Is Gluten the Only Culprit for Non-Celiac Gluten/Wheat Sensitivity?
Abstract
:1. Introduction
2. What about Gluten?
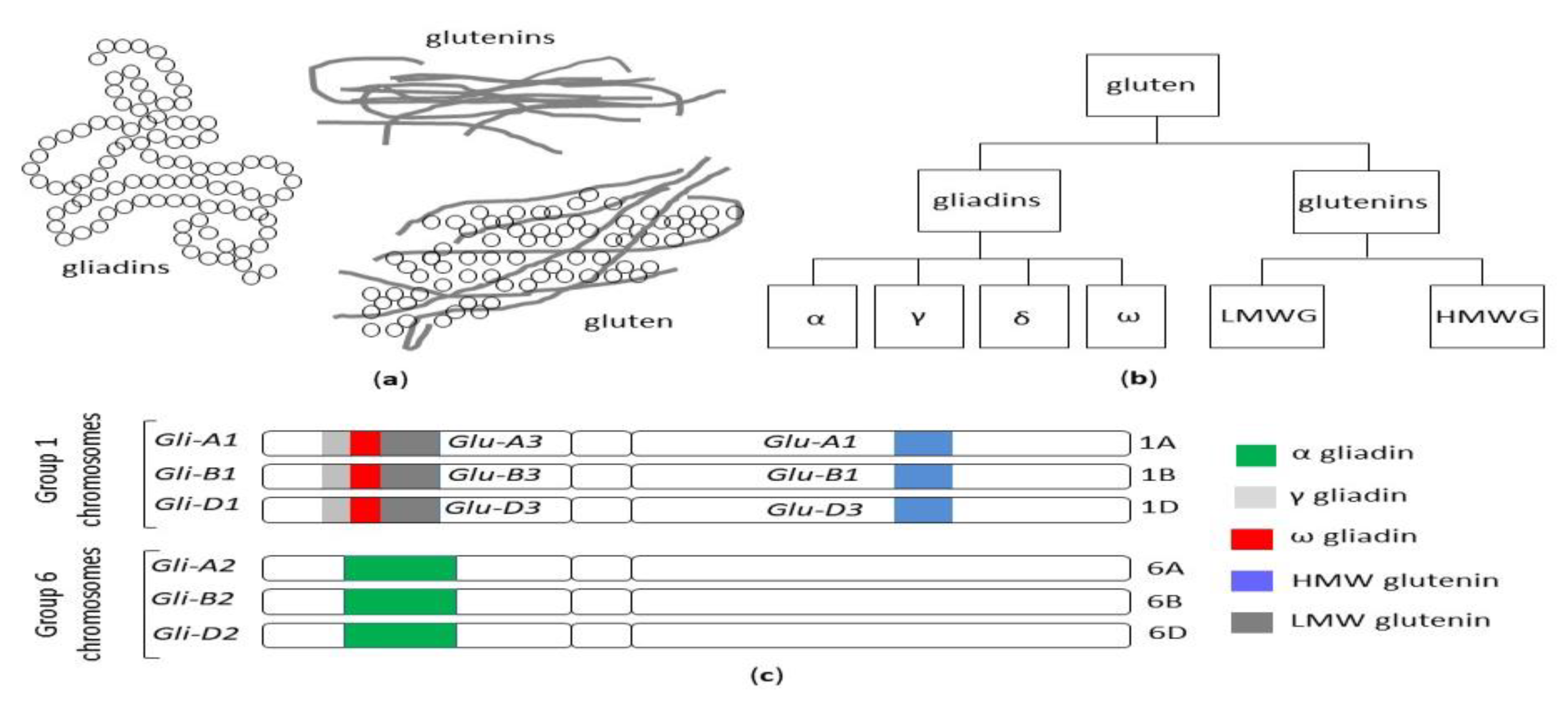
2.1. Gluten Structure and Genetics
2.2. Is There a Role for Microbiota?
2.3. Gluten Consumption
2.4. Gluten Exorphins
3. Not Only Gluten
3.1. Wheat α-Amylase/Trypsin Inhibitors (ATIs)
3.2. Wheat Germ Agglutinin (WGA)
3.3. Fructans
3.4. Wheat Glyphosate
4. Gluten-Related Disorders
4.1. Celiac Disease (CD)
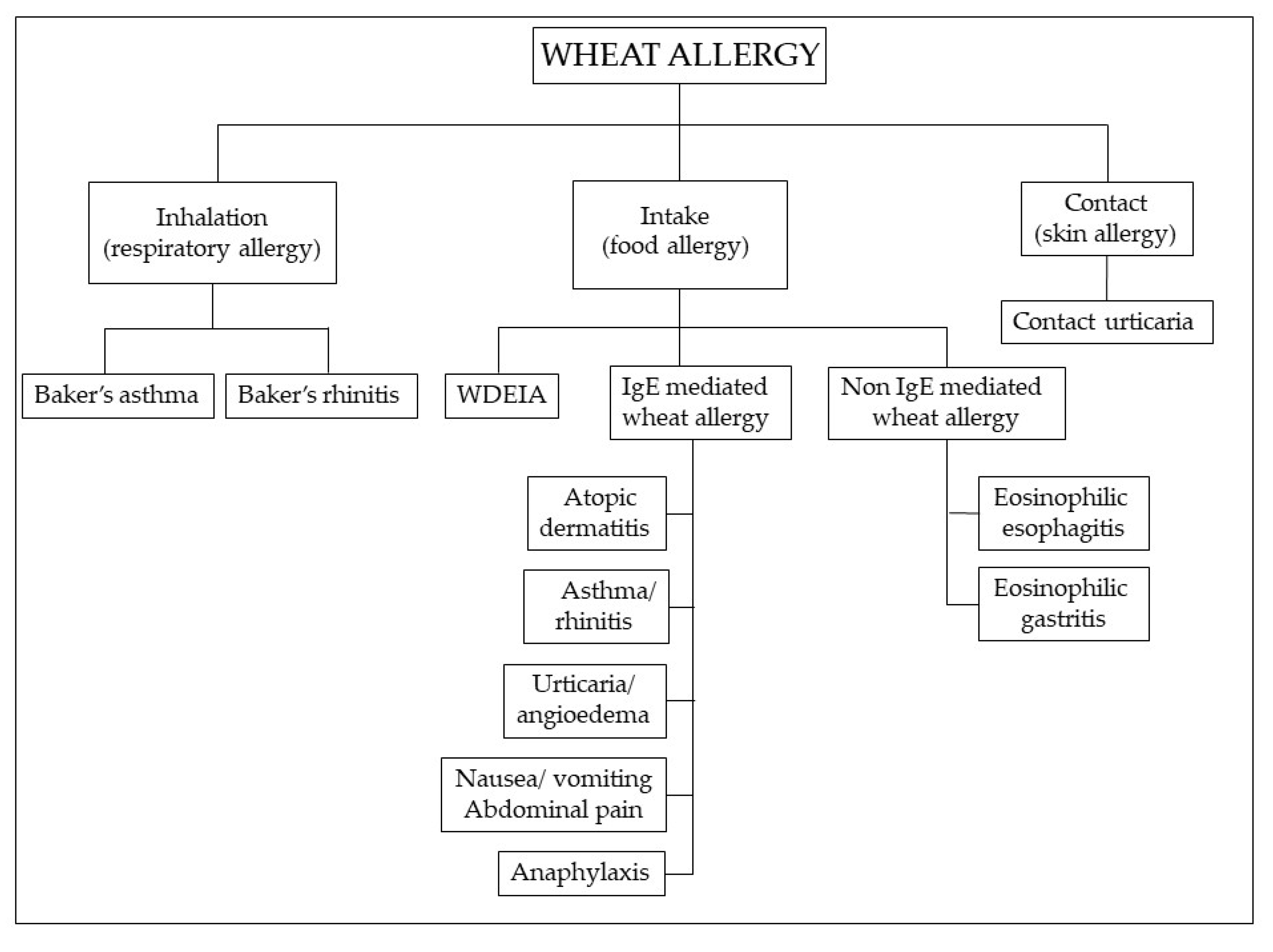
4.2. Wheat Allergy (WA)
4.3. Non-Celiac Gluten/Wheat Sensitivity (NCG/WS)
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rostami, K.; Bold, J.; Parr, A.; Johnson, M.W. Gluten-Free Diet Indications, Safety, Quality, Labels, and Challenges. Nutrients 2017, 9, 846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Research and Markets. Global Gluten-Free Food Market (2018–2023) Report; ID: 4856374; Research and Markets: Dublin, Ireland, 2019. [Google Scholar]
- Kim, H.S.; Patel, K.G.; Orosz, E.; Kothari, N.; Demyen, M.F.; Pyrsopoulos, N.; Ahlawat, S.K. Time Trends in the Prevalence of Celiac Disease and Gluten-Free Diet in the US Population. Results From the National Health and Nutrition Examination Surveys 2009–2014. JAMA Intern. Med. 2016, 176, 1716–1717. [Google Scholar] [CrossRef] [PubMed]
- NPD Group. Percentage of U.S. Adults Trying to Cut Down or Avoid Gluten in Their Diets Reaches New High in 2013. Available online: http://www.npd.com/wps/portal/npd/us/news/press-releases/percentage-of-us-adults-trying-to-cut-down-or-avoid-gluten-in-their-diets-reaches-new-high-in-2013-reports-npd (accessed on 27 June 2020).
