Nutritional Interventions to Improve Asthma-Related Outcomes through Immunomodulation: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
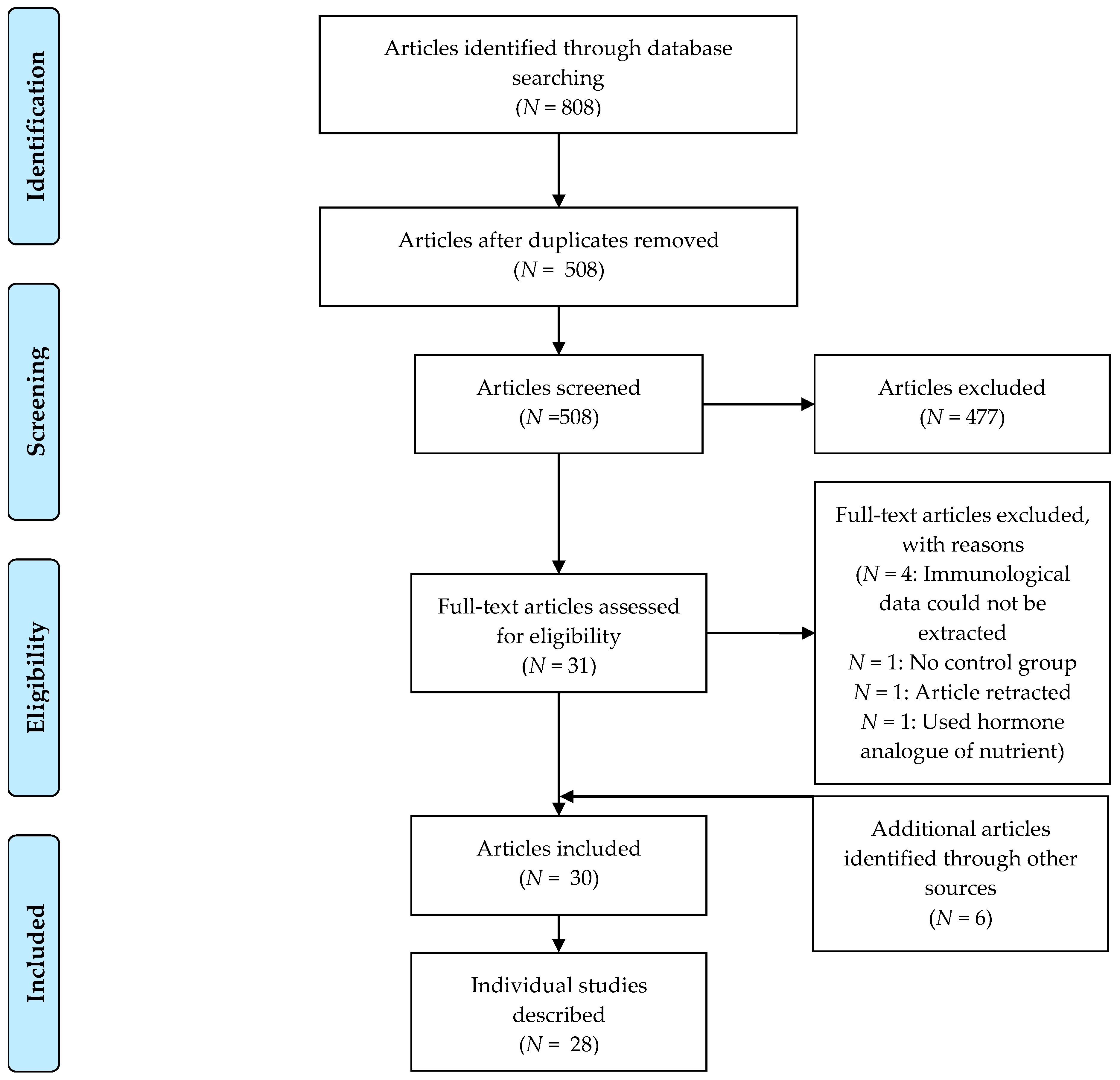
2.2. Selection of Studies
2.3. Data Extraction
2.4. Methodological Quality Assessment
3. Results
3.1. Herbs, Herbal Mixtures and Extracts
3.2. Supplements
3.3. Weight Loss
3.4. Vitamin D3
3.5. Omega-3 LCPUFAs
3.6. Whole Food Approaches
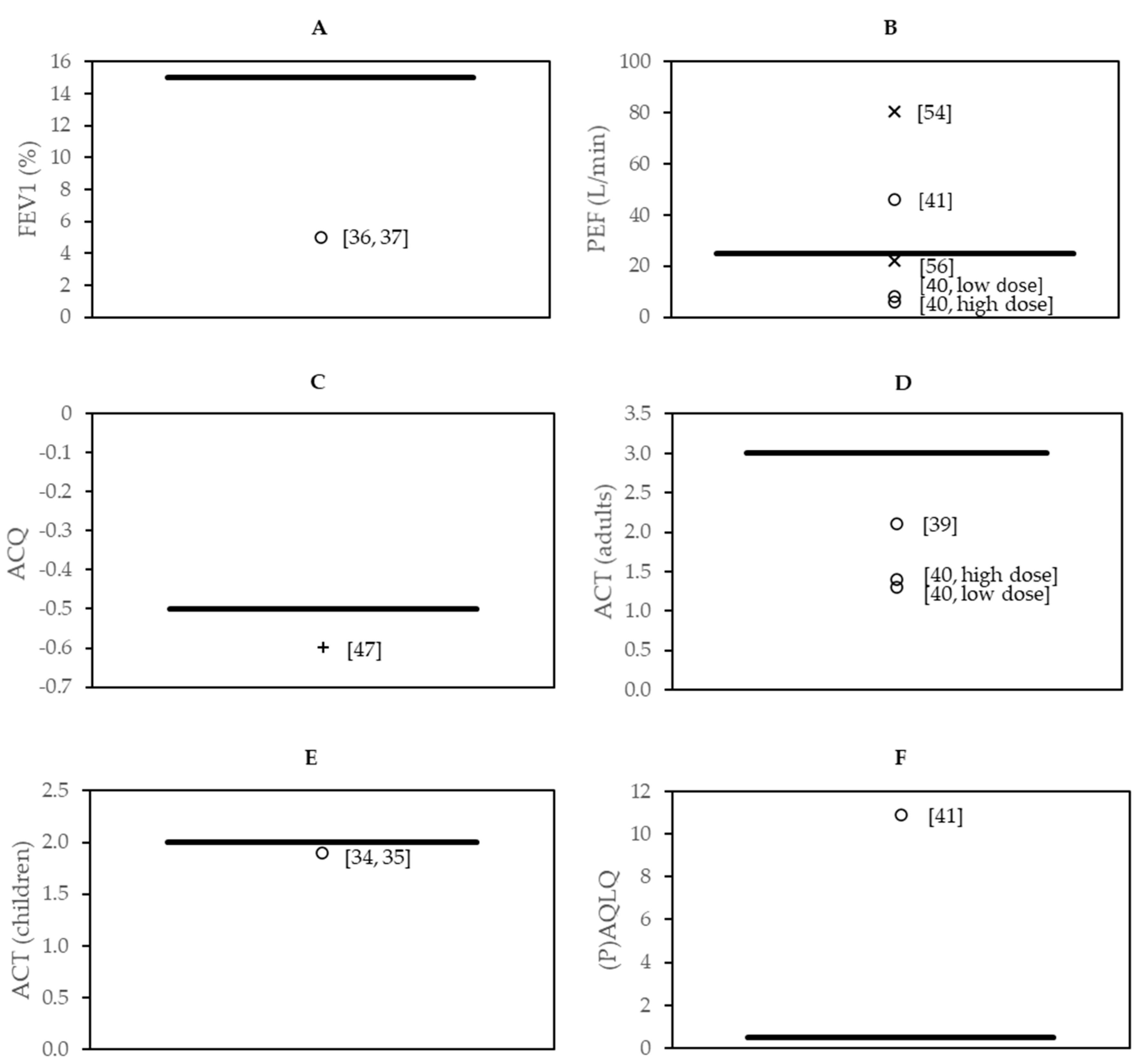
3.7. Effect Sizes in the Context of Minimal Clinically Important Difference
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Global Asthma Network. The Global Asthma Report 2018; Global Asthma Network: Auckland, New Zealand, 2018. [Google Scholar]
- Maslan, J.; Mims, J.W. What is asthma? Pathophysiology, demographics, and health care costs. Otolaryngol. Clin. N. Am. 2014, 47, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2020. 2020. Available online: www.ginasthma.org (accessed on 24 March 2020).
- Amin, S.; Soliman, M.; McIvor, A.; Cave, A.; Cabrera, C. Understanding patient perspectives on medication adherence in asthma: A targeted review of qualitative studies. Patient Prefer. Adherence 2020, 14, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Bårnes, C.B.; Ulrik, C.S. Asthma and adherence to inhaled corticosteroids: Current status and future perspectives. Respir. Care 2015, 60, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Dahl, R. Systemic side effects of inhaled corticosteroids in patients with asthma. Respir. Med. 2006, 100, 1307–1317. [Google Scholar] [CrossRef]
- Tattersall, M.C.; Guo, M.; Korcarz, C.E.; Gepner, A.D.; Kaufman, J.D.; Liu, K.J.; Barr, R.G.; Donohue, K.M.; McClelland, R.L.; Delaney, J.A. Asthma predicts cardiovascular disease events: The multi-ethnic study of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1520–1525. [Google Scholar] [CrossRef]
- Schulze, M.B.; Martínez-González, M.A.; Fung, T.T.; Lichtenstein, A.H.; Forouhi, N.G. Food based dietary patterns and chronic disease prevention. BMJ 2018, 361, k2396. [Google Scholar] [CrossRef]
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D. Low-grade inflammation, diet composition and health: Current research evidence and its translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef]
- Mozaffarian, D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: A comprehensive review. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef]
- Rosato, V.; Temple, N.J.; La Vecchia, C.; Castellan, G.; Tavani, A.; Guercio, V. Mediterranean diet and cardiovascular disease: A systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2019, 58, 173–191. [Google Scholar] [CrossRef]
- Khan, S.U.; Khan, M.U.; Riaz, H.; Valavoor, S.; Zhao, D.; Vaughan, L.; Okunrintemi, V.; Riaz, I.B.; Khan, M.S.; Kaluski, E. Effects of nutritional supplements and dietary interventions on cardiovascular outcomes: An umbrella review and evidence map. Ann. Intern. Med. 2019, 171, 190–198. [Google Scholar] [CrossRef]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—A systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Larsen, V.; Del Giacco, S.R.; Moreira, A.; Bonini, M.; Charles, D.; Reeves, T.; Carlsen, K.H.; Haahtela, T.; Bonini, S.; Fonseca, J. Asthma and dietary intake: An overview of systematic reviews. Allergy 2016, 71, 433–442. [Google Scholar] [CrossRef]
- Allan, K.; Devereux, G. Diet and asthma: Nutrition implications from prevention to treatment. J. Am. Diet. Assoc. 2011, 111, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, M.; Wang, C.; Xiao, Y.; An, T.; Zou, M.; Cheng, G. Association between vitamin D status and asthma control: A meta-analysis of randomized trials. Respir. Med. 2019, 150, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Miyata, J.; Arita, M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol. Int. 2015, 64, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Guilleminault, L.; Williams, E.J.; Scott, H.A.; Berthon, B.S.; Jensen, M.; Wood, L.G. Diet and asthma: Is it time to adapt our message? Nutrients 2017, 9, 1227. [Google Scholar] [CrossRef]
- Chaudhuri, R.; Livingston, E.; McMahon, A.D.; Lafferty, J.; Fraser, I.; Spears, M.; McSharry, C.P.; Thomson, N.C. Effects of smoking cessation on lung function and airway inflammation in smokers with asthma. Am. J. Respir. Crit. Care Med. 2006, 174, 127–133. [Google Scholar] [CrossRef]
