A Comparison of Gene Expression Changes in the Blood of Individuals Consuming Diets Supplemented with Olives, Nuts or Long-Chain Omega-3 Fatty Acids
Abstract
1. Introduction
2. Materials and Methods
2.1. Analysis of PBMC Transcriptomic Studies
2.2. Pathway Enrichment Analysis
2.3. Gene-Transcription Factors Interaction Analysis
2.4. Gene-miRNA Interaction Analysis
2.5. Gene-Disease Association Analysis
2.6. Diet and Dementia Analysis
3. Results
3.1. Gene Expression Comparison of the Diet Supplements
3.2. Pathway Enrichment Analysis
3.3. Gene-Transcription Factors Interaction Analysis
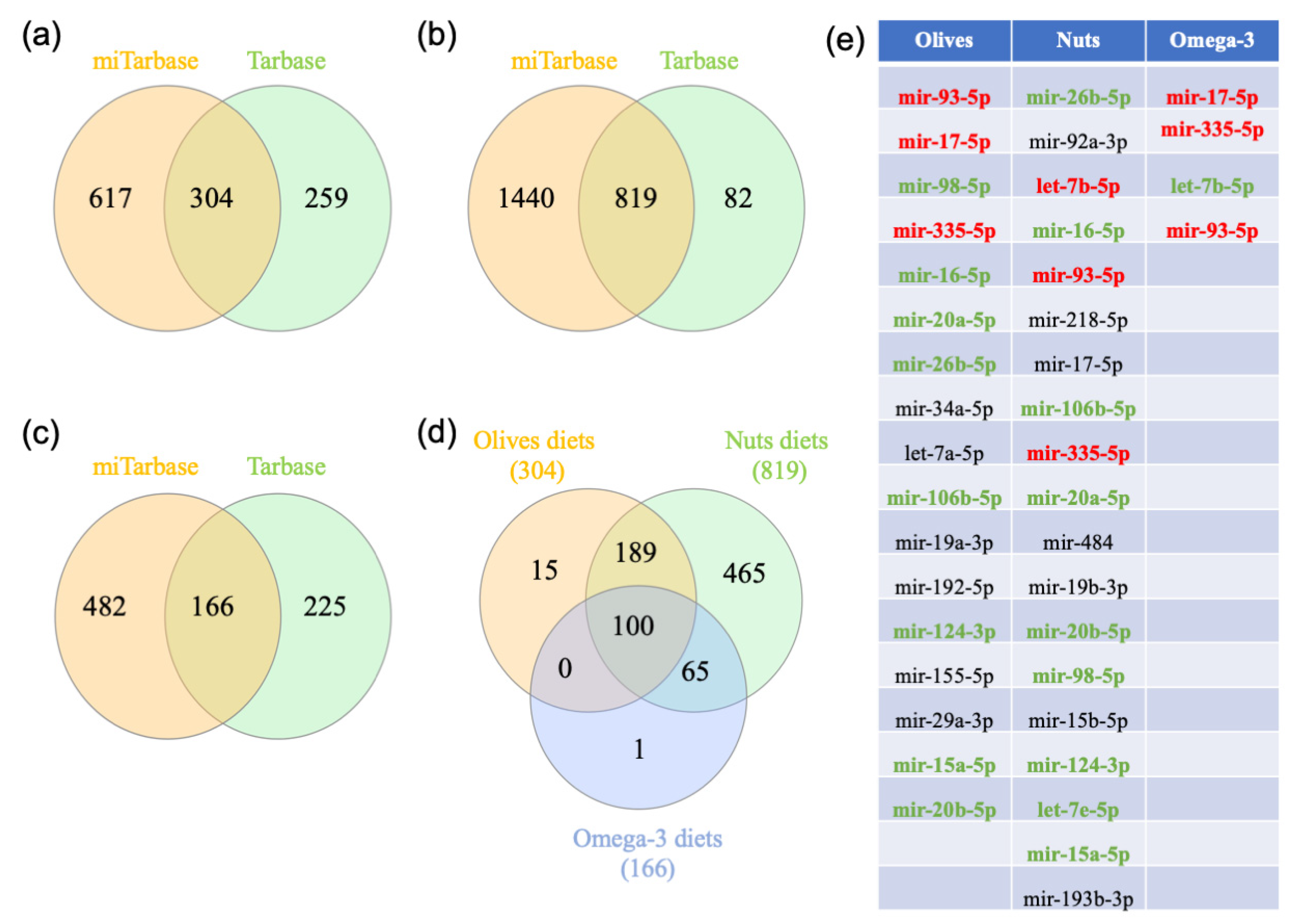
3.4. Gene-miRNA Interaction Analysis
3.5. Gene-Disease Association Analysis
3.6. Diets and Dementia Analysis
4. Discussion
4.1. Regulation of Gene Expression by Components of a Mediterranean Diet
4.2. Gene Expression Regulation by Transcription Factors and miRNA in Mediterranean Diets
4.3. Mediterranean Diets and Disease Association
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sanchez-Sanchez, M.L.; Garcia-Vigara, A.; Hidalgo-Mora, J.J.; Garcia-Perez, M.A.; Tarin, J.; Cano, A. Mediterranean Diet and Health: A Systematic Review of Epidemiological Studies and Intervention Trials. Maturitas 2020, 136, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Nunez-Cordoba, J.M.; Valencia-Serrano, F.; Toledo, E.; Alonso, A.; Martinez-Gonzalez, M.A. The Mediterranean Diet and Incidence of Hypertension: The Seguimiento Universidad de Navarra (SUN) Study. Am. J. Epidemiol. 2009, 169, 339–346. [Google Scholar] [CrossRef]
- Bendinelli, B.; Masala, G.; Bruno, R.M.; Caini, S.; Saieva, C.; Boninsegni, A.; Ungar, A.; Ghiadoni, L.; Palli, D. A Priori Dietary Patterns and Blood Pressure in the EPIC Florence Cohort: A Cross-Sectional Study. Eur. J. Nutr. 2019, 58, 455–466. [Google Scholar] [CrossRef]
- De Pergola, G.; D’Alessandro, A. Influence of Mediterranean Diet on Blood Pressure. Nutrients 2018, 10, 1700. [Google Scholar] [CrossRef]
- Tosatti, J.A.G.; Alves, M.T.; Gomes, K.B. The Role of the Mediterranean Dietary Pattern on Metabolic Control of Patients with Diabetes Mellitus: A Narrative Review. Adv. Exp. Med. Biol. 2020, 1307, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvado, J.; Guasch-Ferre, M.; Lee, C.H.; Estruch, R.; Clish, C.B.; Ros, E. Protective Effects of the Mediterranean Diet on Type 2 Diabetes and Metabolic Syndrome. J. Nutr. 2015, 146, 920S–927S. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Schwedhelm, C.; Galbete, C.; Hoffmann, G. Adherence to Mediterranean Diet and Risk of Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients 2017, 9, 1063. [Google Scholar] [CrossRef]
- Sanchez-Rodriguez, E.; Biel-Glesson, S.; Fernandez-Navarro, J.R.; Calleja, M.A.; Espejo-Calvo, J.A.; Gil-Extremera, B.; de la Torre, R.; Fito, M.; Covas, M.I.; Vilchez, P.; et al. Effects of Virgin Olive Oils Differing in Their Bioactive Compound Contents on Biomarkers of Oxidative Stress and Inflammation in Healthy Adults: A Randomized Double-Blind Controlled Trial. Nutrients 2019, 11, 561. [Google Scholar] [CrossRef]
- Bendinelli, B.; Masala, G.; Saieva, C.; Salvini, S.; Calonico, C.; Sacerdote, C.; Agnoli, C.; Grioni, S.; Frasca, G.; Mattiello, A.; et al. Fruit, Vegetables, and Olive Oil and Risk of Coronary Heart Disease in Italian Women: The EPICOR Study. Am. J. Clin. Nutr. 2011, 93, 275–283. [Google Scholar] [CrossRef]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil, by-Products, and Leaf of Olea europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef]
- Mazzocchi, A.; Leone, L.; Agostoni, C.; Pali-Scholl, I. The Secrets of the Mediterranean Diet. Does [Only] Olive Oil Matter? Nutrients 2019, 11, 2941. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Marfella, R.; Ciotola, M.; Di Palo, C.; Giugliano, F.; Giugliano, G.; D’Armiento, M.; D’Andrea, F.; Giugliano, D. Effect of a Mediterranean-Style Diet on Endothelial Dysfunction and Markers of Vascular Inflammation in the Metabolic Syndrome: A Randomized Trial. JAMA 2004, 292, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Menendez, J.A.; Lupu, R. Mediterranean Dietary Traditions for the Molecular Treatment of Human Cancer: Anti-Oncogenic Actions of the Main Olive Oil’s Monounsaturated Fatty Acid Oleic Acid (18:1n-9). Curr. Pharm. Biotechnol. 2006, 7, 495–502. [Google Scholar] [CrossRef]