- Gatti, S.; Lionetti, E.; Balanzoni, L.; Verma, A.K.; Galeazzi, T.; Gesuita, R.; Scattolo, N.; Cinquetti, M.; Fasano, A.; Catassi, C. Celiac Screening Team. Increased Prevalence of Celiac Disease in School-age Children in Italy. Clin. Gastroenterol. Hepatol. 2020, 18, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Sapone, A.; Zevallos, V.; Schuppan, D. Nonceliac gluten sensitivity. Gastroenterology 2015, 148, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Zannini, E.; Arendt, E.K. Low FODMAPs and gluten-free foods for irritable bowel syndrome treatment: Lights and shadows. Food Res. Int. 2018, 110, 33–41. [Google Scholar] [CrossRef]
- Tanpowpong, P.; Broder-Fingert, S.; Katz, A.J.; Camargo, C.A., Jr. Predictors of dietary gluten avoidance in adults without a prior diagnosis of celiac disease. Nutrition 2015, 31, 236–238. [Google Scholar] [CrossRef]
- Lis, D.M. Exit Gluten-Free and Enter Low FODMAPs: A Novel Dietary Strategy to Reduce Gastrointestinal Symptoms in Athletes. Sports Med. 2019, 49, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Golley, S.; Corsini, N.; Topping, D.; Morell, M.; Mohr, P. Motivations for avoiding wheat consumption in Australia: Results from a population survey. Public Health Nutr. 2015, 18, 490–499. [Google Scholar] [CrossRef] [Green Version]
- The Hartman Group, Inc. The Hartman Group’s Health & Wellness 2015 and Organic & Natural 2014 Reports. Available online: http://www.hartman-group.com/acumenPdfs/gluten-free-2015-09-03.pdf (accessed on 22 December 2015).
- Gorgitano, M.T.; Sodano, V. Gluten-Free Products: From Dietary Necessity to Premium Price Extraction Tool. Nutrients 2019, 11, 1997. [Google Scholar] [CrossRef] [Green Version]
- Zysk, W.; Głąbska, D.; Guzek, D. Role of Front-of-Package Gluten-Free Product Labeling in a Pair-Matched Study in Women with and without Celiac Disease on a Gluten-Free Diet. Nutrients 2019, 11, 398. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, C.; Hieke, S.; Taper, C.; Siegrist, M. European consumer healthiness evaluation of ‘Free-from’ labelled food products. Food Qual. Prefer. 2018, 68, 377–388. [Google Scholar] [CrossRef]
- Di Liberto, D.; Carlisi, D.; D’Anneo, A.; Emanuele, S.; Giuliano, M.; De Blasio, A.; Calvaruso, G.; Lauricella, M. Gluten Free Diet for the Management of Non Celiac Diseases: The Two Sides of the Coin. Healthcare 2020, 8, 400. [Google Scholar] [CrossRef] [PubMed]
- Kreutz, J.M.; Adriaanse, M.P.M.; van der Ploeg, E.M.C.; Vreugdenhil, A.C.E. Narrative Review: Nutrient Deficiencies in Adults and Children with Treated and Untreated Celiac Disease. Nutrients 2020, 12, 500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Sabatino, A.; Corazza, G.R. Nonceliac gluten sensitivity: Sense or sensibility? Ann. Intern. Med. 2012, 156, 309–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niland, B.; Cash, B.D. Health Benefits and Adverse Effects of a Gluten-Free Diet in Non-Celiac Disease Patients. Gastroenterol. Hepatol. 2018, 14, 82–91. [Google Scholar]
- Biesiekierski, J.R. What is gluten? J. Gastroenterol. Hepatol. 2017, 32, 78–81. [Google Scholar] [CrossRef] [Green Version]
- Hoffmanová, I.; Sánchez, D.; Szczepanková, A.; Tlaskalová-Hogenová, H. The Pros and Cons of Using Oat in a Gluten-Free Diet for Celiac Patients. Nutrients 2019, 11, 2345. [Google Scholar] [CrossRef] [Green Version]
- Lundin, K.E.; Nilsen, E.M.; Scott, H.G.; Løberg, E.M.; Gjøen, A.; Bratlie, J.; Skar, V.; Mendez, E.; Løvik, A.; Kett, K. Oats induced villous atrophy in coeliac disease. Gut 2003, 52, 1649–1652. [Google Scholar] [CrossRef] [Green Version]
- Arentz-Hansen, H.; Fleckenstein, B.; Molberg, Ø.; Scott, H.; Koning, F.; Jung, G.; Roepstorff, P.; Lundin, K.E.; Sollid, L.M. The molecular basis for oat intolerance in patients with celiac disease. PLoS Med. 2004, 1, e1. [Google Scholar] [CrossRef]
- Silano, M.; Pozo, E.P.; Uberti, F.; Manferdelli, S.; Del Pinto, T.; Felli, C.; Budelli, A.; Vincentini, O.; Restani, P. Diversity of oat varieties in eliciting the early inflammatory events in celiac disease. Eur. J. Nutr. 2014, 53, 1177–1186. [Google Scholar] [CrossRef] [Green Version]
- Poley, J.R. The Gluten-Free Diet: Can Oats and Wheat Starch Be Part of It? J. Am. Coll. Nutr. 2017, 36, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shewry, P. What Is Gluten-Why Is It Special? Front. Nutr. 2019, 6, 101. [Google Scholar] [CrossRef]
- Sharma, N.; Bhatia, S.; Chunduri, V.; Kaur, S.; Sharma, S.; Kapoor, P.; Kumari, A.; Garg, M. Pathogenesis of Celiac Disease and Other Gluten Related Disorders in Wheat and Strategies for Mitigating Them. Front. Nutr. 2020, 7, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Liu, Y.; Tong, J.; Yu, L.; Ding, M.; Zhang, Z.; Rehman, A.U.; Majzoobi, M.; Wang, Z.; Gao, X. The overexpression of high-molecular-weight glutenin subunit Bx7 improves the dough rheological properties by altering secondary and micro-structures of wheat gluten. Food Res. Int. 2020, 130, 108914. [Google Scholar] [CrossRef] [PubMed]
- Balakireva, A.V.; Zamyatnin, A.A. Properties of Gluten Intolerance: Gluten Structure, Evolution, Pathogenicity and Detoxification Capabilities. Nutrients 2016, 8, 644. [Google Scholar] [CrossRef] [Green Version]
- Lerner, A.; Shoenfeld, Y.; Matthias, T. Adverse effects of gluten ingestion and advantages of gluten withdrawal in nonceliac autoimmune disease. Nutr. Rev. 2017, 75, 1046–1058. [Google Scholar] [CrossRef] [Green Version]
- Balfourier, F.; Bouchet, S.; Robert, S.; De Oliveira, R.; Rimbert, H.; Kitt, J.; Choulet, F.; Paux, E. International Wheat Genome Sequencing Consortium; BreedWheat Consortium. Worldwide phylogeography and history of wheat genetic diversity. Sci. Adv. 2019, 5, eaav0536. [Google Scholar] [CrossRef] [Green Version]
- Venske, E.; Dos Santos, R.S.; Busanello, C.; Gustafson, P.; Costa de Oliveira, A. Bread wheat: A role model for plant domestication and breeding. Hereditas 2019, 156, 16. [Google Scholar] [CrossRef] [Green Version]
- Van den Broeck, H.C.; de Jong, H.C.; Salentijn, E.M.; Dekking, L.; Bosch, D.; Hamer, R.J.; Gilissen, L.J.; van der Meer, I.M.; Smulders, M.J. Presence of celiac disease epitopes in modern and old hexaploid wheat varieties: Wheat breeding may have contributed to increased prevalence of celiac disease. Theor. Appl. Genet. 2010, 12, 1527–1539. [Google Scholar] [CrossRef] [Green Version]
- Huo, N.; Zhang, S.; Zhu, T.; Dong, L.; Wang, Y.; Mohr, T.; Hu, T.; Liu, Z.; Dvorak, J.; Luo, M.C.; et al. Gene Duplication and Evolution Dynamics in the Homeologous Regions Harboring Multiple Prolamin and Resistance Gene Families in Hexaploid Wheat. Front. Plant Sci. 2018, 9, 673. [Google Scholar] [CrossRef] [Green Version]
