Acer tegmentosum Maxim Inhibits Adipogenesis in 3t3-l1 Adipocytes and Attenuates Lipid Accumulation in Obese Rats Fed a High-Fat Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of ATM
2.2. Cell Culture
2.3. Oil Red O Staining and Triglyceride Assay
2.4. RT-PCR
2.5. Western Blot Analysis
2.6. Animal Experiments
2.7. Biochemical Assays
2.8. Histological Analysis
2.9. Statistical Analysis
3. Results
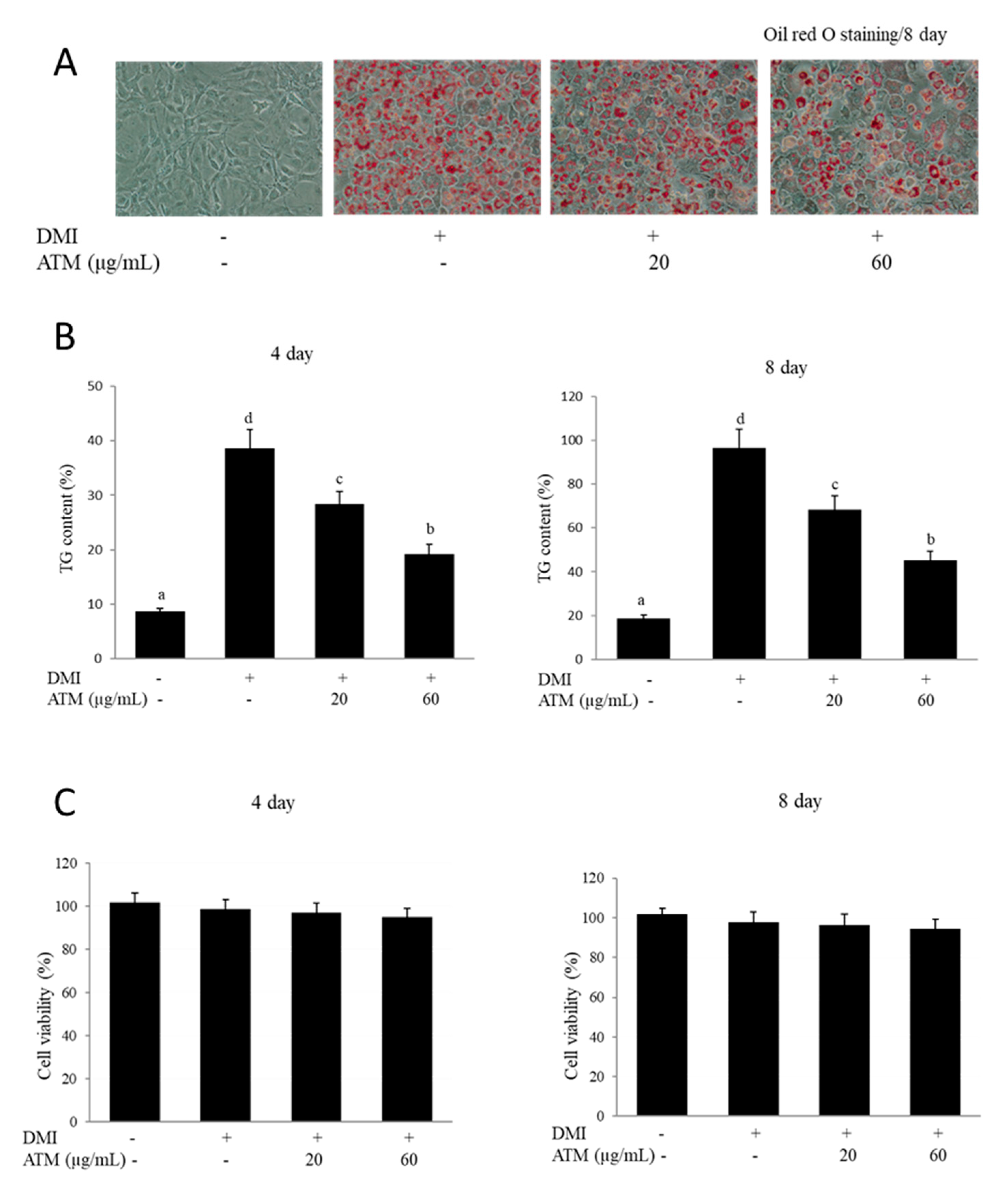
3.1. Effect of ATM on Intracellular Lipid Accumulation and TG Content in 3T3-L1 Cells
3.2. Effect of ATM on Cell Viability
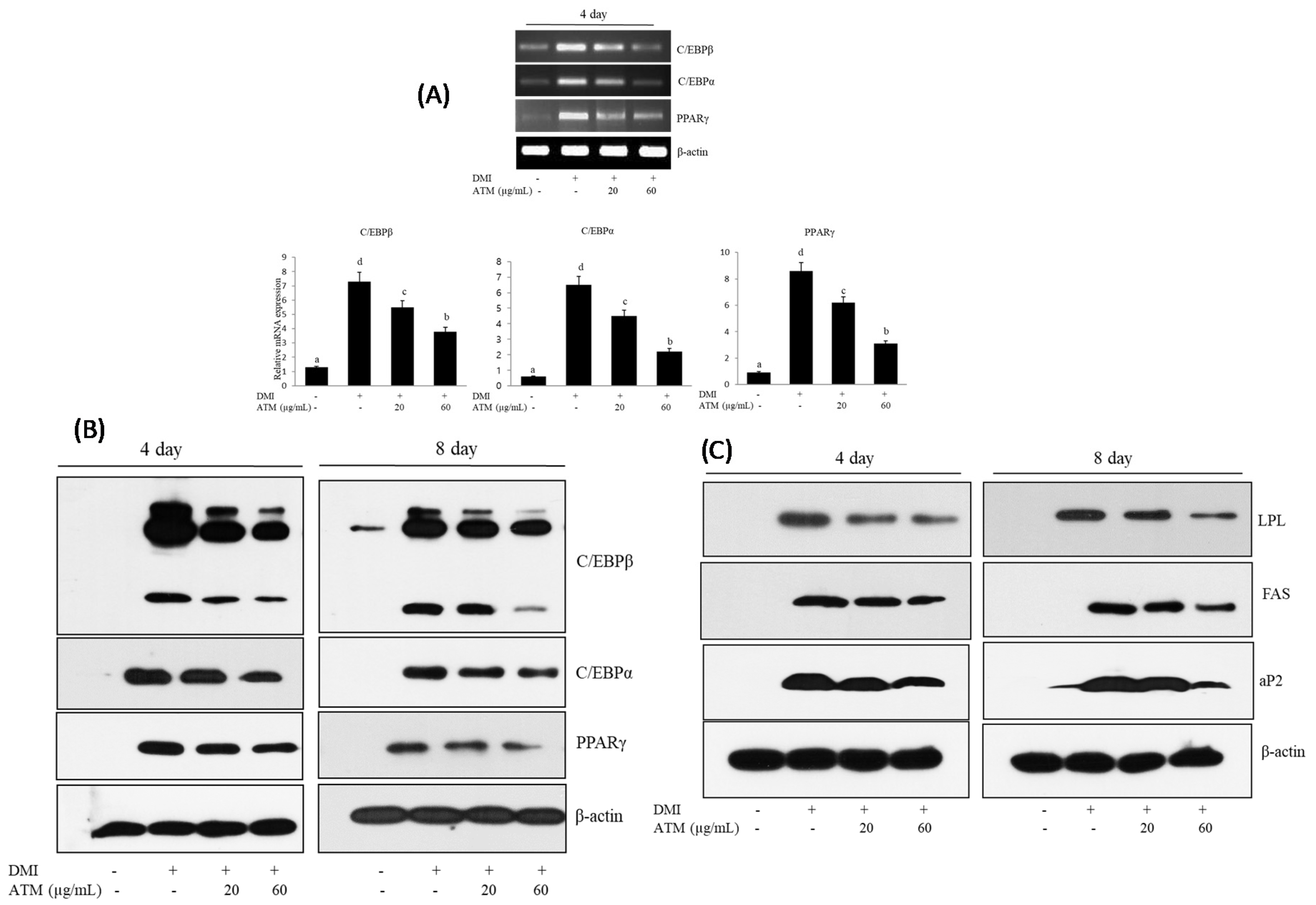
3.3. Effect of ATM on the Expression of Adipogenic-Specific Genes during Adipocyte Differentiation
3.4. Effect of ATM on the Expression of Lipogenesis-Specific Genes during Adipocyte Differentiation
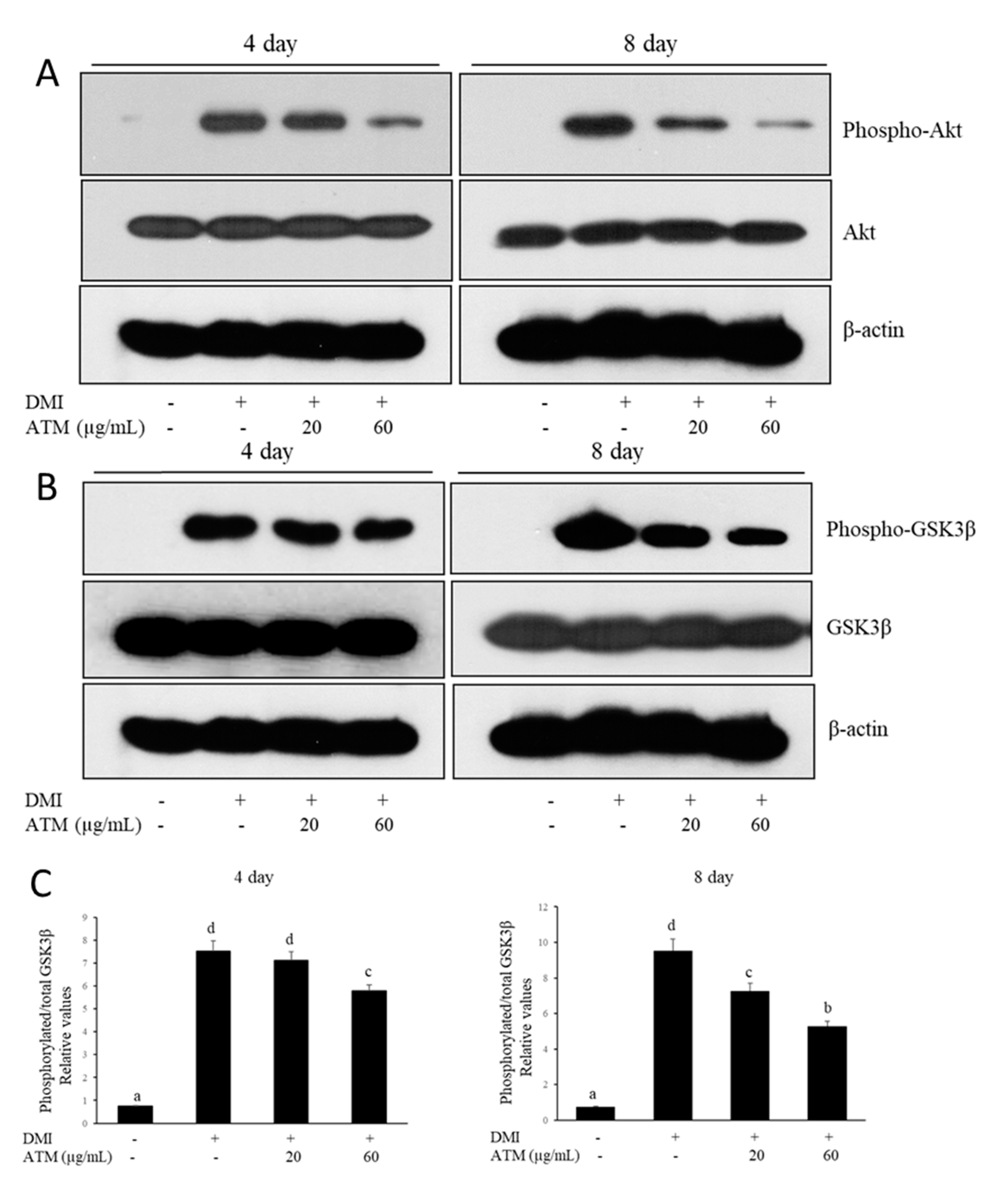
3.5. Effect of ATM on the Regulation of Akt and GSK3β during Adipocyte Differentiation
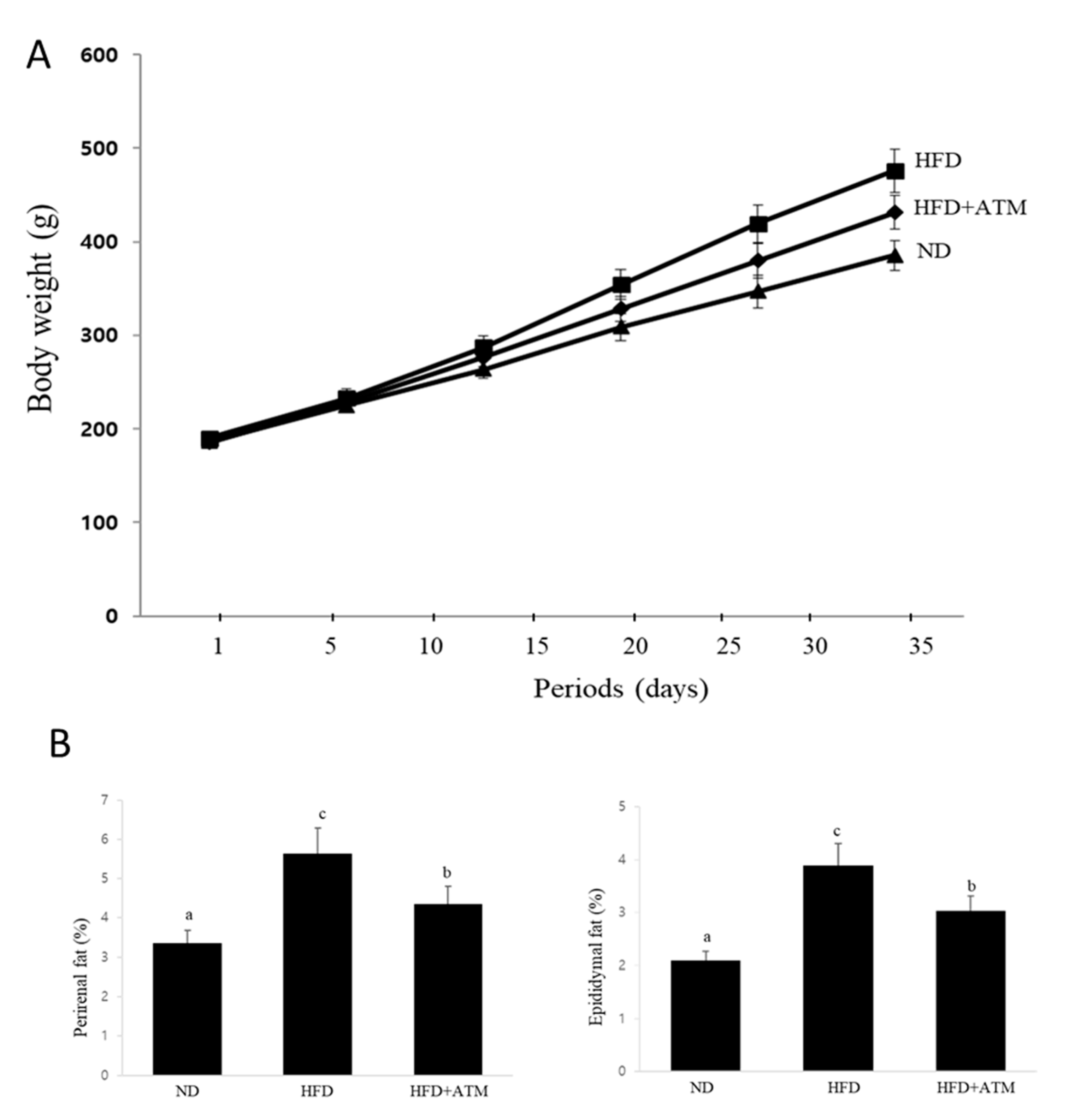
3.6. Effect of ATM on Body Weight and Adipose Tissue Weight in HFD-Induced Obese Rats
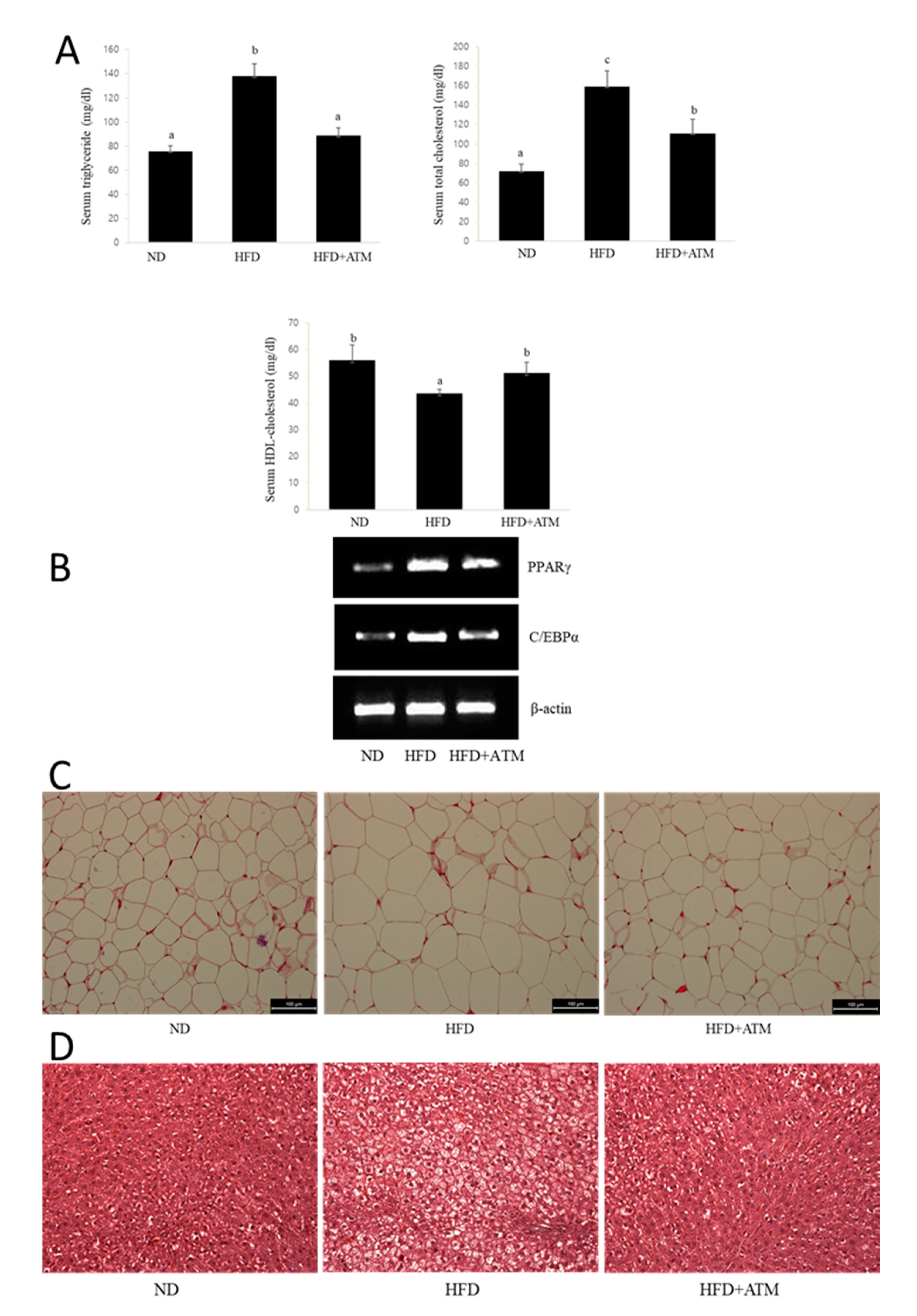
3.7. Effect of ATM on Blood Biochemical Indexes in HFD-Induced Obese Rats
3.8. Effect of ATM on Adipogenic Gene Expression or Lipid Accumulation in Epidydymal Adipose Tissue
