Effects of Multi-Ingredient Preworkout Supplementation across a Five-Day Resistance and Endurance Training Microcycle in Middle-Aged Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.2.1. Familiarization
2.2.2. Baseline Assessments
2.2.3. Body Composition
2.2.4. Medicine Ball Throw (MBT)
2.2.5. Vertical Jump
2.2.6. Maximal Isometric Force (MIF)
2.2.7. Tensiomyography Assessment
2.2.8. Incremental Cycling Test to Exhaustion
2.3. Dietary and Supplementation
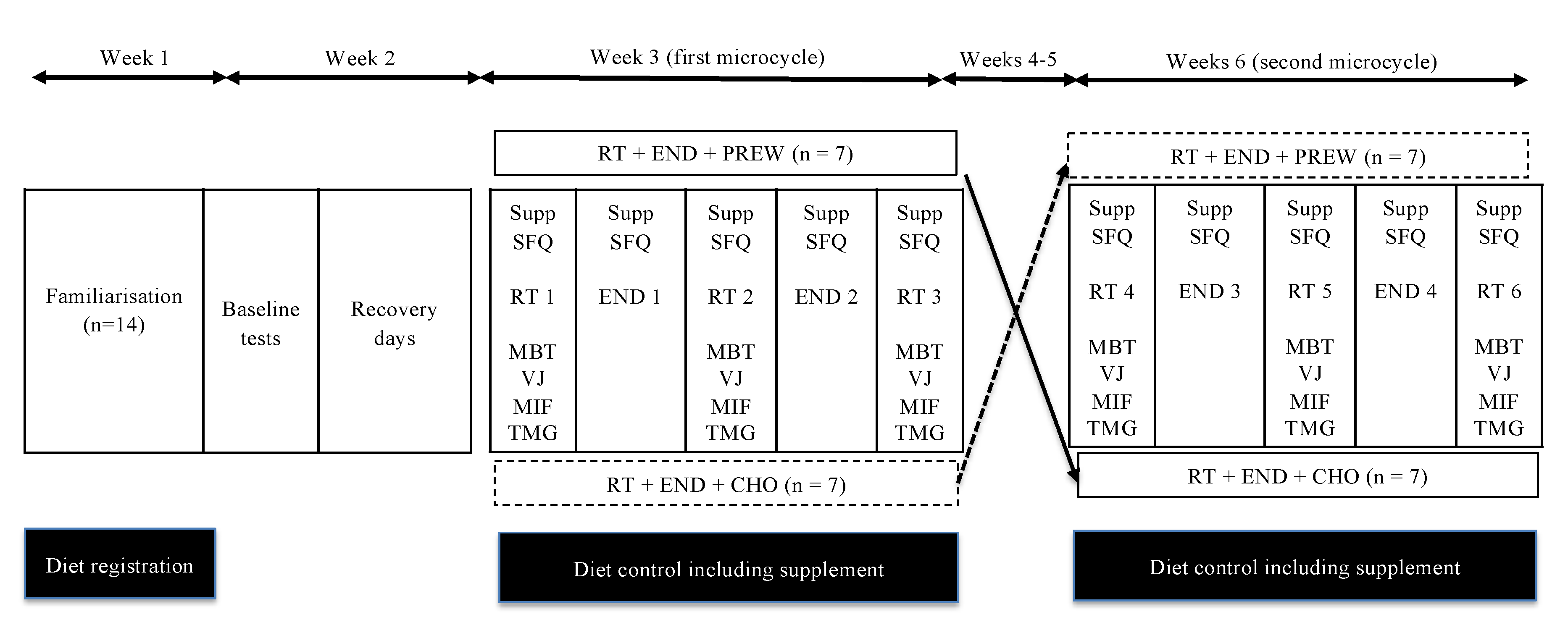
2.4. Exercise Protocols and Postworkout Assessments
2.5. Statistical Analyses
3. Results
3.1. Diet Analysis
3.2. Primary Outcomes
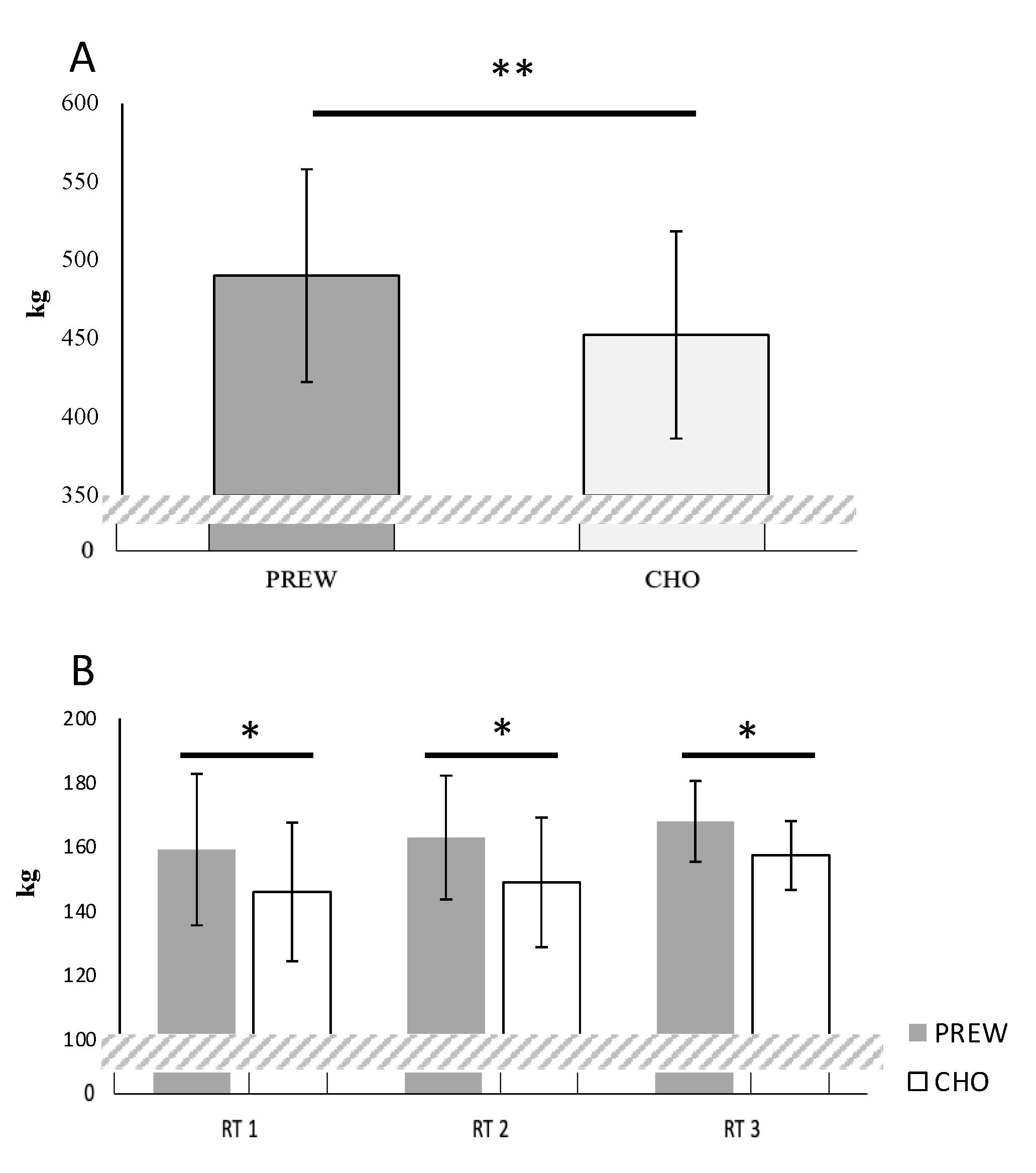
3.2.1. Total Resistance Training Volume Per Session (SVOL)
3.2.2. Subtract Oxidation during Endurance Training Sessions
3.3. Secondary and Exploratory Outcomes
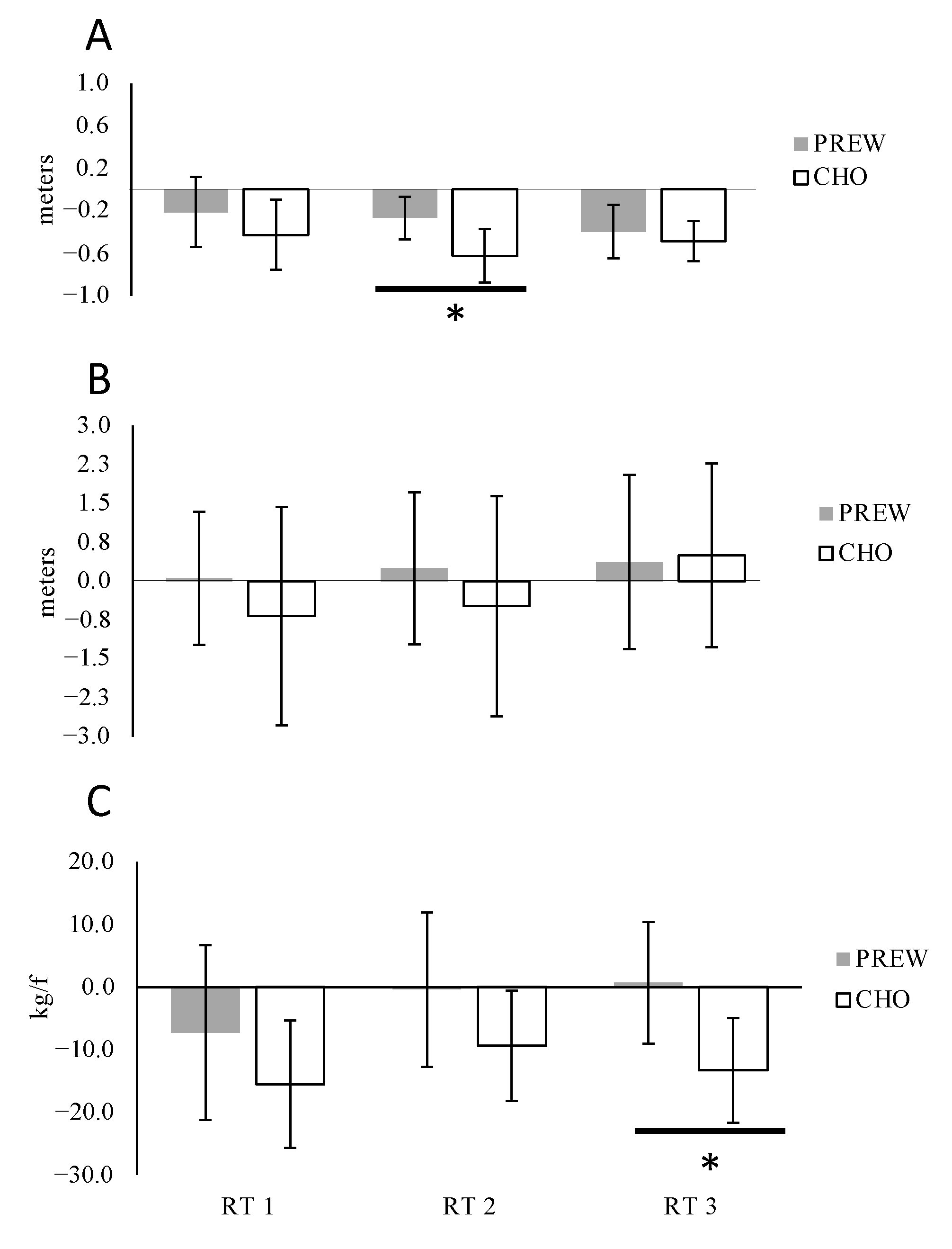
3.3.1. Medicine Ball Throw (MBT)
3.3.2. Vertical Jump (CMJ)
3.3.3. Maximal Isometric Force (MIF)
3.3.4. Tensiomyography
3.3.5. Perception of Effort
3.3.6. Subjective Feelings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jagim, A.R.; Harty, P.S.; Camic, C.L. Common Ingredient Profiles of Multi-Ingredient Pre-Workout Supplements. Nutrients 2019, 11, 254. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.B.; Earnest, C.P.; Dalton, R.; Sowinski, R.J.; Grubic, T.J.; Favot, C.J.; Coletta, A.M.; Rasmussen, C.; Greenwood, M.; Kreider, R.B. Short-Term Effects of a Ready-to-Drink Pre-Workout Beverage on Exercise Performance and Recovery. Nutrients 2017, 9, 823. [Google Scholar] [CrossRef] [PubMed]
- Ormsbee, M.J.; Mandler, W.K.; Thomas, D.D.; Ward, E.G.; Kinsey, A.W.; Simonavice, E.; Panton, L.B.; Kim, J.-S. The effects of six weeks of supplementation with multi-ingredient performance supplements and resistance training on anabolic hormones, body composition, strength, and power in resistance-trained men. J. Int. Soc. Sports Nutr. 2012, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, H.C.; Byrd, M.T.; Wallace, B.; Clasey, J.L. Examination of a Multi-ingredient Preworkout Supplement on Total Volume of Resistance Exercise and Subsequent Strength and Power Performance. J. Strength Cond. Res. 2018, 32, 1479–1490. [Google Scholar] [CrossRef]
- Alkhatib, A.; Seijo, M.; Larumbe-Zabala, E.; Naclerio, F. Acute effectiveness of a “fat-loss” product on substrate utilization, perception of hunger, mood state and rate of perceived exertion at rest and during exercise. J. Int. Soc. Sports Nutr. 2015, 12, 1–8. [Google Scholar] [CrossRef]
