Silletti, E.; Vingerhoeds, M.H. Saliva as research material: Biochemical, physicochemical and practical aspects. Arch. Oral Biol. 2007, 52, 1114–1135. [Google Scholar] [CrossRef] [PubMed]
- Quadros, E.V.; Nakayama, Y.; Sequeira, J.M. The binding properties of the human receptor for the cellular uptake of vitamin B12. Biochem. Biophys. Res. Commun. 2005, 327, 1006–1010. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Woo, J.S.; Schmitz, J.; Prinz, B.; Root, K.; Chen, F.; Bloch, J.S.; Zenobi, R.; Locher, K.P. Structural basis of transcobalamin recognition by human CD320 receptor. Nat. Commun. 2016, 7, 12100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reboul, E.; Goncalves, A.; Comera, C.; Bott, R.; Nowicki, M.; Landrier, J.F.; Jourdheuil-Rahmani, D.; Dufour, C.; Collet, X.; Borel, P. Vitamin D intestinal absorption is not a simple passive diffusion: Evidences for involvement of cholesterol transporters. Mol. Nutr. Food Res. 2011, 55, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Borel, P.; Lietz, G.; Goncalves, A.; de Edelenyi, F.S.; Lecompte, S.; Curtis, P.; Goumidi, L.; Caslake, M.J.; Miles, E.A.; Packard, C.; et al. CD36 and SR-BI are involved in cellular uptake of provitamin A carotenoids by Caco-2 and HEK cells, and some of their genetic variants are associated with plasma concentrations of these micronutrients in humans. J. Nutr. 2013, 143, 448–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adkins, Y.; Lonnerdal, B. Potential host-defense role of a human milk vitamin B-12-binding protein, haptocorrin, in the gastrointestinal tract of breastfed infants, as assessed with porcine haptocorrin in vitro. Am. J. Clin. Nutr. 2003, 77, 1234–1240. [Google Scholar] [CrossRef] [Green Version]
- Arnold, R.R.; MCole, F.; McGhee, J.R. A bactericidal effect for human lactoferrin. Science 1977, 197, 263–265. [Google Scholar] [CrossRef]
- Degnan, P.H.; Taga, M.E.; Goodman, A.L. Vitamin B12 as a modulator of gut microbial ecology. Cell Metab. 2014, 20, 769–778. [Google Scholar] [CrossRef] [Green Version]
- Lloyd-Price, J.; Mahurkar, A.; Rahnavard, G.; Crabtree, J.; Orvis, J.; Hall, A.B.; Brady, A.; Creasy, H.H.; McCracken, C.; Giglio, M.G.; et al. Strains, functions and dynamics in the expanded Human Microbiome Project. Nature 2017, 550, 61–66. [Google Scholar] [CrossRef]
- Fedosov, S.N.; Fedosova, N.U.; Krautler, B.; Nexo, E.; Petersen, T.E. Mechanisms of discrimination between cobalamins and their natural analogues during their binding to the specific B12-transporting proteins. Biochemistry 2007, 46, 6446–6458. [Google Scholar] [CrossRef] [PubMed]
- Naderpoor, N.; Mousa, A.; Arango, L.F.G.; Barrett, H.L.; Nitert, M.D.; de Courten, B. Effect of Vitamin D Supplementation on Faecal Microbiota: A Randomised Clinical Trial. Nutrients 2019, 11, 2888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, Y.; Nichols, R.G.; Cai, J.; Patterson, A.D.; Cantorna, M.T. Vitamin A deficiency in mice alters host and gut microbial metabolism leading to altered energy homeostasis. J. Nutr. Biochem. 2018, 54, 28–34. [Google Scholar] [CrossRef] [PubMed]
- McVoy, L.A.; Kew, R.R. CD44 and annexin A2 mediate the C5a chemotactic cofactor function of the vitamin D binding protein. J. Immunol. 2005, 175, 4754–4760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kew, R.R.; Webster, R.O. Gc-globulin (vitamin D-binding protein) enhances the neutrophil chemotactic activity of C5a and C5a des Arg. J. Clin. Investig. 1988, 82, 364–369. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, N.; Naraparaju, V.R.; Asbell, S.O. Deglycosylation of serum vitamin D3-binding protein leads to immunosuppression in cancer patients. Cancer Res. 1996, 56, 2827–2831. [Google Scholar]
| n = 10 | Resting | Stimulated | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p | r | |
| Flow rate (mL/min) | 0.70 | 0.28 | 1.95 | 0.92 | <0.01 | 0.44 |
| Total protein concentration (mg/mL) | 1.66 | 0.50 | 1.29 | 0.36 | <0.05 | 0.66 |
| Total protein output (mg/min) | 1.85 | 0.26 | 2.35 | 0.98 | <0.01 | 0.45 |
| n = 14 | Mean | SD |
|---|---|---|
| Serum retinol (μmol/L) | 2.08 | 0.39 |
| Serum total vitamin B12 (pmol/L) | 196 | 92 |
| Serum 25-OH-D (nmol/L) | 40 | 22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blakeley, M.; Sobczyńska-Malefora, A.; Carpenter, G. The Origins of Salivary Vitamin A, Vitamin B12 and Vitamin D-Binding Proteins. Nutrients 2020, 12, 3838. https://doi.org/10.3390/nu12123838
Blakeley M, Sobczyńska-Malefora A, Carpenter G. The Origins of Salivary Vitamin A, Vitamin B12 and Vitamin D-Binding Proteins. Nutrients. 2020; 12(12):3838. https://doi.org/10.3390/nu12123838
Chicago/Turabian StyleBlakeley, Matthew, Agata Sobczyńska-Malefora, and Guy Carpenter. 2020. "The Origins of Salivary Vitamin A, Vitamin B12 and Vitamin D-Binding Proteins" Nutrients 12, no. 12: 3838. https://doi.org/10.3390/nu12123838
APA StyleBlakeley, M., Sobczyńska-Malefora, A., & Carpenter, G. (2020). The Origins of Salivary Vitamin A, Vitamin B12 and Vitamin D-Binding Proteins. Nutrients, 12(12), 3838. https://doi.org/10.3390/nu12123838