- Huo, N.; Zhu, T.; Altenbach, S.; Dong, L.; Wang, Y.; Mohr, T.; Liu, Z.; Dvorak, J.; Luo, M.C.; Gu, Y.Q. Dynamic Evolution of α-Gliadin Prolamin Gene Family in Homeologous Genomes of Hexaploid Wheat. Sci. Rep. 2018, 8, 5181. [Google Scholar] [CrossRef] [PubMed]
- Malalgoda, M.; Meinhardt, S.W.; Simsek, S. Detection and quantitation of immunogenic epitopes related to celiac disease in historical and modern hard red spring wheat cultivars. Food Chem. 2018, 264, 101–107. [Google Scholar] [CrossRef]
- Prandi, B.; Tedeschi, T.; Folloni, S.; Galaverna, G.; Sforza, S. Peptides from gluten digestion: A comparison between old and modern wheat varieties. Food Res. Int. 2017, 91, 92–102. [Google Scholar] [CrossRef]
- Ficco, D.B.; Prandi, B.; Amaretti, A.; Anfelli, I.; Leonardi, A.; Raimondi, S.; Pecchioni, N.; De Vita, P.; Faccini, A.; Sforza, S.; et al. Comparison of gluten peptides and potential prebiotic carbohydrates in old and modern Triticum turgidum ssp. genotypes. Food Res. Int. 2019, 120, 568–576. [Google Scholar] [CrossRef]
- Ribeiro, M.; Nunes, F.M. We might have got it wrong: Modern wheat is not more toxic for celiac patients. Food Chem. 2019, 278, 820–822. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Whittaker, A.; Gori, A.M.; Cesari, F.; Surrenti, E.; Abbate, R.; Gensini, G.F.; Benedettelli, S.; Casini, A. Effect of Triticum turgidum subsp. turanicum wheat on irritable bowel syndrome: A double-blinded randomised dietary intervention trial. Br. J. Nutr. 2014, 111, 1992–1999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasarda, D.D. Can an increase in celiac disease be attributed to an increase in the gluten content of wheat as a consequence of wheat breeding? J. Agric. Food Chem. 2013, 61, 1155–1159. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Spencer, S.P.; Fragiadakis, G.K.; Sonnenburg, J.L. Pursuing Human-Relevant Gut Microbiota-Immune Interactions. Immunity 2019, 51, 225–239. [Google Scholar] [CrossRef]
- Fasano, A. All disease begins in the (leaky) gut: Role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Research 2020, 9, F1000 Faculty Rev-69. [Google Scholar] [CrossRef]
- Alam, A.; Neish, A. Role of gut microbiota in intestinal wound healing and barrier function. Tissue Barriers 2018, 6, 1539595. [Google Scholar] [CrossRef] [PubMed]
- Chibbar, R.; Dieleman, L.A. The Gut Microbiota in Celiac Disease and probiotics. Nutrients 2019, 11, 2375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collado, M.C.; Donat, E.; Ribes-Koninckx, C.; Calabuig, M.; Sanz, Y. Specific duodenal and faecal bacterial groups associated with paediatric coeliac disease. J. Clin. Pathol. 2009, 62, 264–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caio, G.; Lungaro, L.; Segata, N.; Guarino, M.; Zoli, G.; Volta, U.; De Giorgio, R. Effect of Gluten-Free Diet on Gut Microbiota Composition in Patients with Celiac Disease and Non-Celiac Gluten/Wheat Sensitivity. Nutrients 2020, 12, 1832. [Google Scholar] [CrossRef] [PubMed]
- Bonder, M.J.; Tigchelaar, E.F.; Cai, X.; Trynka, G.; Cenit, M.C.; Hrdlickova, B.; Zhong, H.; Vatanen, T.; Gevers, D.; Wijmenga, C.; et al. The influence of a short-term gluten-free diet on the human gut microbiome. Genome Med. 2016, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Reddel, S.; Putignani, L.; Del Chierico, F. The Impact of Low-FODMAPs, Gluten-Free, and Ketogenic Diets on Gut Microbiota Modulation in Pathological Conditions. Nutrients 2019, 11, 373. [Google Scholar] [CrossRef] [Green Version]
- Olivares, M.; Neef, A.; Castillejo, G.; Palma, G.D.; Varea, V.; Capilla, A.; Palau, F.; Nova, E.; Marcos, A.; Polanco, I.; et al. The HLA-DQ2 genotype selects for early intestinal microbiota composition in infants at high risk of developing coeliac disease. Gut 2015, 64, 406–417. [Google Scholar] [CrossRef]
- Olivares, M.; Benítez-Páez, A.; de Palma, G.; Capilla, A.; Nova, E.; Castillejo, G.; Varea, V.; Marcos, A.; Garrote, J.A.; Polanco, I.; et al. Increased prevalence of pathogenic bacteria in the gut microbiota of infants at risk of developing celiac disease: The PROFICEL study. Gut Microbes 2018, 9, 551–558. [Google Scholar] [CrossRef] [Green Version]
- Lebwohl, B.; Murray, J.A.; Verdú, E.F.; Crowe, S.E.; Dennis, M.; Fasano, A.; Green, P.H.; Guandalini, S.; Khosla, C. Gluten Introduction, Breastfeeding, and Celiac Disease: Back to the Drawing Board. Am. J. Gastroenterol. 2016, 111, 12–14. [Google Scholar] [CrossRef] [Green Version]
- Koletzko, S.; Lee, H.S.; Beyerlein, A.; Aronsson, C.A.; Hummel, M.; Liu, E.; Simell, V.; Kurppa, K.; Lernmark, Å.; Hagopian, W.; et al. Cesarean Section on the Risk of Celiac Disease in the Offspring: The Teddy Study. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 417–424. [Google Scholar] [CrossRef]
- Kołodziej, M.; Patro-Gołąb, B.; Gieruszczak-Białek, D.; Skórka, A.; Pieścik-Lech, M.; Baron, R.; Szajewska, H. Association between early life (prenatal and postnatal) antibiotic administration and coeliac disease: A systematic review. Arch. Dis. Child. 2019, 104, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Kemppainen, K.M.; Vehik, K.; Lynch, K.F.; Larsson, H.E.; Canepa, R.J.; Simell, V.; Koletzko, S.; Liu, E.; Simell, O.G.; Toppari, J.; et al. Association between Early-Life Antibiotic Use and the Risk of Islet or Celiac Disease Autoimmunity. JAMA Pediatr. 2017, 171, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Verdu, E.F.; Galipeau, H.J.; Jabri, B. Novel players in coeliac disease pathogenesis: Role of the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 497–506. [Google Scholar] [CrossRef]
- Valitutti, F.; Cucchiara, S.; Fasano, A. Celiac Disease and the Microbiome. Nutrients 2019, 11, 2403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cenit, M.C.; Olivares, M.; Codoñer-Franch, P.; Sanz, Y. Intestinal Microbiota and Celiac Disease: Cause, Consequence or Co-Evolution? Nutrients 2015, 7, 6900–6923. [Google Scholar] [CrossRef] [Green Version]
- Caminero, A.; Meisel, M.; Jabri, B.; Verdu, E.F. Mechanisms by which gut microorganisms influence food sensitivities. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Caminero, A.; Verdu, E.F. Celiac disease: Should we care about microbes? Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G161–G170. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Globalization of Food Systems in Developing Countries: Impact on Food Security and Nutrition; FAO Food and Nutrition Paper 2004, n. 83; Food and Agriculture Organization of the United Nations: Rome, Italy, 2004; pp. 1–14, ISSN 0254-4725. [Google Scholar]
- Food and Agriculture Organization of the United Nations. Crop Breeding: The Green Revolution and the Preceding Millennia. Available online: http://www.fao.org/english/newsroom/focus/2003/gmo2.htm (accessed on 28 June 2020).