3.9. Effect of ATM in Fatty Liver Tissue of HFD-Induced Obese Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and Management of the Metabolic Syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebbert, J.O.; Jensen, M.D. Fat Depots, Free Fatty Acids, and Dyslipidemia. Nutrients 2013, 5, 498–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Jeon, T.; Hwang, S.G.; Hirai, S.; Matsui, T.; Yano, H.; Kawada, T.; Lim, B.O.; Park, D.K. Red yeast rice extracts suppress adipogenesis by down-regulating adipogenic transcription factors and gene expression in 3T3-L1 cells. Life Sci. 2004, 75, 3195–3203. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.E.; Erickson, R.L.; Gerin, I.; Derose, P.M.; Bajnok, L.; Longo, K.A.; Misek, D.E.; Kuick, R.; Hanash, S.M.; Atkins, K.B.; et al. Microarray Analyses during Adipogenesis: Understanding the Effects of Wnt Signaling on Adipogenesis and the Roles of Liver X Receptor α in Adipocyte Metabolism. Mol. Cell. Biol. 2002, 22, 5989–5999. [Google Scholar] [CrossRef] [Green Version]
- Rosen, E.D.; MacDougald, O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006, 7, 885–896. [Google Scholar] [CrossRef]
- Lowe, C.E.; O’Rahilly, S.; Rochford, J.J. Adipogenesis at a glance. J. Cell Sci. 2011, 124, 2681–2686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosen, E.D.; Walkey, C.J.; Puigserver, P.; Spiegelman, B.M. Transcriptional regulation of adipogenesis. Genes Dev. 2000, 14, 1293–1307. [Google Scholar]
- Tang, Q.-Q.; Lane, M.D. Adipogenesis: From Stem Cell to Adipocyte. Annu. Rev. Biochem. 2012, 81, 715–736. [Google Scholar] [CrossRef] [Green Version]