- Jang, A.S.; Park, S.W.; Kim, D.J.; Uh, S.; Kim, Y.H.; Whang, H.G.; Lim, G.; Park, C.S. Effects of smoking cessation on airflow obstruction and quality of life in asthmatic smokers. Allergy Asthma Immunol. Res. 2010, 2, 254–259. [Google Scholar] [CrossRef]
- Boulet, L.P.; Turcotte, H.; Martin, J.; Poirier, P. Effect of bariatric surgery on airway response and lung function in obese subjects with asthma. Respir. Med. 2012, 106, 651–660. [Google Scholar] [CrossRef]
- Dixon, A.E.; Pratley, R.E.; Forgione, P.M.; Kaminsky, D.A.; Whittaker-Leclair, L.A.; Griffes, L.A.; Garudathri, J.; Raymond, D.; Poynter, M.E.; Bunn, J.Y. Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation. J. Allergy Clin. Immunol. 2011, 128, 508–515.e502. [Google Scholar] [CrossRef]
- Scott, H.A.; Gibson, P.G.; Garg, M.L.; Pretto, J.J.; Morgan, P.J.; Callister, R.; Wood, L.G. Dietary restriction and exercise improve airway inflammation and clinical outcomes in overweight and obese asthma: A randomized trial. Clin. Exp. Allergy 2013, 43, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D.E.R.; Nicol, C.W.; Bredin, S.S.D. Health benefits of physical activity: The evidence. CMAJ 2006, 174, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.R.; Carroll, D.D.; Workman, L.M.; Carlson, S.A.; Brown, D.W. Physical activity and health-related quality of life: US adults with and without limitations. Qual. Life Res. 2014, 23, 2673–2680. [Google Scholar] [CrossRef] [PubMed]
- Lange, N.E.; Litonjua, A.; Hawrylowicz, C.M.; Weiss, S. Vitamin D, the immune system and asthma. Expert Rev. Clin. Immunol. 2009, 5, 693–702. [Google Scholar] [CrossRef]
- Mann, E.H.; Chambers, E.S.; Pfeffer, P.E.; Hawrylowicz, C.M. Immunoregulatory mechanisms of vitamin D relevant to respiratory health and asthma. Ann. N. Y. Acad. Sci. 2014, 1317, 57–69. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Online Ruler on Screen Pixel Ruler. Available online: https://www.rapidtables.com/web/tools/pixel-ruler.html (accessed on 24 March 2020).
- Ras, R.T.; Hiemstra, H.; Lin, Y.; Vermeer, M.A.; Duchateau, G.S.M.J.E.; Trautwein, E.A. Consumption of plant sterol-enriched foods and effects on plasma plant sterol concentrations—A meta-analysis of randomized controlled studies. Atherosclerosis 2013, 230, 336–346. [Google Scholar] [CrossRef]
- Trabuco, E.C.; Moorman, P.G.; Algeciras-Schimnich, A.; Weaver, A.L.; Cliby, W.A. Association of ovary-sparing hysterectomy with ovarian reserve. Obstet. Gynecol. 2016, 127, 819–827. [Google Scholar] [CrossRef]
- Meropol, S.B.; Metlay, J.P. Accuracy of pneumonia hospital admissions in a primary care electronic medical record database. Pharmacoepidemiol. Drug Saf. 2012, 21, 659–665. [Google Scholar] [CrossRef]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.M.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Barlianto, W.; Rachmawati, M.; Irawan, M.; Wulandari, D. Effects of Nigella sativa oil on Th1/Th2, cytokine balance, and improvement of asthma control in children. Paediatr. Indones 2017, 57, 223–228. [Google Scholar] [CrossRef]
- Barlianto, W.; Wulandari, D.; Chusniyah, M.; Chandra Kusuma, H.M.S.; Prawiro, S.R. Improvement of Th17/Treg balance and asthma control test score by nigella sativa supplementation in asthmatic children: A new approach to managing asthma. Turk. J. Immunol. 2018, 6, 1–7. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Zilaee, M.; Shoushtari, M.H.; Ghasemi dehcheshmeh, M. An evaluation of the effect of saffron supplementation on the antibody titer to heat-shock protein (HSP) 70, hsCRP and spirometry test in patients with mild and moderate persistent allergic asthma: A triple-blind, randomized placebo-controlled trial. Respir. Med. 2018, 145, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Zilaee, M.; Hosseini, S.A.; Jafarirad, S.; Abolnezhadian, F.; Cheraghian, B.; Namjoyan, F.; Ghadiri, A. An evaluation of the effects of saffron supplementation on the asthma clinical symptoms and asthma severity in patients with mild and moderate persistent allergic asthma: A double-blind, randomized placebo-controlled trial. Respir. Res. 2019, 20, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Khayyal, M.T.; El-Ghazaly, M.A.; El-Khatib, A.S.; Hatem, A.M.; De Vries, P.J.F.; El-Shafei, S.; Khattab, M.M. A clinical pharmacological study of the potential beneficial effects of a propolis food product as an adjuvant in asthmatic patients. Fundam. Clin. Pharmacol. 2003, 17, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Koshak, A.; Wei, L.; Koshak, E.; Wali, S.; Alamoudi, O.; Demerdash, A.; Qutub, M.; Pushparaj, P.N.; Heinrich, M. Nigella Sativa supplementation improves asthma control and biomarkers: A randomized, double-blind, placebo-controlled trial. Phytother. Res. 2017, 31, 403–409. [Google Scholar] [CrossRef]