- Hoffman, J.R.; Kang, J.; Ratamess, N.A.; Hoffman, M.W.; Tranchina, C.P.; Faigenbaum, A.D. Examination of a pre-exercise, high energy supplement on exercise performance. J. Int. Soc. Sports Nutr. 2009, 6, 2. [Google Scholar] [CrossRef]
- Harty, P.; Zabriskie, H.A.; Erickson, J.L.; Molling, P.; Kerksick, C.M.; Jagim, A.R. Multi-ingredient pre-workout supplements, safety implications, and performance outcomes: A brief review. J. Int. Soc. Sports Nutr. 2018, 15, 41. [Google Scholar] [CrossRef]
- Ratamess, N.A.; Hoffman, J.R.; Ross, R.; Shanklin, M.; Faigenbaum, A.D.; Kang, J. Effects of an amino acid/creatine energy supplement on the acute hormonal response to resistance exercise. Int. J. Sport Nutr. Exerc. Metab. 2007, 17, 608–623. [Google Scholar] [CrossRef]
- Davis, J.M.; Zhao, Z.; Stock, H.S.; Mehl, K.A.; Buggy, J.; Hand, G.A. Central nervous system effects of caffeine and adenosine on fatigue. Am. J. Physiol. Integr. Comp. Physiol. 2003, 284, R399–R404. [Google Scholar] [CrossRef]
- Hespel, P.; Maughan, R.J.; Greenhaff, P.L. Dietary supplements for football. J. Sports Sci. 2006, 24, 749–761. [Google Scholar] [CrossRef]
- Rekling, J.C.; Funk, G.D.; Bayliss, D.A.; Dong, X.-W.; Feldman, J.L. Synaptic Control of Motoneuronal Excitability. Physiol. Rev. 2000, 80, 767–852. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Angus, D.J.; Cox, G.R.; Cummings, N.K.; Febbraio, M.A.; Gawthorn, K.; Hawley, J.A.; Minehan, M.; Martin, D.T.; Hargreaves, M. Effect of fat adaptation and carbohydrate restoration on metabolism and performance during prolonged cycling. J. Appl. Physiol. 2000, 89, 2413–2421. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, A. Yerba Maté (Illex Paraguariensis) ingestion augments fat oxidation and energy expenditure during exercise at various submaximal intensities. Nutr. Metab. 2014, 11, 42. [Google Scholar] [CrossRef]
- Giannesini, B.; Le Fur, Y.; Cozzone, P.J.; Verleye, M.; Le Guern, M.-E.; Bendahan, D. Citrulline malate supplementation increases muscle efficiency in rat skeletal muscle. Eur. J. Pharmacol. 2011, 667, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Rebouche, C.J. Kinetics, Pharmacokinetics, and Regulation of l-Carnitine and Acetyl-l-carnitine Metabolism. Ann. N. Y. Acad. Sci. 2004, 1033, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Spiering, B.A.; Kraemer, W.J.; Vingren, J.L.; Hatfield, D.L.; Fragala, M.S.; Ho, J.