- Makowski, L.; Brittingham, K.C.; Reynolds, J.M.; Suttles, J.; Hotamisligil, G.S. The Fatty Acid-binding Protein, aP2, Coordinates Macrophage Cholesterol Trafficking and Inflammatory Activity. J. Biol. Chem. 2005, 280, 12888–12895. [Google Scholar] [CrossRef] [Green Version]
- Moseti, D.; Regassa, A.; Kim, W. Molecular Regulation of Adipogenesis and Potential Anti-Adipogenic Bioactive Molecules. Int. J. Mol. Sci. 2016, 17, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magun, R.; Burgering, B.M.; Coffer, P.J.; Pardasani, D.; Lin, Y.; Chabot, J.; Sorisky, A. Expression of a constitutively activated form of protein kinase B (c-Akt) in 3T3-L1 preadipose cells causes spontaneous differentiation. Endocrinol. 1996, 137, 3590–3593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garofalo, R.S.; Orena, S.J.; Rafidi, K.; Torchia, A.J.; Stock, J.L.; Hildebrandt, A.L.; Coskran, T.; Black, S.C.; Brees, D.J.; Wicks, J.R.; et al. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKBβ. J. Clin. Investig. 2003, 112, 197–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, C.J.; Göransson, O.; Kular, G.S.; Leslie, N.R.; Gray, A.; Alessi, D.R.; Sakamoto, K.; Hundal, H.S. Use of Akt Inhibitor and a Drug-resistant Mutant Validates a Critical Role for Protein Kinase B/Akt in the Insulin-dependent Regulation of Glucose and System A Amino Acid Uptake. J. Biol. Chem. 2008, 283, 27653–27667. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.-D.; Xu, P.-Z.; Chen, M.-L.; Hahn-Windgassen, A.; Skeen, J.; Jacobs, J.; Sundararajan, D.; Chen, W.S.; Crawford, S.E.; Coleman, K.G.; et al. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 2003, 17, 1352–1365. [Google Scholar] [CrossRef] [Green Version]
- Kohn, A.D.; Summers, S.A.; Birnbaum, M.J.; Roth, R.A. Expression of a Constitutively Active Akt Ser/Thr Kinase in 3T3-L1 Adipocytes Stimulates Glucose Uptake and Glucose Transporter 4 Translocation. J. Biol. Chem. 1996, 271, 31372–31378. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Liao, K. Protein Kinase B/AKT 1 Plays a Pivotal Role in Insulin-like Growth Factor-1 Receptor Signaling Induced 3T3-L1 Adipocyte Differentiation. J. Biol. Chem. 2004, 279, 35914–35922. [Google Scholar] [CrossRef] [Green Version]
- Baudry, A.; Yang, Z.; Hemmings, B.A. PKB is required for adipose differentiation of mouse embryonic fibroblasts. J. Cell Sci. 2006, 119, 889–897. [Google Scholar] [CrossRef] [Green Version]
- Yu, T.; Lee, J.; Lee, Y.G.; Byeon, S.E.; Kim, M.H.; Sohn, E.-H.; Lee, S.G.; Cho, J.Y. In vitro and in vivo anti-inflammatory effects of ethanol extract from Acer tegmentosum. J. Ethnopharmacol. 2010, 128, 139–147. [Google Scholar] [CrossRef]
- Tung, N.H.; Ding, Y.; Kim, S.K.; Bae, K.; Kim, Y.H. Total Peroxyl Radical-Scavenging Capacity of the Chemical Components from the Stems of Acer tegmentosum Maxim. J. Agric. Food Chem. 2008, 56, 10510–10514. [Google Scholar] [CrossRef]