- Salem, A.M.; Bamosa, A.O.; Qutub, H.O.; Gupta, R.K.; Badar, A.; Elnour, A.; Afzal, M.N. Effect of Nigella sativa supplementation on lung function and inflammatory mediatorsin partly controlled asthma: A randomized controlled trial. Ann. Saudi Med. 2017, 37, 64–71. [Google Scholar] [CrossRef]
- Yugandhar, P.; Rao, K.M.; Sengupta, K. A novel herbal composition containing extracts of Boswellia serrata gum resin and Aegle marmelos fruit alleviates symptoms of asthma in a placebo controlled double-blind clinical study. Phytother. Res. 2017, 32, 140–150. [Google Scholar] [CrossRef]
- Ghaffari, J.; Hossaini, R.F.; Khalilian, A.; Nahanmoghadam, N.; Salehifar, E.; Rafatpanah, H. Vitamin E supplementation, lung functions and clinical manifestations in children with moderate asthma: A randomized double blind placebo- Controlled trial. Iran. J. Allergy Asthma Immunol. 2014, 13, 98–103. [Google Scholar]
- Pearson, P.J.; Lewis, S.A.; Britton, J.; Fogarty, A. Vitamin E supplements in asthma: A parallel group randomised placebo controlled trial. Thorax 2004, 59, 652–656. [Google Scholar] [CrossRef]
- Smith, L.J.; Kalhan, R.; Wise, R.A.; Sugar, E.A.; Lima, J.J.; Irvin, C.G.; Dozor, A.J.; Holbrook, J.T.; Hanania, N.; Sockrider, M.; et al. Effect of a soy isoflavone supplement on lung function and clinical outcomes in patients with poorly controlled asthma: A randomized clinical trial. JAMA 2015, 313, 2033–2043. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.G.; Garg, M.L.; Powell, H.; Gibson, P.G. Lycopene-rich treatments modify noneosinophilic airway inflammation in asthma: Proof of concept. Free Radic. Res. 2008, 42, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Dias-Junior, S.A.; Reis, M.; de Carvalho-Pinto, R.M.; Stelmach, R.; Halpern, A.; Cukier, A. Effects of weight loss on asthma control in obese patients with severe asthma. Eur. Respir. J. 2014, 43, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.E.; Gibson, P.G.; Collins, C.E.; Hilton, J.M.; Wood, L.G. Diet-induced weight loss in obese children with asthma: A randomized controlled trial. Clin. Exp. Allergy 2013, 43, 775–784. [Google Scholar] [CrossRef]
- Toennesen, L.L.; Meteran, H.; Hostrup, M.; Wium Geiker, N.R.; Jensen, C.B.; Porsbjerg, C.; Astrup, A.; Bangsbo, J.; Parker, D.; Backer, V. Effects of exercise and diet in nonobese asthma patients—A randomized controlled trial. J. Allergy Clin. Immunol. Pract. 2018, 6, 803–811. [Google Scholar] [CrossRef]
- Bar Yoseph, R.; Livnat, G.; Schnapp, Z.; Hakim, F.; Dabbah, H.; Goldbart, A.; Bentur, L. The effect of vitamin D on airway reactivity and inflammation in asthmatic children: A double-blind placebo-controlled trial. Pediatr. Pulmonol. 2015, 50, 747–753. [Google Scholar] [CrossRef]
- Castro, M.; King, T.S.; Kunselman, S.J.; Cabana, M.D.; Denlinger, L.; Holguin, F.; Kazani, S.D.; Moore, W.C.; Moy, J.; Sorkness, C.A. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: The VIDA randomized clinical trial. JAMA 2014, 311, 2083–2091. [Google Scholar] [CrossRef]
- De Groot, J.C.; Van Roon, E.N.H.; Storm, H.; Veeger, N.J.G.M.; Zwinderman, A.H.; Hiemstra, P.S.; Bel, E.H.D.; Ten Brinke, A. Vitamin D reduces eosinophilic airway inflammation in nonatopic asthma. J. Allergy Clin. Immunol. 2015, 135, 670–675.e673. [Google Scholar] [CrossRef]
- Kerley, C.P.; Hutchinson, K.; Cormican, L.; Faul, J.; Greally, P.; Coghlan, D.; Elnazir, B. Vitamin D3 for uncontrolled childhood asthma: A pilot study. Pediatr. Allergy Immunol. 2016, 27, 404–412. [Google Scholar] [CrossRef]
- Martineau, A.R.; MacLaughlin, B.D.; Hooper, R.L.; Barnes, N.C.; Jolliffe, D.A.; Greiller, C.L.; Kilpin, K.; McLaughlin, D.; Fletcher, G.; Mein, C.A.; et al. Double-blind randomised placebo-controlled trial of bolus-dose vitamin D3 supplementation in adults with asthma (ViDiAs). Thorax 2015, 70, 451–457. [Google Scholar] [CrossRef]