- Psaltopoulou, T.; Kosti, R.I.; Haidopoulos, D.; Dimopoulos, M.; Panagiotakos, D.B. Olive Oil Intake is Inversely Related to Cancer Prevalence: A Systematic Review and a Meta-Analysis of 13,800 Patients and 23,340 Controls in 19 Observational Studies. Lipids Health Dis. 2011, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Fogli, S.; Arena, C.; Carpi, S.; Polini, B.; Bertini, S.; Digiacomo, M.; Gado, F.; Saba, A.; Saccomanni, G.; Breschi, M.C.; et al. Cytotoxic Activity of Oleocanthal Isolated from Virgin Olive Oil on Human Melanoma Cells. Nutr. Cancer 2016, 68, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Scoditti, E.; Carluccio, M.A.; Calabriso, N.; Santarpino, G.; Verri, T.; De Caterina, R. Effects of Olive Oil on Blood Pressure: Epidemiological, Clinical, and Mechanistic Evidence. Nutrients 2020, 12, 1548. [Google Scholar] [CrossRef]
- Bogani, P.; Galli, C.; Villa, M.; Visioli, F. Postprandial Anti-Inflammatory and Antioxidant Effects of Extra Virgin Olive Oil. Atherosclerosis 2007, 190, 181–186. [Google Scholar] [CrossRef]
- Mitjavila, M.T.; Fandos, M.; Salas-Salvado, J.; Covas, M.I.; Borrego, S.; Estruch, R.; Lamuela-Raventos, R.; Corella, D.; Martinez-Gonzalez, M.A.; Sanchez, J.M.; et al. The Mediterranean Diet Improves the Systemic Lipid and DNA Oxidative Damage in Metabolic Syndrome Individuals. A Randomized, Controlled, Trial. Clin. Nutr. 2013, 32, 172–178. [Google Scholar] [CrossRef]
- De Souza, R.G.M.; Schincaglia, R.M.; Pimentel, G.D.; Mota, J.F. Nuts and Human Health Outcomes: A Systematic Review. Nutrients 2017, 9, 1311. [Google Scholar] [CrossRef]
- Souza, R.G.; Gomes, A.C.; Naves, M.M.; Mota, J.F. Nuts and Legume Seeds for Cardiovascular Risk Reduction: Scientific Evidence and Mechanisms of Action. Nutr. Rev. 2015, 73, 335–347. [Google Scholar] [CrossRef]
- Jackson, C.L.; Hu, F.B. Long-Term Associations of Nut Consumption with Body Weight and Obesity. Am. J. Clin. Nutr. 2014, 100, 408S–411S. [Google Scholar] [CrossRef] [PubMed]
- Mohammadifard, N.; Salehi-Abargouei, A.; Salas-Salvado, J.; Guasch-Ferre, M.; Humphries, K.; Sarrafzadegan, N. The Effect of Tree Nut, Peanut, and Soy Nut Consumption on Blood Pressure: A Systematic Review and Meta-Analysis of Randomized Controlled Clinical Trials. Am. J. Clin. Nutr. 2015, 101, 966–982. [Google Scholar] [CrossRef] [PubMed]
- Viguiliouk, E.; Kendall, C.W.; Blanco Mejia, S.; Cozma, A.I.; Ha, V.; Mirrahimi, A.; Jayalath, V.H.; Augustin, L.S.; Chiavaroli, L.; Leiter, L.A.; et al. Effect of Tree Nuts on Glycemic Control in Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Dietary Trials. PLoS ONE 2014, 9, e103376. [Google Scholar] [CrossRef] [PubMed]
- Blanco Mejia, S.; Kendall, C.W.; Viguiliouk, E.; Augustin, L.S.; Ha, V.; Cozma, A.I.; Mirrahimi, A.; Maroleanu, A.; Chiavaroli, L.; Leiter, L.A.; et al. Effect of Tree Nuts on Metabolic Syndrome Criteria: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. BMJ Open 2014, 4, e004660. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Uriarte, P.; Nogues, R.; Saez, G.; Bullo, M.; Romeu, M.; Masana, L.; Tormos, C.; Casas-Agustench, P.; Salas-Salvado, J. Effect of Nut Consumption on Oxidative Stress and the Endothelial Function in Metabolic Syndrome. Clin. Nutr. 2010, 29, 373–380. [Google Scholar] [CrossRef]
- Lorenzon dos Santos, J.; Quadros, A.S.; Weschenfelder, C.; Garofallo, S.B.; Marcadenti, A. Oxidative Stress Biomarkers, Nut-Related Antioxidants, and Cardiovascular Disease. Nutrients 2020, 12, 682. [Google Scholar] [CrossRef]
- Bitok, E.; Sabate, J. Nuts and Cardiovascular Disease. Prog. Cardiovasc. Dis. 2018, 61, 33–37. [Google Scholar] [CrossRef]
- Parham, M.; Heidari, S.; Khorramirad, A.; Hozoori, M.; Hosseinzadeh, F.; Bakhtyari, L.; Vafaeimanesh, J. Effects of Pistachio Nut Supplementation on Blood Glucose in Patients with Type 2 Diabetes: A Randomized Crossover Trial. Rev. Diabet. Stud. 2014, 11, 190–196. [Google Scholar] [CrossRef]
- Sokola-Wysoczanska, E.; Wysoczanski, T.; Wagner, J.; Czyz, K.; Bodkowski, R.; Lochynski, S.; Patkowska-Sokola, B. Polyunsaturated Fatty Acids and Their Potential Therapeutic Role in Cardiovascular System Disorders—A Review. Nutrients 2018, 10, 1561. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Richter, C.K.; Bowen, K.J.; Skulas-Ray, A.C.; Jackson, K.H.; Petersen, K.S.; Harris, W.S. Recent Clinical Trials Shed New Light on the Cardiovascular Benefits of Omega-3 Fatty Acids. Methodist Debakey Cardiovasc. J. 2019, 15, 171–178. [Google Scholar]
- Lankinen, M.; Schwab, U.; Erkkila, A.; Seppanen-Laakso, T.; Hannila, M.L.; Mussalo, H.; Lehto, S.; Uusitupa, M.; Gylling, H.; Oresic, M. Fatty Fish Intake Decreases Lipids Related to Inflammation and Insulin Signaling—A Lipidomics Approach. PLoS ONE 2009, 4, e5258. [Google Scholar] [CrossRef]