-Y.; Maresh, C.M.; Anderson, J.M.; Volek, J.S. Responses of criterion variables to different supplemental doses of L-carnitine L-tartrate. J. Strength Cond. Res. 2007, 21, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Guthrie, N.; Pezzullo, J.; Sanli, T.; Fielding, R.A.; Bellamine, A. Efficacy of a novel formulation of L-Carnitine, creatine, and leucine on lean body mass and functional muscle strength in healthy older adults: A randomized, double-blind placebo-controlled study. Nutr. Metab. 2017, 14, 7. [Google Scholar] [CrossRef]
- Stevenson, E.J.; Watson, A.; Theis, S.; Holz, A.; Harper, L.D.; Russell, M. A comparison of isomaltulose versus maltodextrin ingestion during soccer-specific exercise. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 117, 2321–2333. [Google Scholar] [CrossRef]
- O’Bryan, K.R.; Doering, T.M.; Morton, R.W.; Coffey, V.G.; Phillips, S.M.; Cox, G.R. Do multi-ingredient protein supplements augment resistance training-induced gains in skeletal muscle mass and strength? A systematic review and meta-analysis of 35 trials. Br. J. Sports Med. 2020, 54, 573–581. [Google Scholar] [CrossRef]
- MacNutt, M.J.; De Souza, M.J.; Tomczak, S.E.; Homer, J.L.; Sheel, A.W. Resting and exercise ventilatory chemosensitivity across the menstrual cycle. J. Appl. Physiol. 2012, 112, 737–747. [Google Scholar] [CrossRef]
- Ross, W.D.; Marfell-Jones, M.J. Kineanthropometry, Chapter 6. In Physiological Testing of High Performance Athlete, 2nd ed.; MacDougal, J.C., Wenger, H.A., Green, H.J., Eds.; Human Kinetics: Champaing, IL, USA, 1991; pp. 223–308. [Google Scholar]
- Dempster, P.; Aitkens, S. A new air displacement method for the determination of human body composition. Med. Sci. Sport Exerc. 1995, 27, 1692–1697. [Google Scholar] [CrossRef]
- Viitasalo, J.T. Evaluation of Explosive Strength for Young and Adult Athletes. Res. Q. Exerc. Sport 1988, 59, 9–13. [Google Scholar] [CrossRef]
- Brown, L.E.; Weir, J.P. ASEP procedures recommendation I: Accurate assessment of muscular strength. J. Exerc. Physiol. Online 2001, 4, 1–21. [Google Scholar]
- Till, K.; Morris, R.; Stokes, K.; Trewartha, G.; Twist, C.; Dobbin, N.; Hunwicks, R.; Jones, B. Validity of an Isometric Midthigh Pull Dynamometer in Male Youth Athletes. J. Strength Cond. Res. 2018, 32, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Haff, G.G.; Carlock, J.M.; Hartman, M.J.; Kilgore, J.L.; Kawamori, N.; Jackson, J.R. Force-time curve characteristics of dynamic and isometric muscle actions of elite women olympic weightlifters. J. Strength Cond. Res. 2005, 19, 741–748. [Google Scholar]