- Emelyanov, A.; Fedoseev, G.; Krasnoschekova, O.; Abulimity, A.; Trendeleva, T.; Barnes, P.J. Treatment of asthma with lipid extract of New Zealand green-lipped mussel: A randomised clinical trial. Eur. Respir. J. 2002, 20, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Hodge, L.; Salome, C.M.; Hughes, J.M.; Liu-Brennan, D.; Rimmer, J.; Allman, M.; Pang, D.; Armour, C.; Woolcock, A.J. Effect of dietary intake of omega-3 and omega-6 fatty acids on severity of asthma in children. Eur. Respir. J. 1998, 11, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Mickleborough, T.D.; Vaughn, C.L.; Shei, R.J.; Davis, E.M.; Wilhite, D.P. Marine lipid fraction PCSO-524TM (lyprinol/omega XL) of the New Zealand green lipped mussel attenuates hyperpnea-induced bronchoconstriction in asthma. Respir. Med. 2013, 107, 1152–1163. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.; Moreira, P.; Delgado, L.; Fonseca, J.; Teixeira, V.; Padrao, P.; Castel-Branco, G. Pilot study of the effects of n-3 polyunsaturated fatty acids on exhaled nitric oxide in patients with stable asthma. J. Investig. Allergol. Clin. Immunol. 2007, 17, 309–313. [Google Scholar] [PubMed]
- Schubert, R.; Kitz, R.; Beermann, C.; Rose, M.A.; Lieb, A.; Sommerer, P.C.; Moskovits, J.; Alberternst, H.; Bohles, H.J.; Schulze, J.; et al. Effect of n-3 polyunsaturated fatty acids in asthma after low-dose allergen challenge. Int. Arch. Allergy Immunol. 2009, 148, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Bseikri, M.; McCann, J.C.; Lal, A.; Fong, E.; Graves, K.; Goldrich, A.; Block, D.; Gildengoren, G.L.; Mietus-Snyder, M.; Shigenaga, M.; et al. A novel nutritional intervention improves lung function in overweight/obese adolescents with poorly controlled asthma: The Supplemental Nutrition in Asthma Control (SNAC) pilot study. FASEB J. 2018, 32, 6643–6654. [Google Scholar] [CrossRef]
- Papamichael, M.M.; Katsardis, C.; Lambert, K.; Tsoukalas, D.; Koutsilieris, M.; Erbas, B.; Itsiopoulos, C. Efficacy of a Mediterranean diet supplemented with fatty fish in ameliorating inflammation in paediatric asthma: A randomised controlled trial. J. Hum. Nutr. Diet. 2019, 32, 185–197. [Google Scholar] [CrossRef]
- Sexton, P.; Black, P.; Metcalf, P.; Wall, C.R.; Ley, S.; Wu, L.; Sommerville, F.; Brodie, S.; Kolbe, J. Influence of mediterranean diet on asthma symptoms, lung function, and systemic inflammation: A randomized controlled trial. J. Asthma 2013, 50, 75–81. [Google Scholar] [CrossRef]
- Sudini, K.; Diette, G.B.; Breysse, P.N.; McCormack, M.C.; Bull, D.; Biswal, S.; Zhai, S.; Brereton, N.; Peng, R.D.; Matsui, E.C. A randomized controlled trial of the effect of broccoli sprouts on antioxidant gene expression and airway inflammation in asthmatics. J. Allergy Clin. Immunol. Pract. 2016, 4, 932–940. [Google Scholar] [CrossRef]
- Wood, L.G.; Garg, M.L.; Smart, J.M.; Scott, H.A.; Barker, D.; Gibson, P.G. Manipulating antioxidant intake in asthma: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 96, 534–543. [Google Scholar] [CrossRef]
- Balaha, M.F.; Tanaka, H.; Yamashita, H.; Rahman, M.N.A.; Inagaki, N. Oral Nigella sativa oil ameliorates ovalbumin-induced bronchial asthma in mice. Int. Immunopharmacol. 2012, 14, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, I.; Nakajima, H.; Endo, H.; Yoshida, S. Interferon gamma regulates antigen-induced eosinophil recruitment into the mouse airways by inhibiting the infiltration of CD4+ T cells. J. Exp. Med. 1993, 177, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.A.; Mahajan, S. Role of cytokines in pathophysiology of asthma. Iran. J. Pharmacol. Ther. 2006, 5, 1–14. [Google Scholar]
- Keyhanmanesh, R.; Boskabady, M.H.; Khamneh, S.; Doostar, Y. Effect of thymoquinone on the lung pathology and cytokine levels of ovalbumin-sensitized guinea pigs. Pharmacol. Rep. 2010, 62, 910–916. [Google Scholar] [CrossRef]
- Rana Keyhanmanesh, L.P.; Omrani, H.; Mirzamohammadi, Z.; Shahbazfar, A.A. The effect of single dose of thymoquinone, the main constituents of Nigella sativa, in guinea pig model of asthma. BioImpacts 2014, 4, 75–81. [Google Scholar]
- Xiong, Y.; Wang, J.; Yu, H.; Zhang, X.; Miao, C. Anti-asthma potential of crocin and its effect on MAPK signaling pathway in a murine model of allergic airway disease. Immunopharmacol. Immunotoxicol. 2015, 37, 236–243. [Google Scholar] [CrossRef]
- Noorbakhsh, M.F.; Shaterzadeh-Yazdi, H.; Hayati, F.; Samarghandian, S.; Farkhondeh, T. Protective effects of thymoquinon on pulmonary disorders in experimental studies. Tanaffos 2018, 17, 211–222. [Google Scholar]