- De Mello, V.D.; Erkkila, A.T.; Schwab, U.S.; Pulkkinen, L.; Kolehmainen, M.; Atalay, M.; Mussalo, H.; Lankinen, M.; Oresic, M.; Lehto, S.; et al. The Effect of Fatty or Lean Fish Intake on Inflammatory Gene Expression in Peripheral Blood Mononuclear Cells of Patients with Coronary Heart Disease. Eur. J. Nutr. 2009, 48, 447–455. [Google Scholar] [CrossRef]
- Kaminski, W.E.; Jendraschak, E.; Kiefl, R.; von Schacky, C. Dietary Omega-3 Fatty Acids Lower Levels of Platelet-Derived Growth Factor mRNA in Human Mononuclear Cells. Blood 1993, 81, 1871–1879. [Google Scholar] [CrossRef]
- Wittwer, J.; Rubio-Aliaga, I.; Hoeft, B.; Bendik, I.; Weber, P.; Daniel, H. Nutrigenomics in Human Intervention Studies: Current Status, Lessons Learned and Future Perspectives. Mol. Nutr. Food Res. 2011, 55, 341–358. [Google Scholar] [CrossRef]
- De Mello, V.D.; Kolehmanien, M.; Schwab, U.; Pulkkinen, L.; Uusitupa, M. Gene Expression of Peripheral Blood Mononuclear Cells as a Tool in Dietary Intervention Studies: What Do We Know So Far? Mol. Nutr. Food Res. 2012, 56, 1160–1172. [Google Scholar] [CrossRef]
- Castaner, O.; Corella, D.; Covas, M.I.; Sorli, J.V.; Subirana, I.; Flores-Mateo, G.; Nonell, L.; Bullo, M.; de la Torre, R.; Portoles, O.; et al. In Vivo Transcriptomic Profile after a Mediterranean Diet in High-Cardiovascular Risk Patients: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2013, 98, 845–853. [Google Scholar]
- D’Amore, S.; Vacca, M.; Cariello, M.; Graziano, G.; D’Orazio, A.; Salvia, R.; Sasso, R.C.; Sabba, C.; Palasciano, G.; Moschetta, A. Genes and miRNA Expression Signatures in Peripheral Blood Mononuclear Cells in Healthy Subjects and Patients with Metabolic Syndrome after Acute Intake of Extra Virgin Olive Oil. Biochim. Biophys. Acta 2016, 1861, 1671–1680. [Google Scholar] [CrossRef]
- Boss, A.; Kao, C.H.; Murray, P.M.; Marlow, G.; Barnett, M.P.; Ferguson, L.R. Human Intervention Study to Assess the Effects of Supplementation with Olive Leaf Extract on Peripheral Blood Mononuclear Cell Gene Expression. Int. J. Mol. Sci. 2016, 17, 2019. [Google Scholar] [CrossRef]
- Myhrstad, M.C.; Ulven, S.M.; Gunther, C.C.; Ottestad, I.; Holden, M.; Ryeng, E.; Borge, G.I.; Kohler, A.; Bronner, K.W.; Thoresen, M.; et al. Fish Oil Supplementation Induces Expression of Genes Related to Cell Cycle, Endoplasmic Reticulum Stress and Apoptosis in Peripheral Blood Mononuclear Cells: A Transcriptomic Approach. J. Intern. Med. 2014, 276, 498–511. [Google Scholar] [CrossRef]
- Bouwens, M.; van de Rest, O.; Dellschaft, N.; Bromhaar, M.G.; de Groot, L.C.; Geleijnse, J.M.; Muller, M.; Afman, L.A. Fish-Oil Supplementation Induces Antiinflammatory Gene Expression Profiles in Human Blood Mononuclear Cells. Am. J. Clin. Nutr. 2009, 90, 415–424. [Google Scholar] [CrossRef]
- Santiago, J.A.; Bottero, V.; Potashkin, J.A. Dissecting the Molecular Mechanisms of Neurodegenerative Diseases through Network Biology. Front. Aging Neurosci. 2017, 9, 166. [Google Scholar] [CrossRef] [PubMed]
- Bottero, V.; Potashkin, J.A. Meta-Analysis of Gene Expression Changes in the Blood of Patients with Mild Cognitive Impairment and Alzheimer’s Disease Dementia. Int. J. Mol. Sci. 2019, 20, 5403. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.A.; Bottero, V.; Potashkin, J.A. Transcriptomic and Network Analysis Highlight the Association of Diabetes at Different Stages of Alzheimer’s Disease. Front. Neurosci. 2019, 13, 1273. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.A.; Bottero, V.; Potashkin, J.A. Transcriptomic and Network Analysis Identifies Shared and Unique Pathways across Dementia Spectrum Disorders. Int. J. Mol. Sci. 2020, 21, 2050. [Google Scholar] [CrossRef]
- Kupershmidt, I.; Su, Q.J.; Grewal, A.; Sundaresh, S.; Halperin, I.; Flynn, J.; Shekar, M.; Wang, H.; Park, J.; Cui, W.; et al. Ontology-based meta-analysis of global collections of high-throughput public data. PLoS ONE 2010, 5, e13066. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Xia, J.; Gill, E.E.; Hancock, R.E. NetworkAnalyst for Statistical, Visual and Network-Based Meta-Analysis of Gene Expression Data. Nat. Protoc. 2015, 10, 823–844. [Google Scholar] [CrossRef]
- Zhou, G.; Soufan, O.; Ewald, J.; Hancock, R.E.W.; Basu, N.; Xia, J. NetworkAnalyst 3.0: A Visual Analytics Platform for Comprehensive Gene Expression Profiling and Meta-Analysis. Nucleic Acids Res. 2019, 47, W234–W241. [Google Scholar] [CrossRef]