- LoTurco, I.; Pereira, L.A.; Kobal, R.; Kitamura, K.; Ramirez-Campillo, R.; Zanetti, V.; Abad, C.C.C.; Nakamura, F.Y. Muscle Contraction Velocity: A Suitable Approach to Analyze the Functional Adaptations in Elite Soccer Players. J. Sports Sci. Med. 2016, 15, 483–491. [Google Scholar]
- Rey, E.; Lago-Peñas, C.; Lago-Ballesteros, J. Tensiomyography of selected lower-limb muscles in professional soccer players. J. Electromyogr. Kinesiol. 2012, 22, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Ezequiel, R.; Lago-Peñas, C.; Lago-Ballesteros, J.; Casais, L. The Effect of Recovery Strategies on Contractile Properties Using Tensiomyography and Perceived Muscle Soreness in Professional Soccer Players. J. Strength Cond. Res. 2012, 26, 3081–3088. [Google Scholar] [CrossRef]
- Martín-Rodríguez, S.; Loturco, I.; Hunter, A.M.; Rodríguez-Ruiz, D.; Munguia-Izquierdo, D. Reliability and measurement error of tensiomyography to assess mechanical muscle function: A systematic review. J. Strength Cond. Res. 2017, 31, 3524–3536. [Google Scholar] [CrossRef]
- Macgregor, L.J.; Hunter, A.M.; Orizio, C.; Fairweather, M.M.; Ditroilo, M. Assessment of Skeletal Muscle Contractile Properties by Radial Displacement: The Case for Tensiomyography. Sport Med. 2018, 48, 1607–1620. [Google Scholar] [CrossRef]
- Karsten, B.; Jobson, S.; Hopker, J.G.; Stevens, L.; Beedie, C.J. Validity and reliability of critical power field testing. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 115, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Ratamess, N.; Alaver, B.A.; Eventoch, T.K.; Housh, T.J.; Kibler, B.; Kraemer, W.J. Progression Models in Resistance training for healthy adults ACSM position Stand. Med. Sci. Sport Exerc. 2009, 41, 687–708. [Google Scholar]
- Robertson, R.J.; Goss, F.L.; Rutkowski, J.; Lenz, B.; Dixon, C.; Timmer, J.; Frazee, K.; Dube, J.; Andreacci, J. Concurrent Validation of the OMNI Perceived Exertion Scale for Resistance Exercise. Med. Sci. Sports Exerc. 2003, 35, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Lodo, L.; Moreira, A.; Zavanela, P.M.; Newton, M.J.; McGuigan, M.R.; Aoki, M.S. Is there a relationship between the total volume of load lifted in bench press exercise and the rating of perceived exertion? J. Sports Med. Phys. Fit. 2012, 52, 483. [Google Scholar]