- Park, K.M.; Yang, M.C.; Lee, K.H.; Kim, K.R.; Choi, S.U.; Lee, K.R. Cytotoxic phenolic constituents of Acer tegmentosum maxim. Arch. Pharmacal Res. 2006, 29, 1086–1090. [Google Scholar] [CrossRef] [PubMed]
- Kim, S. Antioxidant and anti-inflammatory compounds isolated from Acer tegmentosum. J. Med. Plants Res. 2012, 6, 3971–3976. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kang, S.J.; Kim, J.W.; Cho, H.R.; Moon, S.B.; Kim, K.Y.; Lee, H.S.; Han, C.H.; Ku, S.K.; Lee, Y.J. Effects of Polycan, a β-glucan, on experimental periodontitis and alveolar bone loss in Sprague-Dawley rats. J. Periodontal Res. 2012, 47, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Tao, J.; Toguchida, I.; Matsuda, A.H.; Yoshikawa, M. Structures of New Cyclic Diarylheptanoids and Inhibitors of Nitric Oxide Production from Japanese Folk MedicineAcernikoense1. J. Nat. Prod. 2003, 66, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, T.; Lee, J.-W.; Akazawa, H.; Inagaki, M.; Cha, B.-Y.; Nagai, K.; Yagasaki, K.; Akihisa, T.; Woo, J.-T. Osteogenic activity of diphenyl ether-type cyclic diarylheptanoids derived from Acer nikoense. Bioorganic Med. Chem. Lett. 2011, 21, 3248–3251. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Jones, C.P.; Karonen, M.; Salminen, J.-P. Tannin Composition Affects the Oxidative Activities of Tree Leaves. J. Chem. Ecol. 2006, 32, 2235–2251. [Google Scholar] [CrossRef]