- Consortium, E.P. A User’s Guide to the Encyclopedia of DNA Elements (ENCODE). PLoS Biol. 2011, 9, e1001046. [Google Scholar]
- Lachmann, A.; Xu, H.; Krishnan, J.; Berger, S.I.; Mazloom, A.R.; Ma’ayan, A. ChEA: Transcription Factor Regulation Inferred from Integrating Genome-Wide ChIP-X Experiments. Bioinformatics 2010, 26, 2438–2444. [Google Scholar] [CrossRef]
- Khan, A.; Fornes, O.; Stigliani, A.; Gheorghe, M.; Castro-Mondragon, J.A.; van der Lee, R.; Bessy, A.; Cheneby, J.; Kulkarni, S.R.; Tan, G.; et al. JASPAR 2018: Update of the Open-Access Database of Transcription Factor Binding Profiles and Its Web Framework. Nucleic Acids Res. 2018, 46, D1284. [Google Scholar] [CrossRef] [PubMed]
- Karagkouni, D.; Paraskevopoulou, M.D.; Chatzopoulos, S.; Vlachos, I.S.; Tastsoglou, S.; Kanellos, I.; Papadimitriou, D.; Kavakiotis, I.; Maniou, S.; Skoufos, G.; et al. DIANA-TarBase v8: A Decade-Long Collection of Experimentally Supported miRNA-Gene Interactions. Nucleic Acids Res. 2018, 46, D239–D245. [Google Scholar] [CrossRef] [PubMed]
- Sethupathy, P.; Corda, B.; Hatzigeorgiou, A.G. TarBase: A Comprehensive Database of Experimentally Supported Animal microRNA Targets. RNA 2006, 12, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.H.; Shrestha, S.; Yang, C.D.; Chang, N.W.; Lin, Y.L.; Liao, K.W.; Huang, W.C.; Sun, T.H.; Tu, S.J.; Lee, W.H.; et al. miRTarBase Update 2018: A Resource for Experimentally Validated microRNA-Target Interactions. Nucleic Acids Res. 2018, 46, D296–D302. [Google Scholar] [CrossRef] [PubMed]
- Pinero, J.; Bravo, A.; Queralt-Rosinach, N.; Gutierrez-Sacristan, A.; Deu-Pons, J.; Centeno, E.; Garcia-Garcia, J.; Sanz, F.; Furlong, L.I. DisGeNET: A Comprehensive Platform Integrating Information on Human Disease-Associated Genes and Variants. Nucleic Acids Res. 2017, 45, D833–D839. [Google Scholar] [CrossRef]
- Samieri, C.; Sonawane, A.R.; Lefevre-Arbogast, S.; Helmer, C.; Grodstein, F.; Glass, K. Using Network Science Tools to Identify Novel Diet Patterns in Prodromal Dementia. Neurology 2020, 94, e2014–e2025. [Google Scholar] [CrossRef]
- Vinciguerra, F.; Graziano, M.; Hagnas, M.; Frittitta, L.; Tumminia, A. Influence of the Mediterranean and Ketogenic Diets on Cognitive Status and Decline: A Narrative Review. Nutrients 2020, 12, 1019. [Google Scholar] [CrossRef]
- Roman, G.C.; Jackson, R.E.; Gadhia, R.; Roman, A.N.; Reis, J. Mediterranean Diet: The Role of Long-Chain Omega-3 fatty Acids in Fish; Polyphenols in Fruits, Vegetables, Cereals, Coffee, Tea, Cacao and Wine; Probiotics and Vitamins in Prevention of Stroke, Age-Related Cognitive Decline, and Alzheimer Disease. Rev. Neurol. 2019, 175, 724–741. [Google Scholar] [CrossRef]
- Sala-Vila, A.; Valls-Pedret, C.; Rajaram, S.; Coll-Padros, N.; Cofan, M.; Serra-Mir, M.; Perez-Heras, A.M.; Roth, I.; Freitas-Simoes, T.M.; Domenech, M.; et al. Effect of a 2-Year Diet Intervention with Walnuts on Cognitive Decline. The Walnuts and Healthy Aging (WAHA) Study: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2020, 111, 590–600. [Google Scholar] [CrossRef]
- Gorji, N.; Moeini, R.; Memariani, Z. Almond, Hazelnut and Walnut, Three Nuts for Neuroprotection in Alzheimer’s Disease: A Neuropharmacological Review of Their Bioactive Constituents. Pharmacol. Res. 2018, 129, 115–127. [Google Scholar] [CrossRef]
- Gentile, F.; Doneddu, P.E.; Riva, N.; Nobile-Orazio, E.; Quattrini, A. Diet, Microbiota and Brain Health: Unraveling the Network Intersecting Metabolism and Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 7471. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, A.; Walker, D.G.; Terai, K.; McGeer, P.L. Expression of CD43 in Human Microglia and Its Downregulation in Alzheimer’s Disease. J. Neuroimmunol. 1996, 71, 81–86. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, J.; Lu, Q. The Role of Basic Leucine Zipper Transcription Factor E4BP4 in the Immune System and Immune-Mediated Diseases. Clin. Immunol. 2017, 180, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, S.; Yamaguchi, S.; Matsuo, T.; Ishida, Y.; Okamura, H. Antagonistic Role of E4BP4 and PAR Proteins in the Circadian Oscillatory Mechanism. Genes Dev. 2001, 15, 995–1006. [Google Scholar] [CrossRef]
- Tong, X.; Muchnik, M.; Chen, Z.; Patel, M.; Wu, N.; Joshi, S.; Rui, L.; Lazar, M.A.; Yin, L. Transcriptional Repressor E4-Binding Protein 4 (E4BP4) Regulates Metabolic Hormone Fibroblast Growth Factor 21 (FGF21) during Circadian Cycles and Feeding. J. Biol. Chem. 2010, 285, 36401–36409. [Google Scholar] [CrossRef]