- Wellek, S.; Blettner, M. On the Proper Use of the Crossover Design in Clinical Trials. Dtsch. Aerzteblatt Online 2012, 109, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.M.; Walsh, A.L.; Ratamess, N.A.; Kang, J.; Hoffman, J.R. Effect of a Pre-Workout Energy Supplement on Acute Multi-Joint Resistance Exercise. J. Sports Sci. Med. 2011, 10, 261–266. [Google Scholar]
- Jagim, A.R.; Jones, M.T.; Wright, G.A.; Antoine, C.S.; Kovacs, A.; Oliver, J.M. The acute effects of multi-ingredient pre-workout ingestion on strength performance, lower body power, and anaerobic capacity. J. Int. Soc. Sports Nutr. 2016, 13, 11. [Google Scholar] [CrossRef]
- Goldstein, E.R.; Ziegenfuss, T.N.; Kalman, D.S.; Kreider, R.B.; Campbell, B.; Wilborn, C.D.; Taylor, L.W.; Willoughby, D.S.; Stout, J.R.; Graves, B.S.; et al. International society of sports nutrition position stand: Caffeine and performance. J. Int. Soc. Sports Nutr. 2010, 7, 5–15. [Google Scholar] [CrossRef]
- Cooper, R.; Naclerio, F.; Allgrove, J.; Larumbe-Zabala, E. Effects of a carbohydrate and caffeine gel on intermittent sprint performance in recreationally trained males. Eur. J. Sport Sci. 2014, 14, 353–361. [Google Scholar] [CrossRef]
- Lieberman, H.R.; Tharion, W.J.; Shukitt-Hale, B.; Speckman, K.L.; Tulley, R. Effects of caffeine, sleep loss, and stress on cognitive performance and mood during U.S. Navy SEAL training. Psychopharmacology 2002, 164, 250–261. [Google Scholar] [CrossRef]
- McLester, J.R.; Bishop, P.A.; Smith, J.; Wyers, L.; Dale, B.; Kozusko, J.; Richardson, M.; Nevett, M.E.; Lomax, R.G. A Series of Studies—A Practical Protocol for Testing Muscular Endurance Recovery. J. Strength Cond. Res. 2003, 17, 259. [Google Scholar] [CrossRef] [PubMed]
- Bartolomei, S.; Sadres, E.; Church, D.D.; Arroyo, E.; Iii, J.A.G.; Varanoske, A.N.; Wang, R.; Beyer, K.S.; Oliveira, L.P.; Stout, J.R.; et al. Comparison of the recovery response from high-intensity and high-volume resistance exercise in trained men. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 117, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Kerksick, C.M.; Leutholtz, B. Nutrient Administration and Resistance Training. J. Int. Soc. Sports Nutr. 2005, 2, 50–67. [Google Scholar] [CrossRef] [PubMed]
- McLellan, T.M.; Pasiakos, S.M.; Lieberman, H.R. Effects of Protein in Combination with Carbohydrate Supplements on Acute or Repeat Endurance Exercise Performance: A Systematic Review. Sports Med. 2013, 44, 535–550. [Google Scholar] [CrossRef]
- Barbalho, M.; Coswig, V.; Steele, J.; Fisher, J.P.; Paoli, A.; Gentil, P. Evidence for an Upper Threshold for Resistance Training Volume in Trained Women. Med. Sci. Sports Exerc. 2019, 51, 515–522. [Google Scholar] [CrossRef]