- Pashirzad, M.; Shafiee, M.; Avan, A.; Ryzhikov, M.; Fiuji, H.; Bahreyni, A.; Khazaei, M.; Soleimanpour, S.; Hassanian, S.M. Therapeutic potency of crocin in the treatment of inflammatory diseases: Current status and perspective. J. Cell. Physiol. 2019, 234, 14601–14611. [Google Scholar] [CrossRef]
- Berry, M.A.; Shaw, D.E.; Green, R.H.; Brightling, C.E.; Wardlaw, A.J.; Pavord, I.D. The use of exhaled nitric oxide concentration to identify eosinophilic airway inflammation: An observational study in adults with asthma. Clin. Exp. Allergy 2005, 35, 1175–1179. [Google Scholar] [CrossRef]
- Fireman, E.; Shtark, M.; Priel, I.E.; Shiner, R.; Mor, R.; Kivity, S.; Fireman, Z. Hydrogen peroxide in exhaled breath condensate (EBC) vs eosinophil count in induced sputum (IS) in parenchymal vs. airways lung diseases. Inflammation 2007, 30, 44–51. [Google Scholar] [CrossRef]
- Strunk, R.C.; Szefler, S.J.; Phillips, B.R.; Zeiger, R.S.; Chinchilli, V.M.; Larsen, G.; Hodgdon, K.; Morgan, W.; Sorkness, C.A.; Lemanske, R.F., Jr. Relationship of exhaled nitric oxide to clinical and inflammatory markers of persistent asthma in children. J. Allergy Clin. Immunol. 2003, 112, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Kharitonov, S.A.; Yates, D.H.; Barnes, P.J. Inhaled glucocorticoids decrease nitric oxide in exhaled air of asthmatic patients. Am. J. Respir. Crit. Care Med. 1996, 153, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Silkoff, P.E.; McClean, P.; Spino, M.; Erlich, L.A.; Slutsky, A.S.; Zamel, N. Dose-response relationship and reproducibility of the fall in exhaled nitric oxide after inhaled beclomethasone dipropionate therapy in asthma patients. Chest 2001, 119, 1322–1328. [Google Scholar] [CrossRef]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2015, 1851, 469–484. [Google Scholar] [CrossRef]
- Haworth, O.; Cernadas, M.; Yang, R.; Serhan, C.N.; Levy, B.D. Resolvin E1 regulates interleukin 23, interferon-γ and lipoxin A 4 to promote the resolution of allergic airway inflammation. Nat. Immunol. 2008, 9, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, H.; Miyahara, N.; Gelfand, E.W. The role of leukotriene B4 in allergic diseases. Allergol. Int. 2008, 57, 291–298. [Google Scholar] [CrossRef]
- Yates, C.M.; Calder, P.C.; Rainger, G.E. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol. Ther. 2014, 141, 272–282. [Google Scholar] [CrossRef]
- Weaver, K.L.; Ivester, P.; Seeds, M.; Case, L.D.; Arm, J.P.; Chilton, F.H. Effect of dietary fatty acids on inflammatory gene expression in healthy humans. J. Biol. Chem. 2009, 284, 15400–15407. [Google Scholar] [CrossRef]
- Bilal, S.; Haworth, O.; Wu, L.; Weylandt, K.H.; Levy, B.D.; Kang, J.X. Fat-1 transgenic mice with elevated omega-3 fatty acids are protected from allergic airway responses. Biochim. Biophys. Acta Mol. Basis Dis. 2011, 1812, 1164–1169. [Google Scholar] [CrossRef]
- FAO/WHO. Expert Consultation on Fats and Fatty Acids in Human Nutrition: Fats and Fatty Acids in Human Nutrition: Report of an Expert Consultation, 10–14 November 2008, Geneva; Food and Agriculture Organization of the United Nations: Rome, Italy, 2010. [Google Scholar]
- Yin, H.; Liu, W.; Goleniewska, K.; Porter, N.A.; Morrow, J.D.; Peebles, R.S., Jr. Dietary supplementation of ω-3 fatty acid-containing fish oil suppresses F2-isoprostanes but enhances inflammatory cytokine response in a mouse model of ovalbumin-induced allergic lung inflammation. Free Radic. Biol. Med. 2009, 47, 622–628. [Google Scholar] [CrossRef]
- Okoniewski, W.; Lu, K.D.; Forno, E. Weight loss for children and adults with obesity and asthma. A systematic review of randomized controlled trials. Ann. Am. Thorc. Soc. 2019, 16, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Farzan, S. The asthma phenotype in the obese: Distinct or otherwise? J. Allergy 2013, 2013. [Google Scholar] [CrossRef]
- Sideleva, O.; Suratt, B.T.; Black, K.E.; Tharp, W.G.; Pratley, R.E.; Forgione, P.; Dienz, O.; Irvin, C.G.; Dixon, A.E. Obesity and asthma: An inflammatory disease of adipose tissue not the airway. Am. J. Respir. Crit. Care Med. 2012, 186, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Scott, H.A.; Gibson, P.G.; Garg, M.L.; Pretto, J.J.; Morgan, P.J.; Callister, R.; Wood, L.G. Determinants of weight loss success utilizing a meal replacement plan and/or exercise, in overweight and obese adults with asthma. Respirology 2015, 20, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Berthon, B.S.; Macdonald-Wicks, L.K.; Gibson, P.G.; Wood, L.G. Investigation of the association between dietary intake, disease severity and airway inflammation in asthma. Respirology 2013, 18, 447–454. [Google Scholar] [CrossRef]