- Wang, Y.; Kuang, Z.; Yu, X.; Ruhn, K.A.; Kubo, M.; Hooper, L.V. The Intestinal Microbiota Regulates Body Composition through NFIL3 and the Circadian Clock. Science 2017, 357, 912–916. [Google Scholar] [CrossRef]
- Velmurugan, B.K.; Chang, R.L.; Marthandam Asokan, S.; Chang, C.F.; Day, C.H.; Lin, Y.M.; Lin, Y.C.; Kuo, W.W.; Huang, C.Y. A Minireview of E4BP4/NFIL3 in Heart Failure. J. Cell Physiol. 2018, 233, 8458–8466. [Google Scholar] [CrossRef]
- MacGillavry, H.D.; Stam, F.J.; Sassen, M.M.; Kegel, L.; Hendriks, W.T.; Verhaagen, J.; Smit, A.B.; van Kesteren, R.E. NFIL3 and cAMP Response Element-Binding Protein Form a Transcriptional Feedforward Loop That Controls Neuronal Regeneration-Associated Gene Expression. J. Neurosci. 2009, 29, 15542–15550. [Google Scholar] [CrossRef]
- Kim, C.S.; Park, H.S.; Kawada, T.; Kim, J.H.; Lim, D.; Hubbard, N.E.; Kwon, B.S.; Erickson, K.L.; Yu, R. Circulating Levels of MCP-1 and IL-8 Are Elevated in Human Obese Subjects and Associated with Obesity-Related Parameters. Int. J. Obes. 2006, 30, 1347–1355. [Google Scholar] [CrossRef]
- Sharabiani, M.T.; Vermeulen, R.; Scoccianti, C.; Hosnijeh, F.S.; Minelli, L.; Sacerdote, C.; Palli, D.; Krogh, V.; Tumino, R.; Chiodini, P.; et al. Immunologic Profile of Excessive BODY weight. Biomarkers 2011, 16, 243–251. [Google Scholar] [CrossRef]
- Casas, R.; Urpi-Sarda, M.; Sacanella, E.; Arranz, S.; Corella, D.; Castaner, O.; Lamuela-Raventos, R.M.; Salas-Salvado, J.; Lapetra, J.; Portillo, M.P.; et al. Anti-Inflammatory Effects of the Mediterranean Diet in the Early and Late Stages of Atheroma Plaque Development. Mediat. Inflamm. 2017, 2017, 3674390. [Google Scholar] [CrossRef] [PubMed]
- Colombo, F.; Di Lorenzo, C.; Regazzoni, L.; Fumagalli, M.; Sangiovanni, E.; Peres de Sousa, L.; Bavaresco, L.; Tomasi, D.; Bosso, A.; Aldini, G.; et al. Phenolic Profiles and Anti-Inflammatory Activities of Sixteen Table Grape (Vitis vinifera L.) Varieties. Food Funct. 2019, 10, 1797–1807. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Luo, H.; Wu, J. Drak2 Overexpression Results in Increased Beta-Cell Apoptosis after Free Fatty Acid Stimulation. J. Cell Biochem. 2008, 105, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Luo, H.; Han, B.; Bertrand, R.; Wu, J. Drak2 is Upstream of p70S6 Kinase: Its Implication in Cytokine-Induced Islet Apoptosis, Diabetes, and Islet Transplantation. J. Immunol. 2009, 182, 4762–4770. [Google Scholar] [CrossRef]
- Wang, S.; Xu, L.; Lu, Y.T.; Liu, Y.F.; Han, B.; Liu, T.; Tang, J.; Li, J.; Wu, J.; Li, J.Y.; et al. Discovery of Benzofuran-3(2H)-One Derivatives as Novel DRAK2 Inhibitors That Protect Islet Beta-Cells from Apoptosis. Eur. J. Med. Chem. 2017, 130, 195–208. [Google Scholar] [CrossRef]
- Lijnen, H.R.; Frederix, L.; Scroyen, I. Deficiency of Plasminogen Activator Inhibitor-2 Impairs Nutritionally Induced Murine Adipose Tissue Development. J. Thromb. Haemost. 2007, 5, 2259–2265. [Google Scholar] [CrossRef]
- Aissa, A.F.; Amaral, C.L.D.; Venancio, V.P.; Machado, C.D.S.; Hernandes, L.C.; Santos, P.; Curi, R.; Bianchi, M.L.P.; Antunes, L.M.G. Methionine-Supplemented Diet Affects the Expression of Cardiovascular Disease-Related Genes and Increases Inflammatory Cytokines in Mice Heart and Liver. J. Toxicol Environ. Health A 2017, 80, 1116–1128. [Google Scholar] [CrossRef]
- Smyth, D.J.; Plagnol, V.; Walker, N.M.; Cooper, J.D.; Downes, K.; Yang, J.H.; Howson, J.M.; Stevens, H.; McManus, R.; Wijmenga, C.; et al. Shared and Distinct Genetic Variants in Type 1 Diabetes and Celiac Disease. N. Engl. J. Med. 2008, 359, 2767–2777. [Google Scholar] [CrossRef]
- Hunt, K.A.; Zhernakova, A.; Turner, G.; Heap, G.A.; Franke, L.; Bruinenberg, M.; Romanos, J.; Dinesen, L.C.; Ryan, A.W.; Panesar, D.; et al. Newly Identified Genetic Risk Variants for Celiac Disease Related to the Immune Response. Nat. Genet. 2008, 40, 395–402. [Google Scholar] [CrossRef]
- Johnson, B.A.; Wang, J.; Taylor, E.M.; Caillier, S.J.; Herbert, J.; Khan, O.A.; Cross, A.H.; De Jager, P.L.; Gourraud, P.A.; Cree, B.C.; et al. Multiple Sclerosis Susceptibility Alleles in African Americans. Genes Immun. 2010, 11, 343–350. [Google Scholar] [CrossRef]
- Choi, M.S.; Kim, Y.J.; Kwon, E.Y.; Ryoo, J.Y.; Kim, S.R.; Jung, U.J. High-Fat Diet Decreases Energy Expenditure and Expression of Genes Controlling Lipid Metabolism, Mitochondrial Function and Skeletal System Development in the Adipose Tissue, along with Increased Expression of Extracellular Matrix Remodelling- and Inflammation-Related Genes. Br. J. Nutr. 2015, 113, 867–877. [Google Scholar] [PubMed]