- Schoenfeld, B.J.; Contreras, B.; Krieger, J.; Grgic, J.; Delcastillo, K.; Belliard, R.; Alto, A. Resistance Training Volume Enhances Muscle Hypertrophy but Not Strength in Trained Men. Med. Sci. Sports Exerc. 2019, 51, 94–103. [Google Scholar] [CrossRef]
- Westcott, W.L. Resistance training is medicine: Effects of strength training on health. Curr. Sports Med. Rep. 2012, 11, 209–216. [Google Scholar] [CrossRef]
- Bracesco, N.; Sanchez, A.; Contreras, V.; Menini, T.; Gugliucci, A. Recent advances on Ilex paraguariensis research: Minireview. J. Ethnopharmacol. 2011, 136, 378–384. [Google Scholar] [CrossRef]
- Pickering, C.; Grgic, J. Caffeine and Exercise: What Next? Sport Med. 2019, 49, 1007–1030. [Google Scholar] [CrossRef]
- Kumar, N.; Warren, G.L.; Snow, T.K.; Millard-Stafford, M. Caffeine Ingestion with or Without Low-Dose Carbohydrate Improves Exercise Tolerance in Sedentary Adults. Front. Nutr. 2019, 6, 9. [Google Scholar] [CrossRef]
- Schwarz, N.A.; McKinley-Barnard, S.K. Acute Oral Ingestion of a Multi-ingredient Preworkout Supplement Increases Exercise Performance and Alters Postexercise Hormone Responses: A Randomized Crossover, Double-Blinded, Placebo-Controlled Trial. J. Diet. Suppl. 2020, 17, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Sureda, A.; Córdova, A.; Ferrer, M.D.; Pérez, G.; Tur, J.A.; Pons, A. l-Citrulline-malate influence over branched chain amino acid utilization during exercise. Graefe’s Arch. Clin. Exp. Ophthalmol. 2010, 110, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.R.; Ratamess, N.A.; Kang, J.; Rashti, S.L.; Faigenbaum, A.D. Effect of betaine supplementation on power performance and fatigue. J. Int. Soc. Sports Nutr. 2009, 6, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Zaragoza, J.; Tinsley, G.; Urbina, S.; Villa, K.; Santos, E.; Juaneza, A.; Tinnin, M.; Davidson, C.; Mitmesser, S.; Zhang, Z.; et al. Effects of acute caffeine, theanine and tyrosine supplementation on mental and physical performance in athletes. J. Int. Soc. Sports Nutr. 2019, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, C.; Lira, F.S.; Rossi, F.E.; Billaut, F.; Loschi, R.; Padilha, C.D.S. Multi-ingredient pre-workout supplementation changes energy system contribution and improves performance during high-intensity intermittent exercise in physically active individuals: A double-blind and placebo controlled study. J. Int. Soc. Sports Nutr. 2020, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, W.J.; Hooper, D.R.; Szivak, T.K.; Kupchak, B.R.; Dunn-Lewis, C.; Comstock, B.A.; Flanagan, S.D.; Looney, D.P.; Sterczala, A.J.; Dupont, W.H.; et al. The Addition of Beta-hydroxy-beta-methylbutyrate and Isomaltulose to Whey Protein Improves Recovery from Highly Demanding Resistance Exercise. J. Am. Coll. Nutr. 2015, 34, 91–99. [Google Scholar] [CrossRef]