- Karim, M.B. The Green Revolution: An International Bibliography; Greenwood: Westport, CT, USA, 1986; pp. 1–28. [Google Scholar]
- Enghiad, A.; Ufer, D.; Countryman, A.M.; Thilmany, D.D. An Overview of Global Wheat Market Fundamentals in an Era of Climate Concerns. Int. J. Agron. 2017, 2017, 3931897. [Google Scholar] [CrossRef]
- Tiwari, H. Wheat Gluten Market: Global Industry Analysis 2014–2018 and Opportunity Assessment 2019–2029; Future Market Insights: London, UK, 2020. [Google Scholar]
- MarketsandMarkets. Wheat Protein Market; Report Code: FB 5949; MarketsandMarkets: Pune, India, 2018. [Google Scholar]
- Day, L.; Augustin, M.A.; Batey, I.L.; Wrigley, C.W. Wheat-gluten uses and industry needs. Trends Food Sci. Technol. 2006, 17, 82–90. [Google Scholar] [CrossRef]
- Dhiraj, B.; Prabhasankar, P. Influence of Wheat-Milled Products and Their Additive Blends on Pasta Dough Rheological, Microstructure, and Product Quality Characteristics. Int. J. Food Sci. 2013, 2013, 538070. [Google Scholar] [CrossRef] [Green Version]
- Giannou, V.; Tzia, C. Addition of Vital Wheat Gluten to Enhance the Quality Characteristics of Frozen Dough Products. Foods 2016, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Teschemacher, H. Opioid receptor ligands derived from food proteins. Curr. Pharm. Des. 2003, 9, 1331–1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Udenigwe, C.C. Role of food-derived opioid peptides in the central nervous and gastrointestinal systems. J. Food Biochem. 2019, 43, e12629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lister, J.; Fletcher, P.J.; Nobrega, J.N.; Remington, G. Behavioral effects of food-derived opioid-like peptides in rodents: Implications for schizophrenia? Pharmacol. Biochem. Behav. 2015, 134, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Glass, M.J.; Grace, M.; Cleary, J.P.; Billington, C.J.; Levine, A.S. Potency of naloxone’s anorectic effect in rats is dependent on diet preference. Am. J. Physiol. 1996, 271, R217–R221. [Google Scholar] [CrossRef] [PubMed]
- Boggiano, M.M.; Chandler, P.C.; Viana, J.B.; Oswald, K.D.; Maldonado, C.R.; Wauford, P.K. Combined dieting and stress evoke exaggerated responses to opioids in binge-eating rats. Behav. Neurosci. 2005, 119, 1207–1214. [Google Scholar] [CrossRef]
- Morley, J.E.; Levine, A.S.; Yamada, T.; Gebhard, R.L.; Prigge, W.F.; Shafer, R.B.; Goetz, F.C.; Silvis, S.E. Effect of exorphins on gastrointestinal function, hormonal release, and appetite. Gastroenterology 1983, 84, 1517–1523. [Google Scholar] [CrossRef]
- Corazza, G.R.; Frazzoni, M.; Strocchi, A.; Prati, C.; Sarchielli, P.; Capelli, M. Alimentary exorphin actions on motility and hormonal secretion of gastrointestinal tract. In Opioid Peptides in the Periphery; Fraioli, F., Isidori, A., Mazzetti, M., Eds.; Elsevier Sciences Publisher: Amsterdam, NL, USA, 1984; pp. 243–247. [Google Scholar]
- Takahashi, M.; Fukunaga, H.; Kaneto, H.; Fukudome, S.; Yoshikawa, M. Behavioral and pharmacological studies on gluten exorphin A5, a newly isolated bioactive food protein fragment, in mice. Jpn. J. Pharmacol. 2000, 84, 259–265. [Google Scholar] [CrossRef] [Green Version]
- Bressan, P.; Kramer, P. Bread and Other Edible Agents of Mental Disease. Front. Hum. Neurosci. 2016, 10, 130. [Google Scholar] [CrossRef] [Green Version]
- Dupont, F.M.; Vensel, W.H.; Tanaka, C.K.; Hurkman, W.J.; Altenbach, S.B. Deciphering the complexities of the wheat flour proteome using quantitative two-dimensional electrophoresis, three proteases and tandem mass spectrometry. Proteome Sci. 2011, 9, 10. [Google Scholar] [CrossRef] [Green Version]
- Guo, G.; Lv, D.; Yan, X.; Subburaj, S.; Ge, P.; Li, X.; Hu, Y.; Yan, Y. Proteome characterization of developing grains in bread wheat cultivars (Triticum aestivum L.). BMC Plant Biol. 2012, 12, 147. [Google Scholar] [CrossRef] [Green Version]
- Finnie, C.; Melchior, S.; Roepstorff, P.; Svensson, B. Proteome analysis of grain filling and seed maturation in barley. Plant Physiol. 2002, 129, 1308–1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junker, Y.; Zeissig, S.; Kim, S.J.; Barisani, D.; Wieser, H.; Leffler, D.A.; Zevallos, V.; Libermann, T.A.; Dillon, S.; Freitag, T.L.; et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J. Exp. Med. 2012, 209, 2395–2408. [Google Scholar] [CrossRef] [PubMed]
- Caminero, A.; McCarville, J.L.; Zevallos, V.F.; Pigrau, M.; Xuechen, B.Y.; Jury, J.; Galipeau, H.J.; Clarizio, A.V.; Casqueiro, J.; Murray, J.A.; et al. Lactobacilli Degrade Wheat Amylase Trypsin Inhibitors to Reduce Intestinal Dysfunction Induced by Immunogenic Wheat Proteins. Gastroenterology 2019, 156, 2266–2280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zevallos, V.F.; Raker, V.; Tenzer, S.; Jimenez-Calvente, C.; Ashfaq-Khan, M.; Russel, N.; Pickert, G.; Schild, H.; Steinbrink, K.; Schuppan, D. Nutritional wheat amylase-trypsin inhibitors promote intestinal inflammation via activation of myeloid cells. Gastroenterology 2017, 152, 1100–1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leccioli, V.; Oliveri, M.; Romeo, M.; Berretta, M.; Rossi, P. A New Proposal for the Pathogenic Mechanism of Non-Coeliac/Non-Allergic Gluten/Wheat Sensitivity: Piecing Together the Puzzle of Recent Scientific Evidence. Nutrients 2017, 9, 1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstein, I.J.; Winter, H.C.; Poretz, R.D. Glicoproteins II. Wheat germ agglutinin. New Comprehensive. Biochemistry 1997, 29 Pt B, 403–474. [Google Scholar]
- Peumans, W.J.; Van Damme, E.J.M. Prevalence, biological activity and genetic manipulation of lectins in foods. Trends Food Sci. Technol. 1996, 7, 132–138. [Google Scholar] [CrossRef]
- Haas, H.; Falcone, F.H.; Schramm, G.; Haisch, K.; Gibbs, B.F.; Klaucke, J.; Poppelmann, M.; Becker, W.M.; Gabius, H.J.; Schlaak, M. Dietary lectins can induce in vitro release of IL-4 and IL-13 from human basophils. Eur. J. Immunol. 1999, 29, 918–927. [Google Scholar] [CrossRef]
- Dalla Pellegrina, C.; Perbellini, O.; Scupoli, M.T.; Tomelleri, C.; Zanetti, C.; Zoccatelli, G.; Fusi, M.; Peruffo, A.; Rizzi, C.; Chignola, R. Effects of wheat germ agglutinin on human gastrointestinal epithelium: Insights from an experimental model of immune/epithelial cell interaction. Toxicol. Appl. Pharmacol. 2009, 237, 146–153. [Google Scholar] [CrossRef]
- Muraille, E.; Pajak, B.; Urbain, J.; Leo, O. Carbohydrate-bearing cell surface receptors involved in innate immunity: Interleukin-12 induction by mitogenic and nonmitogenic lectins. Cell Immunol. 1999, 191, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, A.; Kesherwani, V. Production of TNF-alpha, IL-1beta, IL-12 and IFN-gamma in murine peritoneal macrophages on treatment with wheat germ agglutinin in vitro: Involvement of tyrosine kinase pathways. Glycoconj. J. 2007, 24, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Alaedini, A.; Bojarski, C.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; De Magistris, L.; Dieterich, W.; Di Liberto, D.; et al. The overlapping area of non-celiac gluten sensitivity (NCGS) and wheat-sensitive irritable bowel syndrome (IBS): An update. Nutrients 2017, 9, 1268. [Google Scholar] [CrossRef] [Green Version]