- Moreno-Viedma, V.; Amor, M.; Sarabi, A.; Bilban, M.; Staffler, G.; Zeyda, M.; Stulnig, T.M. Common Dysregulated Pathways in Obese Adipose Tissue and Atherosclerosis. Cardiovasc. Diabetol. 2016, 15, 120. [Google Scholar] [CrossRef] [PubMed]
- Leandro, G.S.; Evangelista, A.F.; Lobo, R.R.; Xavier, D.J.; Moriguti, J.C.; Sakamoto-Hojo, E.T. Changes in Expression Profiles Revealed by Transcriptomic Analysis in Peripheral Blood Mononuclear Cells of Alzheimer’s Disease Patients. J. Alzheimers Dis. 2018, 66, 1483–1495. [Google Scholar] [CrossRef] [PubMed]
- Grewal, R.; Reutzel, M.; Dilberger, B.; Hein, H.; Zotzel, J.; Marx, S.; Tretzel, J.; Sarafeddinov, A.; Fuchs, C.; Eckert, G.P. Purified Oleocanthal and Ligstroside Protect against Mitochondrial Dysfunction in Models of Early Alzheimer’s Disease and Brain Ageing. Exp. Neurol. 2020, 328, 113248. [Google Scholar] [CrossRef]
- Kim, S.E.; Choo, J.; Yoon, J.; Chu, J.R.; Bae, Y.J.; Lee, S.; Park, T.; Sung, M.K. Genome-Wide Analysis Identifies Colonic Genes Differentially Associated with Serum Leptin and Insulin Concentrations in C57BL/6J Mice Fed a High-Fat Diet. PLoS ONE 2017, 12, e0171664. [Google Scholar] [CrossRef][Green Version]
- Anghel, S.I.; Wahli, W. Fat Poetry: A Kingdom for PPAR Gamma. Cell Res. 2007, 17, 486–511. [Google Scholar] [CrossRef]
- Chmurzynska, A.; Muzsik, A.; Krzyzanowska-Jankowska, P.; Madry, E.; Walkowiak, J.; Bajerska, J. PPARG and FTO Polymorphism Can Modulate the Outcomes of a Central European Diet and a Mediterranean Diet in Centrally Obese Postmenopausal Women. Nutr. Res. 2019, 69, 94–100. [Google Scholar] [CrossRef]
- Rafehi, H.; Smith, A.J.; Balcerczyk, A.; Ziemann, M.; Ooi, J.; Loveridge, S.J.; Baker, E.K.; El-Osta, A.; Karagiannis, T.C. Investigation into the Biological Properties of the Olive Polyphenol, Hydroxytyrosol: Mechanistic Insights by Genome-Wide mRNA-Seq Analysis. Genes Nutr. 2012, 7, 343–355. [Google Scholar] [CrossRef]
- Rosillo, M.A.; Alcaraz, M.J.; Sanchez-Hidalgo, M.; Fernandez-Bolanos, J.G.; Alarcon-de-la-Lastra, C.; Ferrandiz, M.L. Anti-Inflammatory and Joint Protective Effects of Extra-Virgin Olive-Oil Polyphenol Extract in Experimental Arthritis. J. Nutr. Biochem. 2014, 25, 1275–1281. [Google Scholar] [CrossRef]
- Phillips, C.M.; Goumidi, L.; Bertrais, S.; Field, M.R.; Peloso, G.M.; Shen, J.; McManus, R.; Hercberg, S.; Lairon, D.; Planells, R.; et al. Dietary Saturated Fat Modulates the Association between STAT3 Polymorphisms and Abdominal Obesity in Adults. J. Nutr. 2009, 139, 2011–2017. [Google Scholar] [CrossRef]
- Dongiovanni, P.; Meroni, M.; Longo, M.; Fargion, S.; Fracanzani, A.L. miRNA Signature in NAFLD: A Turning Point for a Non-Invasive Diagnosis. Int. J. Mol. Sci. 2018, 19, 3966. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, M.; Zheng, M.; Hayashi, M.; Lee, J.D.; Yoshino, O.; Lin, S.; Han, J. Impaired microRNA Processing Causes Corpus Luteum Insufficiency and Infertility in Mice. J. Clin. Invest. 2008, 118, 1944–1954. [Google Scholar] [CrossRef] [PubMed]
- Bobbili, M.R.; Mader, R.M.; Grillari, J.; Dellago, H. OncomiR-17–5p: Alarm Signal in Cancer? Oncotarget 2017, 8, 71206–71222. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Song, Z.; Shao, W.; Du, W.W.; Zhao, L.R.; Zeng, K.; Yang, B.B.; Jin, T. Curcumin Represses Mouse 3T3-L1 Cell Adipogenic Differentiation via Inhibiting miR-17–5p and Stimulating the Wnt Signalling Pathway Effector Tcf7l2. Cell Death Dis. 2017, 8, e2559. [Google Scholar] [CrossRef]
- Coucha, M.; Mohamed, I.N.; Elshaer, S.L.; Mbata, O.; Bartasis, M.L.; El-Remessy, A.B. High Fat Diet Dysregulates microRNA-17–5p and Triggers Retinal Inflammation: Role of Endoplasmic-Reticulum-Stress. World J. Diabetes 2017, 8, 56–65. [Google Scholar] [CrossRef]
- Tan, L.; Liu, L.; Jiang, Z.; Hao, X. Inhibition of microRNA-17-5p Reduces the Inflammation and Lipid Accumulation, and up-Regulates ATP-Binding Cassette TransporterA1 in Atherosclerosis. J. Pharmacol. Sci. 2019, 139, 280–288. [Google Scholar] [CrossRef]
- Dellago, H.; Bobbili, M.R.; Grillari, J. MicroRNA-17–5p: At the Crossroads of Cancer and Aging—A Mini-Review. Gerontology 2017, 63, 20–28. [Google Scholar] [CrossRef]
- Inukai, S.; de Lencastre, A.; Turner, M.; Slack, F. Novel microRNAs Differentially Expressed during Aging in the Mouse Brain. PLoS ONE 2012, 7, e40028. [Google Scholar] [CrossRef]
- Hebert, S.S.; Horre, K.; Nicolai, L.; Bergmans, B.; Papadopoulou, A.S.; Delacourte, A.; De Strooper, B. MicroRNA Regulation of Alzheimer’s Amyloid Precursor Protein Expression. Neurobiol. Dis. 2009, 33, 422–428. [Google Scholar] [CrossRef]