- Kim, E.-C.; Kim, S.H.; Piao, S.-J.; Kim, T.-J.; Bae, K.; Kim, H.S.; Hong, S.-S.; Lee, B.I.; Nam, M.-S. Antiangiogenic Activity of Acer tegmentosum Maxim Water Extract in Vitro and in Vivo. J. Korean Med Sci. 2015, 30, 979–987. [Google Scholar] [CrossRef] [Green Version]
- Poulos, S.P.; Dodson, M.V.; Hausman, G.J. Cell line models for differentiation: Preadipocytes and adipocytes. Exp. Biol. Med. 2010, 235, 1185–1193. [Google Scholar] [CrossRef]
- Wang, N.D.; Finegold, M.J.; Bradley, A.; Ou, C.N.; Abdelsayed, S.V.; Wilde, M.D.; Taylor, L.R.; Wilson, D.R.; Darlington, G.J. Impaired energy homeostasis in C/EBP alpha knockout mice. Science 1995, 269, 1108–1112. [Google Scholar] [CrossRef]
- He, W.; Barak, Y.; Hevener, A.; Olson, P.; Liao, D.; Le, J.; Nelson, M.; Ong, E.; Olefsky, J.M.; Evans, R.M. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc. Natl. Acad. Sci. USA 2003, 100, 15712–15717. [Google Scholar] [CrossRef] [Green Version]
- Gwon, S.Y.; Ahn, J.Y.; Jung, C.H.; Moon, B.K.; Ha, T.Y. Shikonin suppresses ERK 1/2 phosphorylation during the early stages of adipocyte differentiation in 3T3-L1 cells. BMC Complementary Altern. Med. 2013, 13, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heikkinen, S.; Auwerx, J.; Argmann, C.A. PPARgamma in human and mouse physiology. Biochim Biophys Acta. 2007, 1771, 999–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tontonoz, P.; Hu, E.; A Graves, R.; I Budavari, A.; Spiegelman, B.M. mPPAR gamma 2: Tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994, 8, 1224–1234. [Google Scholar] [CrossRef] [Green Version]