- Kuruvilla, M.E.; Lee, F.E.H.; Lee, G.B. Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clin. Rev. Allergy Immunol. 2019, 56, 219–233. [Google Scholar] [CrossRef]
- Han, Y.Y.; Forno, E.; Holguin, F.; Celedón, J.C. Diet and asthma: An update. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 369–374. [Google Scholar] [CrossRef]
| Cluster | First Author (Year) | Study Design | Population | Asthma Diagnosis | Intervention and Dose ## | Study Duration | n | Age (Years) | Male (%) |
|---|---|---|---|---|---|---|---|---|---|
| Herbs, herbal mixtures and extracts | Barlianto (2017) * Barlianto (2018) * [34,35] | Parallel | Children with asthma | GINA guidelines | Nigella sativa oil 15–30 mg/kg/day | 8 weeks | 28 | 9 # | 39 # |
| Hosseini (2018) ** Zilaee (2019) ** [36,37] | Parallel | Adults with mild-to-moderate asthma | GINA guidelines | Saffron 100 mg/day | 8 weeks | 76 | 41 # | 63 # | |
| Khayyal (2003) [38] | Parallel | Adults with mild-to-moderate asthma | National Institutes of Health and GINA guidelines | Aqueous extract of propolis 13% solution, equivalent to active constituents in 2 mL of aqueous extract of propolis per day | 2 months | 46 | Range: 19–52 | 78 # | |
| Koshak (2017) [39] | Parallel | Adults with asthma | GINA guidelines and ACT score | Nigella sativa oil 1 g/day | 4 weeks | 80 | 41 # | 41 # | |
| Salem (2017) [40] | Parallel, 3 arms | Adults with asthma | Previous physician’s diagnosis and National Institutes of Health criteria | Nigella sativa (low dose) 1 g/day | 12 weeks | 76 | 38 # | 34 # | |
| Nigella sativa (high dose) 2 g/day | |||||||||
| Yugandhar (2017) [41] | Parallel | Adults with bronchial asthma | Previous physician’s diagnosis | Extract of B. serrata gum resin and A. marmelos fruit 200 mg/day | 56 days | 29 | 39 # | 41 # | |
| Supplements | Ghaffari (2014) [42] | Parallel | Children with moderate asthma | Previous physician’s diagnosis | Vitamin E 50 mg/day | 8 weeks | 240 | 9 # | 54 # |
| Pearson (2004) [43] | Parallel | Adults with asthma | Previous physician’s diagnosis and medication use | Vitamin E 500 mg/day | 6 weeks | 72 | 48 | 46 | |
| Smith (2015) [44] | Parallel | Children and adults with asthma | Previous physician’s diagnosis, symptoms and medication use | Soy isoflavone 100 mg/day | 6 months | 386 | 36 | 34 | |
| Wood (2008) [45] | Crossover, 3 arms | Adults with stable asthma | Previous physician’s diagnosis, symptoms and airway hyper-responsiveness | Tomato extract 45 mg lycopene/day | 7 days | 22 | 52 | 36 | |
| Tomato juice 45 mg lycopene/day | |||||||||
| Weight loss | Dias-Junior (2014) [46] | Parallel | Obese adults with severe asthma | Previous physician’s diagnosis and treatment according to GINA guidelines | Low-calorie intake, use of sibutramine (10 mg/day) and use of orlistat (max. 120 mg/day) | 6 months | 33 | 43 # | 6 # |
| Jensen (2013) [47] | Parallel | Obese children with asthma | Previous physician’s diagnosis | Energy reduction (−500 kcal/day) and counseling sessions | 10 weeks | 28 | 12 # | 61 # | |
| Toennesen (2018) 1 [48] | Parallel, 4 arms | Adults with asthma | ACQ score and positive diagnostic test | High protein and low glycemic index diet | 8 weeks | 125 | 40 # | 31 # | |
| Combination of diet and exercise | |||||||||
| Vitamin D3 | Bar Yoseph (2015) [49] | Parallel | Children with mild asthma | Previous physician’s diagnosis, positive methacholine challenge test | Vitamin D3 14.000 IU/week | 6 weeks | 39 | 13 # | 64 # |
| Castro (2014) [50] | Parallel | Adults with symptomatic asthma | Previous physician’s diagnosis, evidence of bronchodilator reversibility or airway hyper-responsiveness | Vitamin D3 100.000 IU once, followed by 4000 IU/day | 28 weeks | 408 | 40 # | 32 # | |
| de Groot (2015) [51] | Parallel | Adults with nonatopic asthma | Evidence of bronchodilator reversibility or airway hyper-responsiveness | Vitamin D3 (cholecalciferol) 400.000 IU single dose | 9 weeks | 44 | 56 # | 59 # | |
| Kerley (2016) [52] | Parallel | Children with uncontrolled asthma | Previous physician’s diagnosis and medication use according to GINA guidelines | Vitamin D3 2000 IU/day | 15 weeks | 39 | 8 # | 62 # | |
| Martineau (2015) [53] | Parallel | Adults with asthma | Previous physician’s diagnosis, evidence of bronchodilator reversibility | Vitamin D3 (Vigantol oil) 120.000 IU/2 months | 1 year | 250 | 48 # | 44 # | |