- Ruskovska, T.; Maksimova, V.; Milenkovic, D. Polyphenols in Human Nutrition: From the In Vitro Antioxidant Capacity to the Beneficial Effects on Cardiometabolic Health and Related Inter-Individual Variability—An Overview and Perspective. Br. J. Nutr. 2020, 123, 241–254. [Google Scholar] [CrossRef]
- Plummer, J.; Park, M.; Perodin, F.; Horowitz, M.C.; Hens, J.R. Methionine-Restricted Diet Increases miRNAs That Can Target RUNX2 Expression and Alters Bone Structure in Young Mice. J. Cell Biochem. 2017, 118, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Oger, F.; Gheeraert, C.; Mogilenko, D.; Benomar, Y.; Molendi-Coste, O.; Bouchaert, E.; Caron, S.; Dombrowicz, D.; Pattou, F.; Duez, H.; et al. Cell-Specific Dysregulation of microRNA Expression in Obese White Adipose Tissue. J. Clin. Endocrinol. Metab. 2014, 99, 2821–2833. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, J.F.; Neylon, A.; McGorrian, C.; Blake, G.J. miRNA-93-5p and Other miRNAs as Predictors of Coronary Artery Disease and STEMI. Int. J. Cardiol. 2016, 224, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Bushe, C.; Haddad, P.; Peveler, R.; Pendlebury, J. The Role of Lifestyle Interventions and Weight Management in Schizophrenia. J. Psychopharmacol. 2005, 19, 28–35. [Google Scholar] [CrossRef]
- Strassnig, M.; Brar, J.S.; Ganguli, R. Nutritional Assessment of Patients with Schizophrenia: A Preliminary Study. Schizophr. Bull. 2003, 29, 393–397. [Google Scholar] [CrossRef]
- McCreadie, R.G. Diet, Smoking and Cardiovascular Risk in People with Schizophrenia: Descriptive Study. Br. J. Psychiatry 2003, 183, 534–539. [Google Scholar] [CrossRef]
- Joseph, J.; Depp, C.; Shih, P.B.; Cadenhead, K.S.; Schmid-Schonbein, G. Modified Mediterranean Diet for Enrichment of Short Chain Fatty Acids: Potential Adjunctive Therapeutic to Target Immune and Metabolic Dysfunction in Schizophrenia? Front. Neurosci. 2017, 11, 155. [Google Scholar] [CrossRef]
- Bogomolova, S.; Zarnowiecki, D.; Wilson, A.; Fielder, A.; Procter, N.; Itsiopoulos, C.; O’Dea, K.; Strachan, J.; Ballestrin, M.; Champion, A.; et al. Dietary Intervention for People with Mental Illness in South Australia. Health Promot. Int. 2018, 33, 71–83. [Google Scholar] [CrossRef]
- Peet, M. The Metabolic Syndrome, Omega-3 Fatty Acids and Inflammatory Processes in Relation to Schizophrenia. Prostaglandins Leukot. Essent. Fatty Acids 2006, 75, 323–327. [Google Scholar] [CrossRef]
- Emsley, R.; Myburgh, C.; Oosthuizen, P.; van Rensburg, S.J. Randomized, Placebo-Controlled Study of Ethyl-Eicosapentaenoic Acid as Supplemental Treatment in Schizophrenia. Am. J. Psychiatry 2002, 159, 1596–1598. [Google Scholar] [CrossRef]
- Chen, A.T.; Chibnall, J.T.; Nasrallah, H.A. A Meta-Analysis of Placebo-Controlled Trials of Omega-3 Fatty Acid Augmentation in Schizophrenia: Possible Stage-Specific Effects. Ann. Clin. Psychiatry 2015, 27, 289–296. [Google Scholar] [PubMed]
- Skoldstam, L.; Hagfors, L.; Johansson, G. An Experimental Study of a Mediterranean Diet Intervention for Patients with Rheumatoid Arthritis. Ann. Rheum. Dis. 2003, 62, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Sepodes, B.; Rocha, J.; Direito, R.; Fernandes, A.; Brites, D.; Freitas, M.; Fernandes, E.; Bronze, M.R.; Figueira, M.E. Protective Effects of Hydroxytyrosol-Supplemented Refined Olive Oil in Animal Models of Acute Inflammation and Rheumatoid Arthritis. J. Nutr. Biochem. 2015, 26, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Sugioka, Y.; Tada, M.; Okano, T.; Mamoto, K.; Inui, K.; Habu, D.; Koike, T. Monounsaturated Fatty Acids Might Be Key Factors in the Mediterranean Diet that Suppress Rheumatoid Arthritis Disease Activity: The TOMORROW Study. Clin. Nutr. 2018, 37, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, C.; Kouvari, M.; D’Cunha, N.M.; Georgousopoulou, E.N.; Panagiotakos, D.B.; Mellor, D.D.; Kellett, J.; Naumovski, N. The Effects of the Mediterranean Diet on Rheumatoid Arthritis Prevention and Treatment: A Systematic Review of Human Prospective Studies. Rheumatol. Int. 2018, 38, 737–747. [Google Scholar] [CrossRef]
- Petersson, S.; Philippou, E.; Rodomar, C.; Nikiphorou, E. The Mediterranean Diet, Fish Oil Supplements and Rheumatoid Arthritis Outcomes: Evidence from Clinical Trials. Autoimmun. Rev. 2018, 17, 1105–1114. [Google Scholar] [CrossRef]
- Porras, M.; Rada, G.; Duran, J. Effects of Mediterranean diet on the Treatment of Rheumatoid Arthritis. Medwave 2019, 19, e7640. [Google Scholar] [CrossRef]