- Tchernychev, B.; Wilchek, M. Natural human antibodies to dietary lectins. FEBS Lett. 1996, 397, 139–142. [Google Scholar] [CrossRef] [Green Version]
- Sollid, L.M.; Kolberg, J.; Scott, H.; Ek, J.; Fausa, O.; Brandtzaeg, P. Antibodies to wheat germ agglutinin in coeliac disease. Clin. Exp. Immunol. 1986, 63, 95–100. [Google Scholar]
- Troncone, R.; Auricchio, S.; De Vincenzi, M.; Donatiello, A.; Farris, E.; Silano, V. An analysis of cereals that react with serum antibodies in patients with coeliac disease. J. Pediatr. Gastroenterol. Nutr. 1987, 6, 346–350. [Google Scholar] [CrossRef]
- Volta, U.; Lazzari, R.; Bianchi, F.B.; Lenzi, M.; Baldoni, A.M.; Cassani, F.; Collina, A.; Pisi, E. Antibodies to dietary antigens in coeliac disease. Scand. J. Gastroenterol. 1986, 21, 935–940. [Google Scholar] [CrossRef]
- Pusztai, A.; Ewen, S.W.; Grant, G.; Brown, D.S.; Stewart, J.C.; Peumans, W.J.; Van Damme, E.J.; Bardocz, S. Antinutritive effects of wheat- germ agglutinin and other N-acetylglucosamine-specific lectins. Br. J. Nutr. 1993, 70, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Lorenzsonn, V.; Olsen, W.A. In vivo responses of rat intestinal epithelium to intraluminal dietary lectins. Gastroenterology 1982, 82, 838–848. [Google Scholar] [CrossRef]
- Gibson, P.R.; Muir, J.G.; Newnham, E.D. Other Dietary Confounders: FODMAPS et al. Dig. Dis. 2015, 33, 269–276. [Google Scholar] [CrossRef]
- Gibson, P.R.; Shepherd, S.J. Personal view: Food for thought—Western lifestyle and susceptibility to Crohn’s disease. The FODMAP hypothesis. Aliment Pharmacol. Ther. 2005, 21, 1399–1409. [Google Scholar] [CrossRef]
- Bellini, M.; Gambaccini, D.; Bazzichi, L.; Bassotti, G.; Mumolo, M.G.; Fani, B.; Costa, F.; Ricchiuti, A.; de Bortoli, N.; Mosca, M.; et al. Bioelectrical impedance vector analysis in patients with irritable bowel syndrome on a low FODMAP diet: A pilot study. Tech. Coloproctol. 2017, 21, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.S.; Gearry, R.B.; Muir, J.G.; Irving, P.M.; Rose, R.; Rosella, O.; Haines, M.L.; Shepherd, S.J.; Gibson, P.R. Dietary poorly absorbed, short-chain carbohydrates increase delivery of water and fermentable substrates to the proximal colon. Aliment Pharmacol. Ther. 2010, 31, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Bonfrate, L.; de Bari, O.; Lembo, A.; Ballou, S. Irritable bowel syndrome and diet. Gastroenterol. Rep. 2017, 5, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Nemati, F.; Ghanati, F.; Gavlighi, H.A.; Sharifi, M. Fructan dynamics and antioxidant capacity of 4-day-old seedlings of wheat (Triticum aestivum) cultivars during drought stress and recovery. Funct. Plant Biol. 2018, 45, 1000–1008. [Google Scholar] [CrossRef]
- Biesiekierski, J.R.; Rosella, O.; Rose, R.; Liels, K.; Barrett, J.S.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. Quantification of fructans, galacto-oligosaccharides and other short-chain carbohydrates in processed grains and cereals. J. Hum. Nutr. Diet. 2011, 24, 154–176. [Google Scholar] [CrossRef] [PubMed]
- Whelan, K.; Abrahmsohn, O.; David, G.J.; Staudacher, H.; Irving, P.; Lomer, M.C.; Ellis, P.R. Fructan content of commonly consumed wheat, rye and gluten-free breads. Int. J. Food Sci. Nutr. 2011, 62, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O. The history and current status of glyphosate. Pest Manag. Sci. 2018, 74, 1027–1034. [Google Scholar] [CrossRef]
- Samsel, A.; Seneff, S. Glyphosate, pathways to modern diseases II: Celiac sprue and gluten intolerance. Interdiscip. Toxicol. 2013, 6, 159–184. [Google Scholar] [CrossRef] [Green Version]
- Mesnage, R.; Teixeira, M.; Mandrioli, D.; Falcioni, L.; Ducarmon, Q.R.; Zwittink, R.D.; Amiel, C.; Panoff, J.M.; Belpoggi, F.; Antoniou, M.N. Shotgun metagenomics and metabolomics reveal glyphosate alters the gut microbiome of Sprague-Dawley rats by inhibiting the shikimate pathway. BioRxiv 2019. [Google Scholar] [CrossRef] [Green Version]
- Chłopecka, M.; Mendel, M.; Dziekan, N.; Karlik, W. Glyphosate affects the spontaneous motoric activity of intestine at very low doses—In vitro study. Pestic. Biochem. Physiol. 2014, 113, 25–30. [Google Scholar] [CrossRef]
- Vasiluk, L.; Pinto, L.J.; Moore, M.M. Oral bioavailability of glyphosate: Studies using two intestinal cell lines. Environ. Toxicol. Chem. 2005, 24, 153–160. [Google Scholar] [CrossRef]
- Drago, S.; El Asmar, R.; Di Pierro, M.; Grazia Clemente, M.; Tripathi, A.; Sapone, A.; Thakar, M.; Iacono, G.; Carroccio, A.; D’Agate, C.; et al. Gliadin, zonulin and gut permeability: Effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand. J. Gastroenterol. 2006, 41, 408–419. [Google Scholar] [CrossRef]
- Dubois, P.C.; Trynka, G.; Franke, L.; Hunt, K.A.; Romanos, J.; Curtotti, A.; Zhernakova, A.; Heap, G.A.; Adány, R.; Aromaa, A.; et al. Multiple common variants for celiac disease influencing immune gene expression. Nat. Genet. 2010, 42, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Stene, L.C.; Honeyman, M.C.; Hoffenberg, E.J.; Haas, J.E.; Sokol, R.J.; Emery, L.; Taki, I.; Norris, J.M.; Erlich, H.A.; Eisenbarth, G.S.; et al. Rotavirus Infection Frequency and Risk of Celiac Disease Autoimmunity in Early Childhood: A Longitudinal Study. Am. J. Gastroenterol. 2006, 101, 2333–2340. [Google Scholar] [CrossRef] [PubMed]
- Bouziat, R.; Hinterleitner, R.; Brown, J.J.; Stencel-Baerenwald, J.E.; Ikizler, M.; Mayassi, T.; Meisel, M.; Kim, S.M.; Discepolo, V.; Pruijssers, A.J.; et al. Reovirus infection triggers inflammatory responses to dietary antigens and development of celiac disease. Science 2017, 356, 44–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auricchio, R.; Cielo, D.; de Falco, R.; Galatola, M.; Bruno, V.; Malamisura, B.; Limongelli, M.G.; Troncone, R.; Greco, L. Respiratory Infections and the Risk of Celiac Disease. Pediatrics 2017, 140, e20164102. [Google Scholar] [CrossRef] [Green Version]
- Caminero, A.; McCarville, J.L.; Galipeau, H.J.; Deraison, C.; Bernier, S.P.; Constante, M.; Rolland, C.; Meisel, M.; Murray, J.A.; Yu, X.B.; et al. Duodenal bacterial proteolytic activity determines sensitivity to dietary antigen through protease-activated receptor-2. Nat. Commun. 2019, 10, 1198. [Google Scholar] [CrossRef]
- Pozo-Rubio, T.; Olivares, M.; Nova, E.; De Palma, G.; Mujico, J.R.; Ferrer, M.D.; Marcos, A.; Sanz, Y. Immune development and intestinal microbiota in celiac disease. Clin. Dev. Immunol. 2012, 2012, 654143. [Google Scholar] [CrossRef]
- Olivares, M.; Walker, A.W.; Capilla, A.; Benítez-Páez, A.; Palau, F.; Parkhill, J.; Castillejo, G.; Sanz, Y. Gut Microbiota Trajectory in Early Life May Predict Development of Celiac Disease. Microbiome 2018, 6, 36. [Google Scholar] [CrossRef] [Green Version]