- Jeacocke, N.A.; Burke, W. Methods to standardize dietary intake before performance testing. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 87–103. [Google Scholar] [CrossRef]
- Knowles, O.E.; Aisbett, B.; Main, L.C.; Drinkwater, E.J.; Orellana, L.; Lamon, S. Resistance Training and Skeletal Muscle Protein Metabolism in Eumenorrheic Females: Implications for Researchers and Practitioners. Sports Med. 2019, 49, 1637–1650. [Google Scholar] [CrossRef]
- Pallavi, L.; Souza, U.J.D.; Shivaprakash, G. Assessment of Musculoskeletal Strength and Levels of Fatigue during Different Phases of Menstrual Cycle in Young Adults. J. Clin. Diagn. Res. 2017, 11, CC11. [Google Scholar] [CrossRef]

| Description | Multi-Ingredient (40 g dose) | Placebo (27 g dose) |
|---|---|---|
| Energy value (kcal) | 100 | 102 |
| Macronutrients | ||
| Total carbohydrates (g) of which Isomaltulose (g) Maltodextrin (g) | ~16 (14) (1.9) | 25 (maltodextrin) |
| Total proteins included added amino acids (g) | 9 | - |
| Amino acids and other ingredients | ||
| Betaine Hydrochloride (g) | 2 | - |
| L-Carnitine L-tartrate (g) | 1.5 | - |
| L-Citrulline-DL-malate (g) | 2.5 | - |
| L-Leucine (g) | 3 | - |
| L-Lysine (g) | 2.7 | - |
| L-Arginine Base (g) | 2.5 | - |
| L-Isoleucine (g) | 1.5 | - |
| L-Methionine (g) | 0.7 | - |
| L-Phenylalanine (g) | 1.1 | - |
| Taurine (g) | 1 | - |
| L-Threonine (g) | 1.2 | - |
| L-Tryptophan (g) | 0.3 | - |
| L-Tyrosine (g) | 1 | - |
| L-Valine (g) | 1.5 | - |
| Caffeine (mg) | 400 | |
| Yerba Mate extract (mg) | 300 | |
| Measures | Males (n = 7) Mean ± SD (Range) | Females (n = 7) Mean ± SD (Range) |
|---|---|---|
| Age (yrs) | 49 ± 5 (45–58) | 49 ± 4 (45–55) |
| Height (cm) | 178 ± 5 (168–185) | 161 ± 3 (156–164) |
| Body mass (kg) | 85.8 ± 14 (67–107) | 71 ± 8 (58–82) |
| Fat-Free mass (kg) | 63.3 ± 5 (52–69) | 45.3 ± 5 (54–38) |
| Fat mass (kg) | 25.1 ± 10 (12–38) | 27.4 ± 9 (15–42) |
| Experience in RT (yrs) | 2.4 ± 1 (1–5) | 1.6 ± 1 (1–5) |
| Overhead Medicine ball Throw (m) | 6.1 ± 1.2 (4.5–7.9) | 4.6 ± 0.6 (3.6–5.4) |
| Countermovement jump (cm) | 26.4 ± 3.4 (21.4–30.7) | 17.1 ± 2.6 (12.9–20.4) |