- Tontonoz, P.; Hu, E.; Spiegelman, B.M. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell 1994, 79, 1147–1156. [Google Scholar] [CrossRef]
- Auwerx, J. PPARγ, the ultimate thrifty gene. Diabetologia 1999, 42, 1033–1049. [Google Scholar] [CrossRef] [Green Version]
- Lodhi, I.J.; Wei, X.; Semenkovich, C.F. Lipoexpediency: De novo lipogenesis as a metabolic signal transmitter. Trends Endocrinol. Metab. 2011, 22, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kajno, E.; McGraw, T.E.; González, E. Development of a new model system to dissect isoform specific Akt signalling in adipocytes. Biochem. J. 2015, 468, 425–434. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.H.; Huang, J.; Düvel, K.; Boback, B.; Wu, S.; Squillace, R.M.; Wu, C.-L.; Manning, B.D. Insulin Stimulates Adipogenesis through the Akt-TSC2-mTORC1 Pathway. PLoS ONE 2009, 4, e6189. [Google Scholar] [CrossRef] [PubMed]
- Yoshiga, D.; Sato, N.; Torisu, T.; Mori, H.; Yoshida, R.; Nakamura, S.; Takaesu, G.; Kobayashi, T.; Yoshimura, A. Adaptor Protein SH2-B Linking Receptor-Tyrosine Kinase and Akt Promotes Adipocyte Differentiation by Regulating Peroxisome Proliferator-Activated Receptor γ Messenger Ribonucleic Acid Levels. Mol. Endocrinol. 2007, 21, 1120–1131. [Google Scholar] [CrossRef] [Green Version]