| Omega-3 LCPUFA | Emelyanov (2002) [54] | Parallel | Adults with mild-to-moderate atopic asthma | American Thoracic Society asthma definition | Lipid extract of the New Zealand green-lipped mussel 200 mg/day EPA + DHA | 8 weeks | 46 | 39 # | 26 # |
| Hodge (1998) [55] | Parallel | Children with asthma and a history of episodic wheeze in the last 12 months and airway hyperresponsiveness to histamine | Symptoms and airway hyper-responsiveness | Omega-3 fatty acid-rich diet and omega-3 fatty acid supplementation 1200 mg/day EPA + DHA | 6 months | 39 | 10 # | 41 # | |
| Mickleborough (2013) [56] | Crossover | Adults with mild-to-moderate persistent asthma | Previous physician’s diagnosis | Marine lipid fraction PCSO-524™ 400 mg/day omega-3 LCPUFA, of which 120 mg/day EPA + DHA | 3 weeks | 20 | 23 | 60 | |
| Moreira (2007) [57] | Parallel | Adults with stable, persistent asthma | Previous physician’s diagnosis and use of inhaled corticosteroids | N-3 PUFA 780 mg/day EPA+DHA 10 mg/day vitamin E | 2 weeks | 20 | 38 # | 0 | |
| Schubert (2009) 2 [58] | Parallel | Adults with asthma and house dust mite allergy | Unknown | N-3 PUFA-enriched fat blend 750 mg/day (of which 630 mg/day EPA + DHA) | 3 weeks | 23 | 24 # | 43 # | |
| Whole food approaches | Bseikri (2018) [59] | Parallel | Obese adolescents with asthma | Previous physician’s diagnosis and ACQ score | Nutrient-dense bar (CHORI-bar) 2 bars/day | 2 months | 56 | 15 # | 55 # |
| Papamichael (2019) [60] | Parallel | Children with mild asthma | Previous physician’s diagnosis and GINA guidelines | Two meals with fatty fish per week as part of the Greek Mediterranean diet | 6 months | 64 | 8 # | 52 # | |
| Sexton (2013) [61] | Parallel, 3 arms | Adults with symptomatic asthma | Previous physician’s diagnosis, bronchodilator reversibility or PEFR variability during run-in | High-intervention: encouraged to adopt a Mediterranean diet and received intensive initial advice and 41 h of consultation sessions with a dietitian | 12 weeks | 35 | 38 # | 29 # | |
| Low intervention: received less intensive advice and spent 2 h with a dietitian | |||||||||
| Sudini (2016) [62] | Parallel | Adults with asthma and a positive skin test to an indoor allergen | Previous physician’s diagnosis | Broccoli sprouts 100 g/day | 3 days | 40 | 34 # | 40 # | |
| Wood (2012) 2 [63] | Parallel | Adults with stable asthma | Previous physician’s diagnosis, symptoms and airway hyper-responsiveness | High antioxidant diet | 14 days | 137 | 57 # | 42 # |
| First Author (Year) | Jadad Score *** | Intervention and Dose | Asthma-Related Outcomes | Immunological Parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lung Function | Asthma Control | QoL | |||||||||||
| FEV1 | FVC | PEF | ACT | (P)AQLQ | FeNO | Cells (Sputum, Blood) | Th1 | Th2, IgE | Treg | Pro-Inflammatory Markers | |||
| Barlianto (2017) * Barlianto (2018) * [34,35] | 2 | Nigella sativa oil 15–30 mg/kg/day | ↑ 1 | ↑ | ↓ | ||||||||
| Hosseini (2018) ** Zilaee (2019) ** [36,37] | 5 | Saffron 100 mg/day | ↑ | ↑ | = | ↓ | |||||||
| Khayyal (2003) [38] | 2 | Aqueous extract of propolis 13% solution, equivalent to active constituents in 2 mL of aqueous extract of propolis per day | N/A | N/A | N/A | N/A | N/A | N/A | |||||
| Koshak (2017) [39] | 5 | Nigella sativa oil 1 g/day | = | = | ↑ | ↓ | = | ||||||
| Salem (2017) [40] | 3 | Nigella sativa (low dose) 1 g/day | = | = | ↑ | ↑ | = | ↑ | = | = | = | ||
| Nigella sativa (high dose) 2 g/day | = | = | ↑ | ↑ | = | ↑ | = | = | = | ||||
| Yugandhar (2017) [41] | 4 | Extract of B. serrata gum resin and A. marmelos fruit 200 mg/day | = | ↑ | ↑ | ↑ | ↓ | ||||||
| First Author (Year) | Jadad Score * | Intervention and Dose | Asthma-Related Outcomes | Immunological Parameters | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lung Function | Asthma Control | ||||||||||
| FEV1 | FVC | PEF | ACT | ACQ | FeNO | Cells (Sputum, Blood) | Th2, IgE | Pro-Inflammatory Markers | |||
| Ghaffari (2014) [42] | 3 | Vitamin E 50 mg/day | N/A | N/A | N/A | ||||||
| Pearson (2004) [43] | 5 | Vitamin E 500 mg/day | = | = | = | = | |||||
| Smith (2015) [44] | 5 | Soy isoflavone 100 mg/day | = | ↓ | = | = | ↑ | = | = | ||
| Wood (2008) [45] | 3 | Tomato extract 45 mg lycopene/day | = | = | = | = | ↓; = | ||||
| Tomato juice 45 mg lycopene/day | = | = | = | = | ↓; = | ||||||