- Rintala, A.; Riikonen, I.; Toivonen, A.; Pietilä, S.; Munukka, E.; Pursiheimo, J.P.; Elo, L.L.; Arikoski, P.; Luopajärvi, K.; Schwab, U.; et al. Early fecal microbiota composition in children who later develop celiac disease and associated autoimmunity. Scand. J. Gastroenterol. 2018, 53, 403–409. [Google Scholar] [CrossRef] [Green Version]
- Lionetti, E.; Castellaneta, S.; Francavilla, R.; Pulvirenti, A.; Tonutti, E.; Amarri, S.; Barbato, M.; Barbera, C.; Barera, G.; Bellantoni, A.; et al. SIGENP (Italian Society of Pediatric Gastroenterology, Hepatology, and Nutrition) Working Group on Weaning and CD Risk. Introduction of gluten, HLA status, and the risk of celiac disease in children. N. Engl. J. Med. 2014, 371, 1295–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aronsson, C.A.; Lee, H.S.; Liu, E.; Uusitalo, U.; Hummel, S.; Yang, J.; Hummel, M.; Rewers, M.; She, J.X.; Simell, O.; et al. Age at gluten introduction and risk of celiac disease. Pediatrics 2015, 135, 239–245. [Google Scholar] [CrossRef] [Green Version]
- Leonard, M.M.; Fasano, A. Gluten and celiac disease risk: Is it just a matter of quantity? JAMA 2019, 322, 510–511. [Google Scholar] [CrossRef]
- Lund-Blix, N.A.; Mårild, K.; Tapia, G.; Norris, J.M.; Stene, L.C.; Størdal, K. Gluten Intake in Early Childhood and Risk of Celiac Disease in Childhood: A Nationwide Cohort Study. Am. J. Gastroenterol. 2019, 114, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Dydensborg Sander, S.; Hansen, A.V.; Størdal, K.; Andersen, A.N.; Murray, J.A.; Husby, S. Mode of delivery is not associated with celiac disease. Clin. Epidemiol. 2018, 10, 323–332. [Google Scholar] [CrossRef] [Green Version]
- Sander, S.D.; Andersen, A.M.; Murray, J.A.; Karlstad, Ø.; Husby, S.; Størdal, K. Association Between Antibiotics in the First Year of Life and Celiac Disease. Gastroenterology 2019, 156, 2217–2229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collin, P.; Vilppula, A.; Luostarinen, L.; Holmes, G.K.T.; Kaukinen, K. Review article: Coeliac disease in later life must not be missed. Aliment Pharmacol. Ther. 2018, 47, 563–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebwohl, B.; Sanders, D.S.; Green, P.H.R. Coeliac disease. Lancet 2018, 391, 70–81. [Google Scholar] [CrossRef]
- Denham, J.M.; Hill, I.D. Celiac disease and autoimmunity: Review and controversies. Curr. Allergy Asthma Rep. 2013, 13, 347–353. [Google Scholar] [CrossRef] [Green Version]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.; Kurppa, K.; Mearin, M.L.; Ribes-Koninckx, C.; Shamir, R.; Troncone, R.; Auricchio, R.; Castillejo, G.; et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosing Coeliac Disease 2020. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 141–156. [Google Scholar] [CrossRef] [Green Version]
- Caio, G.; Volta, U.; Sapone, A.; Leffler, D.A.; De Giorgio, R.; Catassi, C.; Fasano, A. Celiac disease: A comprehensive current review. BMC Med. 2019, 17, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eigner, W.; Bashir, K.; Primas, C.; Kazemi-Shirazi, L.; Wrba, F.; Trauner, M.; Vogelsang, H. Dynamics of occurrence of refractory coeliac disease and associated complications over 25 years. Aliment Pharmacol. Ther. 2017, 45, 364–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Sabatino, A.; Rosado, M.M.; Cazzola, P.; Riboni, R.; Biagi, F.; Carsetti, R.; Corazza, G.R. Splenic Hypofunction and the Spectrum of Autoimmune and Malignant Complications in Celiac Disease. Clin. Gastroenterol. Hepatol. 2006, 4, 179–186. [Google Scholar] [CrossRef]
- Zuidmeer, L.; Goldhahn, K.; Rona, R.J.; Gislason, D.; Madsen, C.; Summers, C.; Sodergren, E.; Dahlstrom, J.; Lindner, T.; Sigurdardottir, S.T.; et al. The prevalence of plant food allergies: A systematic review. J. Allergy Clin. Immunol. 2008, 121, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Matricardi, P.M.; Bockelbrink, A.; Beyer, K.; Keil, T.; Niggemann, B.; Grüber, C.; Wahn, U.; Lau, S. Primary versus secondary immunoglobulin E sensitization to soy and wheat in the Multi-Centre Allergy Study cohort. Clin. Exp. Allergy 2008, 38, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Keet, C.A.; Matsui, E.C.; Dhillon, G.; Lenehan, P.; Paterakis, M.; Wood, R.A. The natural history of wheat allergy. Ann. Allergy Asthma Immunol. 2009, 102, 410–415. [Google Scholar] [CrossRef]
- Devonshire, A.L.; Durrani, S.; Assa‘ad, A. Non-IgE mediated food allergy during infancy. Curr. Opin. Allergy Clin. Immunol. 2020, 20, 292–298. [Google Scholar] [CrossRef]
- Anvari, S.; Miller, J.; Yeh, C.Y.; Davis, C.M. IgE-Mediated Food Allergy. Clin. Rev. Allergy Immunol. 2019, 57, 244–260. [Google Scholar] [CrossRef] [Green Version]
- Ricci, G.; Andreozzi, L.; Cipriani, F.; Giannetti, A.; Gallucci, M.; Caffarelli, C. Wheat Allergy in Children: A Comprehensive Update. Medicina 2019, 55, 400. [Google Scholar] [CrossRef] [Green Version]
- Elli, L.; Branchi, F.; Tomba, C.; Villalta, D.; Norsa, L.; Ferretti, F.; Roncoroni, L.; Bardella, M.T. Diagnosis of gluten related disorders: Celiac disease, wheat allergy and non-celiac gluten sensitivity. World J Gastroenterol. 2015, 21, 7110–7119. [Google Scholar] [CrossRef] [PubMed]
- Scherf, K.A.; Brockow, K.; Biedermann, T.; Koehler, P.; Wieser, H. Wheat-dependent exercise-induced anaphylaxis. Clin. Exp. Allergy 2016, 46, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Tatham, A.S.; Shewry, P.R. Allergens to wheat and related cereals. Clin. Exp. Allergy 2008, 38, 1712–1726. [Google Scholar] [CrossRef] [PubMed]
- Salcedo, G.; Quirce, S.; Diaz-Perales, A. Wheat allergens associated with Baker’s asthma. J. Investig. Allergol. Clin. Immunol. 2011, 21, 81–94. [Google Scholar]
- Le, T.A.; Al Kindi, M.; Tan, J.A.; Smith, A.; Heddle, R.J.; Kette, F.E.; Hissaria, P.; Smith, W.B. The clinical spectrum of omega-5-gliadin allergy. Intern. Med. J. 2016, 46, 710–716. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Jiang, N.N.; Wen, L.P.; Li, H.; Yin, J. Wheat allergy in patients with recurrent urticaria. World Allergy Organ. J. 2019, 12, 100013. [Google Scholar] [CrossRef] [Green Version]
- Cianferoni, A. Wheat allergy: Diagnosis and management. J. Asthma Allergy 2016, 9, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Catassi, C.; Elli, L.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; Cellier, C.; Cristofori, F.; de Magistris, L.; Dolinsek, J.; et al. Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): The Salerno Experts’ Criteria. Nutrients 2015, 7, 4966–4977. [Google Scholar] [CrossRef]
- Dolan, R.; Chey, W.D.; Eswaran, S. The role of diet in the management of irritable bowel syndrome: A focus on FODMAPs. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 607–615. [Google Scholar] [CrossRef]
- Cooper, B.T.; Holmes, G.K.; Ferguson, R.; Thompson, R.A.; Allan, R.N.; Cooke, W.T. Gluten-sensitive diarrhea without evidence of celiac disease. Gastroenterology 1980, 79, 801–806. [Google Scholar] [CrossRef]