| Maximal Isometric Mid-Thigh Pull (kgF) | 179 ± 41 (114–240) | 108.4 ± 18.8 (90.50–145.5) |
| peak (mL/kg/min) | 47 ± 11 (34–71) | 31 ± 5 (25–38) |
| Fat max intensity (Watts) | 103 ± 45 (66–196) | 55 ± 12 (41–74) |
| Macronutrients | No Supplementation (n = 14) | With PreWorkout (n = 14) | With Maltodextrin (n = 14) |
|---|---|---|---|
| Proteins | |||
| g·d−1 | 83.3 ± 31.4 | 92.9 ± 31.4 * φ | 83.3 ± 31.4 φ |
| g·kg−1.d−1 | 1.1 ± 0.3 | 1.2 ± 0.33 * φ | 1.1 ± 0.3 φ |
| % of total energy | 16.5 ± 4.4 | 17.5 ± 4.24 * φ | 15.7 ± 4.2 * φ |
| Carbohydrate | |||
| g·d−1 | 242.0 ± 82.3 | 260.1 ± 83.1 * φ | 267.4 ± 82.4 * φ |
| g·kg−1.d−1 | 3.2 ± 1.3 | 3.4 ± 1.3 * φ | 3.5 ± 1.3 * φ |
| % of total energy | 48.3 ± 13.3 | 49.0 ± 12.7 * | 50.8 ± 12.7 * φ |
| Fats | |||
| g·d−1 | 77.4 ± 26.2 | 77.4 ± 26.2 | 77.4 ± 26.2 |
| g·kg−1.d−1 | 0.97 ± 0.2 | 0.97 ± 0.2 | 0.97 ± 0. |
| % of total energy | 35.2 ± 9.9 | 33.5 ± 9.4 | 33.4 ± 9.4 |
| Energy | |||
| Total daily energy | 2053.2 ± 407.7 | 2167.4 ± 407.3 * | 2157.3 ± 407.6 * |
| Kcal·kg−1.d−1 | 26.4 ± 4.9 | 27.9 ± 5.0 * | 27.8 ± 5.0 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puente-Fernández, J.; Seijo, M.; Larumbe-Zabala, E.; Jiménez, A.; Liguori, G.; Rossato, C.J.L.; Mayo, X.; Naclerio, F. Effects of Multi-Ingredient Preworkout Supplementation across a Five-Day Resistance and Endurance Training Microcycle in Middle-Aged Adults. Nutrients 2020, 12, 3778. https://doi.org/10.3390/nu12123778
Puente-Fernández J, Seijo M, Larumbe-Zabala E, Jiménez A, Liguori G, Rossato CJL, Mayo X, Naclerio F. Effects of Multi-Ingredient Preworkout Supplementation across a Five-Day Resistance and Endurance Training Microcycle in Middle-Aged Adults. Nutrients. 2020; 12(12):3778. https://doi.org/10.3390/nu12123778
Chicago/Turabian StylePuente-Fernández, Joel, Marcos Seijo, Eneko Larumbe-Zabala, Alfonso Jiménez, Gary Liguori, Claire J. L. Rossato, Xian Mayo, and Fernando Naclerio. 2020. "Effects of Multi-Ingredient Preworkout Supplementation across a Five-Day Resistance and Endurance Training Microcycle in Middle-Aged Adults" Nutrients 12, no. 12: 3778. https://doi.org/10.3390/nu12123778
APA StylePuente-Fernández, J., Seijo, M., Larumbe-Zabala, E., Jiménez, A., Liguori, G., Rossato, C. J. L., Mayo, X., & Naclerio, F. (2020). Effects of Multi-Ingredient Preworkout Supplementation across a Five-Day Resistance and Endurance Training Microcycle in Middle-Aged Adults. Nutrients, 12(12), 3778. https://doi.org/10.3390/nu12123778