| First Author (Year) | Jadad Score * | Intervention and Dose | Asthma-Related Outcomes | Immunological Parameters | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lung Function | Asthma Control | QoL | |||||||||
| FEV1 | FVC | ACT | ACQ | (P)AQLQ | FeNO | Cells (Sputum, Blood) | Th2, IgE | Pro-Inflammatory Markers | |||
| Dias-Junior (2014) [46] | 3 | Low-calorie intake, use of sibutramine (10 mg/day) and use of orlistat (max. 120 mg/day) | = | ↑ | ↑ | ↓ | = | = | = | = | |
| Jensen (2013) [47] | 3 | Energy reduction (−500 kcal/day) and counseling sessions | = | = | ↓ | = | = | ↓; = | ↓; = | ||
| Toennesen (2018) [48] | 3 | High protein and low glycemic index diet | = | = | = | = | = | = | = | ||
| Combination of diet and exercise | = | = | ↓ | ↑ | = | = | = | ||||
| First Author (Year) | Jadad Score * | Intervention and Dose | Asthma-Related Outcomes | Immunological Parameters | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lung Function | Asthma Control | QoL | ||||||||||||
| FEV1 | FVC | PEF | ACT | ACQ | (P)AQLQ | Other | FeNO | Cells (Sputum, Blood) | Th2, IgE | Treg | Pro-Inflammatory Markers | |||
| Bar Yoseph (2015) [49] | 4 | Vitamin D3 14.000 IU/week | = 1 | = | = | = | = | |||||||
| Castro (2014) [50] | 5 | Vitamin D3 100.000 IU once, followed by 4000 IU/day | = | = | = | |||||||||
| de Groot (2015) [51] | 4 | Vitamin D3 (Cholecalciferol) 400.000 IU single dose | = | = | = | = | ↓ 2 | = | ||||||
| Kerley (2016) [52] | 3 | Vitamin D3 2000 IU/day | = | = | = | = | = | ↑ | ||||||
| Martineau (2015) [53] | 5 | Vitamin D3 (Vigantol oil) 120.000 IU/2 months | = | = | = | = | ||||||||
| First Author (Year) | Jadad Score * | Intervention and Dose | Asthma-Related Outcomes | Immunological Parameters | ||||
|---|---|---|---|---|---|---|---|---|
| Lung Function | Asthma Control | |||||||
| FEV1 | PEF | ACQ | FeNO | Cells (Sputum, Blood) | Other | |||
| Emelyanov (2002) [54] | 5 | Lipid extract of the New Zealand green-lipped mussel 200 mg/day EPA+DHA | = | ↑; = | ↓ 1 | |||
| Hodge (1998) [55] | 4 | Omega-3 fatty acid-rich diet and omega-3 fatty acid supplementation 1200 mg/day EPA+DHA | = | = | ||||
| Mickleborough (2013) [56] | 5 | Marine lipid fraction PCSO-524™ 400 mg/day omega-3 LCPUFA, of which 120 mg/day EPA+DHA | ↑ | ↓ | ||||
| Moreira (2007) [57] | 5 | N-3 PUFA 780 mg/day EPA+DHA 10 mg/day vitamin E | = | = | = | |||
| Schubert (2009) [58] | 4 | N-3 PUFA-enriched fat blend 750 mg/day (of which 630 mg/day EPA+DHA) | = | ↓ | = | |||
| First Author (Year) | Jadad Score * | Intervention and Dose | Asthma-Related Outcomes | Immunological Parameters | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lung Function | Asthma Control | QoL | ||||||||||||
| FEV1 | FVC | PEF | ACT | ACQ | (P)AQLQ | FeNO | Cells (Sputum, Blood) | Th1 | Th2, IgE | Treg | Pro-Inflammatory Markers | |||
| Bseikri (2018) [59] | 2 | Nutrient-dense bar (CHORI-bar) 2 bars/day | = | = | = | = | = | = | ||||||
| Papamichael (2019) [60] | 3 | Mediterranean diet | = | = | = | = | = | ↓ 1 | ||||||
| Sexton (2013) [61] | 2 | Mediterranean diet (high intervention) 2 | = | = | = | = | = | = | = | = | ||||
| Mediterranean diet (low intervention) 2 | = | = | = | = | = | = | = | = | ||||||
| Sudini (2016) [62] | 4 | Broccoli sprouts 100 g/day | = | = | = | = | = | = | ||||||
| Wood (2012) [63] | 3 | High antioxidant diet | ↑ | ↑ | = | = | = | = | = | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Brakel, L.; Mensink, R.P.; Wesseling, G.; Plat, J. Nutritional Interventions to Improve Asthma-Related Outcomes through Immunomodulation: A Systematic Review. Nutrients 2020, 12, 3839. https://doi.org/10.3390/nu12123839
van Brakel L, Mensink RP, Wesseling G, Plat J. Nutritional Interventions to Improve Asthma-Related Outcomes through Immunomodulation: A Systematic Review. Nutrients. 2020; 12(12):3839. https://doi.org/10.3390/nu12123839
Chicago/Turabian Stylevan Brakel, Lieve, Ronald P. Mensink, Geertjan Wesseling, and Jogchum Plat. 2020. "Nutritional Interventions to Improve Asthma-Related Outcomes through Immunomodulation: A Systematic Review" Nutrients 12, no. 12: 3839. https://doi.org/10.3390/nu12123839
APA Stylevan Brakel, L., Mensink, R. P., Wesseling, G., & Plat, J. (2020). Nutritional Interventions to Improve Asthma-Related Outcomes through Immunomodulation: A Systematic Review. Nutrients, 12(12), 3839. https://doi.org/10.3390/nu12123839