- Laudisio, D.; Castellucci, B.; Barrea, L.; Pugliese, G.; Savastano, S.; Colao, A.; Muscogiuri, G. Mediterranean Diet and Breast Cancer Risk: A Narrative Review. Minerva Endocrinol. 2020. [Google Scholar] [CrossRef]
- Dianatinasab, M.; Rezaian, M.; HaghighatNezad, E.; Bagheri-Hosseinabadi, Z.; Amanat, S.; Rezaeian, S.; Masoudi, A.; Ghiasvand, R. Dietary Patterns and Risk of Invasive Ductal and Lobular Breast Carcinomas: A Systematic Review and Meta-Analysis. Clin. Breast Cancer 2020, 20, e516–e528. [Google Scholar] [CrossRef]
- Russo, G.I.; Solinas, T.; Urzi, D.; Privitera, S.; Campisi, D.; Cocci, A.; Carini, M.; Madonia, M.; Cimino, S.; Morgia, G. Adherence to Mediterranean Diet and Prostate Cancer Risk in Sicily: Population-Based Case-Control Study. Int. J. Impot. Res. 2019, 31, 269–275. [Google Scholar] [CrossRef]
- Schneider, L.; Su, L.J.; Arab, L.; Bensen, J.T.; Farnan, L.; Fontham, E.T.H.; Song, L.; Hussey, J.; Merchant, A.T.; Mohler, J.L.; et al. Dietary Patterns Based on the Mediterranean Diet and DASH Diet Are Inversely Associated with High Aggressive Prostate Cancer in PCaP. Ann. Epidemiol. 2019, 29, 16–22.e1. [Google Scholar] [CrossRef] [PubMed]
- Sealy, N.; Hankinson, S.E.; Houghton, S.C. Olive Oil and Risk of Breast Cancer: A Systematic Review and Dose-Response Meta-Analysis of Observational Studies. Br. J. Nutr. 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Urquiza-Salvat, N.; Pascual-Geler, M.; Lopez-Guarnido, O.; Rodrigo, L.; Martinez-Burgos, A.; Cozar, J.M.; Ocana-Peinado, F.M.; Alvarez-Cubero, M.J.; Rivas, A. Adherence to Mediterranean Diet and Risk of Prostate Cancer. Aging Male 2019, 22, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Porciello, G.; Montagnese, C.; Crispo, A.; Grimaldi, M.; Libra, M.; Vitale, S.; Palumbo, E.; Pica, R.; Calabrese, I.; Cubisino, S.; et al. Mediterranean Diet and Quality of Life in Women Treated for Breast Cancer: A Baseline Analysis of DEDiCa Multicentre Trial. PLoS ONE 2020, 15, e0239803. [Google Scholar] [CrossRef] [PubMed]
- Baguley, B.J.; Skinner, T.L.; Jenkins, D.G.; Wright, O.R.L. Mediterranean-Style Dietary Pattern Improves Cancer-Related Fatigue and Quality of Life in Men with Prostate Cancer Treated with Androgen Deprivation Therapy: A Pilot Randomised Control Trial. Clin. Nutr. 2020. [Google Scholar] [CrossRef]






| Diets | Datasets | Platform | Sample # | Age | Sex (%F) | Health | Reference |
|---|---|---|---|---|---|---|---|
| Mediterranean diet + Olive oil | GSE28358 | GPL571 [HG-U133A_2] Affymetrix Human Genome U133A 2.0 Array | 12 | 62 ± 8 | 45 | High risk of coronary artery disease | [36] |
| Olive oil | GSE75025 | GPL10558 Illumina HumanHT-12 V4.0 expression beadchip | 12 | 29 ± 2 | 50 | Healthy | [37] |
| Olive leaf extract | GSE87300 | GPL13667 [HG-U219] Affymetrix Human Genome U219 Array | 15 | 32 | 0 | Healthy | [38] |
| Mediterranean diet + Nuts | GSE28358 | GPL571 [HG-U133A_2] Affymetrix Human Genome U133A 2.0 Array | 10 | 63 ± 6 | 45 | High risk of coronary artery disease | [36] |
| Fish oil 3 weeks | GSE48368 | GPL10558 Illumina HumanHT-12 V4.0 expression beadchip | 17 | 27.2 ± 6.9 | 71 | Healthy | [39] |
| Fish oil 7 weeks | GSE48368 | GPL10558 Illumina HumanHT-12 V4.0 expression beadchip | 17 | 27.2 ± 6.9 | 71 | Healthy | [39] |
| EPA + DHA | GSE12375 | GPL7144 NuGO array (human) NuGO_Hs1a520180 | 23 | 69.9 (67-76) | 35 | Healthy | [40] |
| EPA + DHA | E-MTAB-48 | A-AFFY-111—Affymetrix Custom Array | 23 | 69.9 (67-76) | 35 | Healthy | [40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bottero, V.; Potashkin, J.A. A Comparison of Gene Expression Changes in the Blood of Individuals Consuming Diets Supplemented with Olives, Nuts or Long-Chain Omega-3 Fatty Acids. Nutrients 2020, 12, 3765. https://doi.org/10.3390/nu12123765
Bottero V, Potashkin JA. A Comparison of Gene Expression Changes in the Blood of Individuals Consuming Diets Supplemented with Olives, Nuts or Long-Chain Omega-3 Fatty Acids. Nutrients. 2020; 12(12):3765. https://doi.org/10.3390/nu12123765
Chicago/Turabian StyleBottero, Virginie, and Judith A. Potashkin. 2020. "A Comparison of Gene Expression Changes in the Blood of Individuals Consuming Diets Supplemented with Olives, Nuts or Long-Chain Omega-3 Fatty Acids" Nutrients 12, no. 12: 3765. https://doi.org/10.3390/nu12123765
APA StyleBottero, V., & Potashkin, J. A. (2020). A Comparison of Gene Expression Changes in the Blood of Individuals Consuming Diets Supplemented with Olives, Nuts or Long-Chain Omega-3 Fatty Acids. Nutrients, 12(12), 3765. https://doi.org/10.3390/nu12123765