- Kaukinen, K.; Turjanmaa, K.; Mäki, M.; Partanen, J.; Venäläinen, R.; Reunala, T.; Collin, P. Intolerance to cereals is not specific for coeliac disease. Scand. J. Gastroenterol. 2000, 35, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Sapone, A.; Bai, J.C.; Ciacci, C.; Dolinsek, J.; Green, P.H.R.; Hadjivassiliou, M.; Kaukinen, K.; Rostami, K.; Sanders, D.S.; Schumann, M.; et al. Spectrum of gluten-related disorders: Consensus on new nomenclature and classification. BMC Med. 2012, 10, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volta, U.; Bardella, M.T.; Calabro, A.; Troncone, R.; Corazza, G.R. Study Group for Non-Celiac Gluten Sensitivity. An Italian prospective multicenter survey on patients suspected of having non-celiac gluten sensitivity. BMC Med. 2014, 12, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uhde, M.; Caio, G.; De Giorgio, R.; Green, P.H.; Volta, U.; Alaedini, A. Subclass Profile of IgG Antibody Response to Gluten Differentiates Nonceliac Gluten Sensitivity From Celiac Disease. Gastroenterology 2020, 159, 1965–1967. [Google Scholar] [CrossRef] [PubMed]
- Clemente, E.; Efthymakis, K.; Carletti, E.; Capone, V.; Sperduti, S.; Bologna, G.; Marchisio, M.; Di Nicola, M.; Neri, M.; Sallese, M. An explorative study identifies miRNA signatures for the diagnosis of non-celiac wheat sensitivity. PLoS ONE 2019, 14, e0226478. [Google Scholar] [CrossRef]
- Aziz, I.; Lewis, N.R.; Hadjivassiliou, M.; Winfield, S.N.; Rugg, N.; Kelsall, A.; Newrick, L.; Sanders, D.S. A UK study assessing the population prevalence of self-reported gluten sensitivity and referral characteristics to secondary care. Eur. J. Gastroenterol. Hepatol. 2014, 26, 33–39. [Google Scholar] [CrossRef]
- Carroccio, A.; Giambalvo, O.; Blasca, F.; Iacobucci, R.; D’Alcamo, A.; Mansueto, P. Self-Reported Non-Celiac Wheat Sensitivity in High School Students: Demographic and Clinical Characteristics. Nutrients 2017, 9, 771. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Suarez, M.G.; Murray, J.A. Nonceliac Gluten and Wheat Sensitivity. Clin. Gastroenterol. Hepatol. 2020, 18, 1913–1922. [Google Scholar] [CrossRef]
- Molina-Infante, J.; Carroccio, A. Suspected Nonceliac Gluten Sensitivity Confirmed in Few Patients After Gluten Challenge in Double-Blind, Placebo-Controlled Trials. Clin. Gastroenterol. Hepatol. 2017, 15, 339–348. [Google Scholar] [CrossRef] [Green Version]
- Ong, D.K.; Mitchell, S.B.; Barrett, J.S.; Shepherd, S.J.; Irving, P.M.; Biesiekierski, J.R.; Smith, S.; Gibson, P.R.; Muir, J.G. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J. Gastroenterol. Hepatol. 2010, 25, 1366–1373. [Google Scholar] [CrossRef]
- Biesiekierski, J.R.; Peters, S.L.; Newnham, E.D.; Rosella, O.; Muir, J.G.; Gibson, P.R. No Effects of Gluten in Patients with Self-Reported Non-Celiac Gluten Sensitivity After Dietary Reduction of Fermentable, Poorly Absorbed, Short-Chain Carbohydrates. Gastroenterology 2013, 145, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Skodje, G.I.; Sarna, V.K.; Minelle, I.H.; Rolfsen, K.L.; Muir, J.G.; Gibson, P.R.; Veierød, M.B.; Henriksen, C.; Lundin, K.E.A. Fructan, rather than gluten, induces symptoms in patients with self-reported non-celiac gluten sensitivity. Gastroenterology 2018, 154, 529–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volta, U.; Caio, G.; De Giorgio, R. More Than One Culprit for Nonceliac Gluten/Wheat Sensitivity. Gastroenterology 2018, 155, 227. [Google Scholar] [CrossRef] [PubMed]
- Skodje, G.I.; Henriksen, C.; Veierød, M.B.; Gibson, P.R.; Lundin, K.E.A. More Than One Culprit for Nonceliac Gluten/Wheat Sensitivity Reply. Gastroenterology 2018, 155, 228. [Google Scholar] [CrossRef] [PubMed]
- Laudisi, F.; Stolfi, C.; Monteleone, G. Impact of Food Additives on Gut Homeostasis. Nutrients 2019, 1, 2334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bischoff, S.; Crowe, S.E. Gastrointestinal food allergy: New insights into pathophysiology and clinical perspectives. Gastroenterology 2005, 128, 1089–1113. [Google Scholar] [CrossRef]
- Carroccio, A.; Mansueto, P.; D’Alcamo, A.; Iacono, G. Non-celiac wheat sensitivity as an allergic condition: Personal experience and narrative review. Am. J. Gastroenterol. 2013, 108, 1845–1853. [Google Scholar] [CrossRef]
- Carroccio, A.; Mansueto, P.; Iacono, G.; Soresi, M.; D’Alcamo, A.; Cavataio, F.; Brusca, I.; Florena, A.M.; Ambrosiano, G.; Seidita, A.; et al. Non-celiac wheat sensitivity diagnosed by double-blind placebo-controlled challenge: Exploring a new clinical entity. Am. J. Gastroenterol. 2012, 107, 1898–1906. [Google Scholar] [CrossRef] [Green Version]
- Sapone, A.; Lammers, K.M.; Casolaro, V.; Cammarota, M.; Giuliano, M.T.; De Rosa, M.; Stefanile, R.; Mazzarella, G.; Tolone, C.; Russo, M.I.; et al. Divergence of gut permeability and mucosal immune gene expression in two gluten-associated conditions: Celiac disease and gluten sensitivity. BMC Med. 2011, 9, 23. [Google Scholar] [CrossRef] [Green Version]
- Di Liberto, D.; Mansueto, P.; D’Alcamo, A.; Lo Pizzo, M.; Lo Presti, E.; Geraci, G.; Fayer, F.; Guggino, G.; Iacono, G.; Dieli, F.; et al. Predominance of type 1 innate lymphoid cells in the rectal mucosa of patients with non-celiac wheat sensitivity: Reversal after a wheat-free diet. Clin. Transl. Gastroenterol. 2016, 7, e178. [Google Scholar] [CrossRef] [Green Version]
- Volta, U.; Caio, G.; Karunaratne, T.B.; Alaedini, A.; De Giorgio, R. Non-coeliac gluten/wheat sensitivity: Advances in knowledge and relevant questions. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Fritscher-Ravens, A.; Schuppan, D.; Ellrichmann, M.; Schoch, S.; Röcken, C.; Brasch, J.; Bethge, J.; Böttner, M.; Klose, J.; Milla, P.J. Confocal endomicroscopy shows food-associated changes in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology 2014, 147, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Cardoso-Silva, D.; Delbue, D.; Itzlinger, A.; Moerkens, R.; Withoff, S.; Branchi, F.; Schumann, M. Intestinal Barrier Function in Gluten-Related Disorders. Nutrients 2019, 11, 2325. [Google Scholar] [CrossRef] [Green Version]
- Volta, U.; De Giorgio, R.; Caio, G.; Uhde, M.; Manfredini, R.; Alaedini, A. Nonceliac Wheat Sensitivity: An Immune-Mediated Condition with Systemic Manifestations. Gastroenterol. Clin. N. Am. 2019, 48, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Rotondi Aufiero, V.; Fasano, A.; Mazzarella, G. Non-Celiac Gluten Sensitivity: How Its Gut Immune Activation and Potential Dietary Management Differ from Celiac Disease. Mol. Nutr. Food Res. 2018, 62, e1700854. [Google Scholar] [CrossRef] [PubMed]
- Vazquez–Roque, M.I.; Camilleri, M.; Smyrk, T.; Murray, J.A.; Marietta, E.; O’Neill, J.; Carlson, P.; Lamsam, J.; Janzow, D.; Eckert, D.; et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: Effects on bowel frequency and intestinal function. Gastroenterology 2013, 144, 903–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volta, U.; Tovoli, F.; Cicola, R.; Parisi, C.; Fabbri, A.; Piscaglia, M.; Fiorini, E.; Caio, G. Serological tests in gluten sensitivity (nonceliac gluten intolerance). J. Clin. Gastroenterol. 2012, 46, 680–685. [Google Scholar] [CrossRef]