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell. Mol. Life Sci. 2018, 75, 3313–3327. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, H.-H.; Lee, S.-J.; Kim, S.-H.; Jang, S.-H.; Won, C.; Kim, H.-D.; Kim, T.H.; Cho, J.-H. Acer tegmentosum Maxim Inhibits Adipogenesis in 3t3-l1 Adipocytes and Attenuates Lipid Accumulation in Obese Rats Fed a High-Fat Diet. Nutrients 2020, 12, 3753. https://doi.org/10.3390/nu12123753
Cho H-H, Lee S-J, Kim S-H, Jang S-H, Won C, Kim H-D, Kim TH, Cho J-H. Acer tegmentosum Maxim Inhibits Adipogenesis in 3t3-l1 Adipocytes and Attenuates Lipid Accumulation in Obese Rats Fed a High-Fat Diet. Nutrients. 2020; 12(12):3753. https://doi.org/10.3390/nu12123753
Chicago/Turabian StyleCho, Hang-Hee, Soo-Jung Lee, Sung-Ho Kim, Sun-Hee Jang, Chungkil Won, Hong-Duck Kim, Tae Hoon Kim, and Jae-Hyeon Cho. 2020. "Acer tegmentosum Maxim Inhibits Adipogenesis in 3t3-l1 Adipocytes and Attenuates Lipid Accumulation in Obese Rats Fed a High-Fat Diet" Nutrients 12, no. 12: 3753. https://doi.org/10.3390/nu12123753
APA StyleCho, H.-H., Lee, S.-J., Kim, S.-H., Jang, S.-H., Won, C., Kim, H.-D., Kim, T. H., & Cho, J.-H. (2020). Acer tegmentosum Maxim Inhibits Adipogenesis in 3t3-l1 Adipocytes and Attenuates Lipid Accumulation in Obese Rats Fed a High-Fat Diet. Nutrients, 12(12), 3753. https://doi.org/10.3390/nu12123753