- Uhde, M.; Ajamian, M.; Caio, G.; De Giorgio, R.; Indart, A.; Green, P.H.; Verna, E.C.; Volta, U.; Alaedini, A. Intestinal cell damage and systemic immune activation in individuals reporting sensitivity to wheat in the absence of coeliac disease. Gut 2016, 65, 1930–1937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollon, J.; Puppa, E.L.; Greenwald, B.; Goldberg, E.; Guerrerio, A.; Fasano, A. Effect of gliadin on permeability of intestinal biopsy explants from celiac disease patients and patients with non-celiac gluten sensitivity. Nutrients 2015, 7, 1565–1576. [Google Scholar] [CrossRef]
- Usai-Satta, P.; Bassotti, G.; Bellini, M.; Oppia, F.; Lai, M.; Cabras, F. Irritable Bowel Syndrome and Gluten-Related Disorders. Nutrients 2020, 12, 1117. [Google Scholar] [CrossRef] [Green Version]
- Lovell, R.M.; Ford, A.C. Global prevalence of and risk factors for irritable bowel syndrome: A meta-analysis. Clin. Gastroenterol. Hepatol. 2012, 10, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Lacy, B.E.; Patel, N.K. Rome criteria and a diagnostic approach to irritable bowel syndrome. J. Clin. Med. 2017, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Lacy, B.E.; Talley, N.J. Irritable bowel syndrome. N. Engl. J. Med. 2017, 376, 2566–2578. [Google Scholar] [CrossRef] [Green Version]
- De Giorgio, R.; Volta, U.; Gibson, P.R. Sensitivity to wheat, gluten and FODMAPs in IBS: Facts or fiction? Gut 2016, 65, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Czaja-Bulsa, G. Non coeliac gluten sensitivity—A new disease with gluten intolerance. Clin. Nutr. 2015, 34, 189–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makharia, A.; Catassi, C.; Makharia, G.K. The overlap between irritable bowel syndrome and non-celiac gluten sensitivity: A clinical dilemma. Nutrients 2015, 7, 10417–10426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, P.A.; Fraher, M.H.; Quigley, E.M. Irritable bowel syndrome: The role of food in pathogenesis and management. Gastroenterol. Hepatol. 2014, 10, 164–174. [Google Scholar]
- Simrén, M.; Månsson, A.; Langkilde, A.M.; Svedlund, J.; Abrahamsson, H.; Bengtsson, U.; Björnsson, E.S. Food-Related Gastrointestinal Symptoms in the Irritable Bowel Syndrome. Digestion 2001, 63, 108–115. [Google Scholar] [CrossRef]
- Bellini, M.; Gambaccini, D.; Stasi, C.; Urbano, M.T.; Marchi, S.; Usai-Satta, P. Irritable bowel syndrome: A disease still searching for pathogenesis, diagnosis and therapy. World J. Gastroenterol. 2014, 20, 8807–8820. [Google Scholar] [CrossRef]
- Lacy, B.E.; Mearin, F.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407. [Google Scholar] [CrossRef] [Green Version]
- Bellini, M.; Tonarelli, S.; Nagy, A.G.; Pancetti, A.; Costa, F.; Ricchiuti, A.; de Bortoli, N.; Mosca, M.; Marchi, S.; Rossi, A. Low FODMAP Diet: Evidence, Doubts, and Hopes. Nutrients 2020, 12, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, P.R. History of the low FODMAP diet. J. Gastroenterol. Hepatol. 2017, 32, 5–7. [Google Scholar] [CrossRef] [Green Version]
- Marsh, A.; Eslick, E.M.; Eslick, G.D. Does a diet low in FODMAPs reduce symptoms associated with functional gastrointestinal disorders? A comprehensive systematic review and meta-analysis. Eur. J. Nutr. 2016, 55, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Schumann, D.; Klose, P.; Lauche, R.; Dobos, G.; Langhorst, J.; Cramer, H. Low fermentable, oligo-, di-, mono-saccharides and polyol diet in the treatment of irritable bowel syndrome: A systematic review and meta-analysis. Nutrition 2018, 45, 24–31. [Google Scholar] [CrossRef]
- Dionne, J.; Ford, A.C.; Yuan, Y.; Chey, W.D.; Lacy, B.E.; Saito, Y.A.; Quigley, E.M.M.; Moayyedi, P. A Systematic Review and Meta-Analysis Evaluating the Efficacy of a Gluten-Free Diet and a Low FODMAPs Diet in Treating Symptoms of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2018, 113, 1290–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellini, M.; Tonarelli, S.; Barracca, F.; Morganti, R.; Pancetti, A.; Bertani, L.; de Bortoli, N.; Costa, F.; Mosca, M.; Marchi, S.; et al. A Low-FODMAP Diet for Irritable Bowel Syndrome: Some Answers to the Doubts from a Long-Term Follow-Up. Nutrients 2020, 12, 2360. [Google Scholar] [CrossRef]
- AtkinsD, B.; Briss, P.A.; Eccles, M.; Falck-Ytter, Y.; Flottorp, S. Grading quality of evidence and strength of recommendations. BMJ 2004, 328, 1490. [Google Scholar] [CrossRef] [Green Version]
- McKenzie, Y.A.; Bowyer, R.K.; Leach, H.; Gulia, P.; Horobin, J.; O’Sullivan, N.A.; Pettitt, C.; Reeves, L.B.; Seamark, L.; Williams, M.; et al. British Dietetic Association systematic review and evidence based practice guidelines for the dietary management of irritable bowel syndrome in adults (2016 update). J. Hum. Nutr. Diet. 2016, 29, 549–575. [Google Scholar] [CrossRef] [Green Version]
- Morcos, A.; Dinan, T.; Quigley, E.M. Irritable bowel syndrome: Role of food in pathogenesis and management. J. Dig. Dis. 2009, 10, 237–246. [Google Scholar] [CrossRef]
- Bohn, L.; Storsrud, S.; Tornblom, H.; Bengtsson, U.; Simren, M. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am. J. Gastroenterol. 2013, 108, 634–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, R.L.; Vazquez-Roque, M.I.; Carlson, P.; Burton, D.; Grover, M.; Camilleri, M.; Turner, J.R. Gluten-induced symptoms in diarrhea-predominant irritable bowel syndrome are associated with increased myosin light chain kinase activity and claudin-15 expression. Lab. Investig. 2017, 97, 14–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto-Sanchez, M.I.; Nardelli, A.; Borojevic, R.; De Palma, G.; Calo, N.C.; McCarville, J.; Caminero, A.; Basra, D.; Mordhorst, A.; Ignatova, E.; et al. Gluten-free Diet Reduces Symptoms, Particularly Diarrhea, in Patients With Irritable Bowel Syndrome and Anti-gliadin IgG. Clin. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Volta, U.; Caio, G.; Tovoli, F.; De Giorgio, R. Non-celiac gluten sensitivity: Questions still to be answered despite increasing awareness. Cell Mol. Immunol. 2013, 10, 383–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mumolo, M.G.; Rettura, F.; Melissari, S.; Costa, F.; Ricchiuti, A.; Ceccarelli, L.; de Bortoli, N.; Marchi, S.; Bellini, M. Is Gluten the Only Culprit for Non-Celiac Gluten/Wheat Sensitivity? Nutrients 2020, 12, 3785. https://doi.org/10.3390/nu12123785
Mumolo MG, Rettura F, Melissari S, Costa F, Ricchiuti A, Ceccarelli L, de Bortoli N, Marchi S, Bellini M. Is Gluten the Only Culprit for Non-Celiac Gluten/Wheat Sensitivity? Nutrients. 2020; 12(12):3785. https://doi.org/10.3390/nu12123785
Chicago/Turabian StyleMumolo, Maria Gloria, Francesco Rettura, Sara Melissari, Francesco Costa, Angelo Ricchiuti, Linda Ceccarelli, Nicola de Bortoli, Santino Marchi, and Massimo Bellini. 2020. "Is Gluten the Only Culprit for Non-Celiac Gluten/Wheat Sensitivity?" Nutrients 12, no. 12: 3785. https://doi.org/10.3390/nu12123785






